Abstract
Abstract 4202
It is unknown whether patients with cancer who develop VTE after a surgical procedure have the same risk of recurrent VTE as clinical patients cancer-associated thrombosis. VTE recurrence risk in non-cancer patients with VTE after surgery is approximately 1% in the 3 months following completion of anticoagulation. It is unknown whether surgical patients with cancer follow the low risk of recurrence as other provoked VTEs or whether they have the high recurrence risk typical of cancer patients.
We performed a post-hoc analysis of a single centre retrospective cohort study conducted at the Thrombosis Unit of the Ottawa Hospital. The charts of patients with cancer and VTE followed from 2002 to 2004 and from 2007 to 2008 were reviewed. We sought to compare the risk of recurrent VTE between patients with cancer who developed a first VTE after major surgery with all other patients with cancer-associated thrombosis. We included patients > or = 18 years of age with active malignancy and objectively diagnosed index VTE [pulmonary embolism (PE), proximal deep venous thrombosis (DVT) of the legs or arms, PE + DVT; unusual site thrombosis]. After the first VTE, all patients received a minimum of 6 months of anticoagulation. In the surgery group, index VTE was considered associated with the intervention if it occurred within the first 3 months after the procedure.
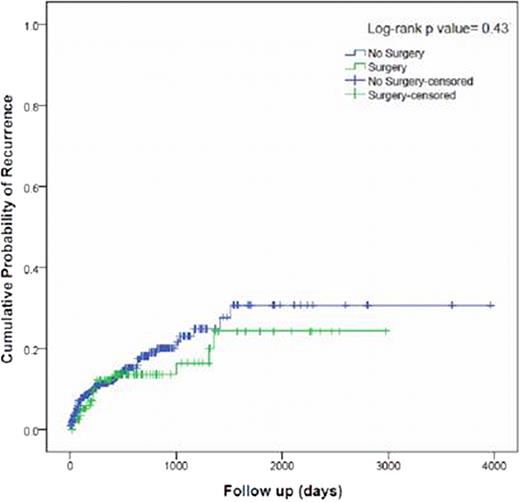
543 patients were included. 121 patients had VTE after surgery and 17 (13.1%) developed a recurrence during therapeutic anticoagulation. Of 422 clinical patients, 61 (14.7%) had a recurrent VTE (Table). The relative risk of recurrent VTE comparing patients who had and who did not have surgery was non-significant (RR= 0.97 (95%CI: 0.587 – 1.574; p= 1.000) suggesting that patients with cancer who undergo surgery have similar risk of developing a recurrent VTE during anticoagulation as patients with cancer-associated VTE who do not undergo surgery. VTE recurrence occurred predominantly within the first 6 months of anticoagulation [Surgery: 9 of 17 patients (52.9 %); no surgery: 45 of 61 (73.7%) patients (p=0.1377)] (Figure). There was no significant difference in VTE recurrence risk according to anticoagulant strategy, tumor site, histology, TNM stage, age or gender between surgery and no surgery groups.
Patients with cancer who develop VTE after surgery have similar risk of developing a recurrent VTE during the anticoagulation period as clinical patients with cancer-associated VTE.
Baseline characteristics of patients with malignancy-associated thrombosis according to previous surgical history.
| Characteristics . | Surgery (N=121) . | No surgery (N=422) . | P . |
|---|---|---|---|
| Age in years [mean (SD)] | 62.7 (13.5) | 63.1 (13.6) | 0.774 |
| Male gender [N(%)] | 55 (45.45) | 184 (43.6) | 0.796 |
| Primary Tumor Site [N(%)] | <0.0001 | ||
| Hematological | 4 (3.31) | 54 (12.8) | |
| Thorax# | 11 (9.09) | 88 (20.85) | |
| Gastrointestinal | 50 (41.32) | 99 (23.46) | |
| Breast | 12 (9.92) | 73 (17.3) | |
| Central nervous system | 15 (12.4) | 15 (3.55) | |
| Genito-urinary | 11 (9.09) | 36 (8.53) | |
| Gynecological | 12 (9.92) | 38 (9) | |
| Musculo-skeletal | 6 (4.96) | 14 (3.32) | |
| Mouth/salivary glands | 0 (0) | 5 (1.18) | |
| Histology [N(%)] | 0.017 | ||
| Adenocarcinoma | 75 (61.98) | 231 (54.74) | |
| Non adenocarcinoma | 42 (34.71) | 140 (33.18) | |
| Not applicable | 4 (3.31) | 51 (12.09) | |
| TNM Stage [N(%)] | <0.001 | ||
| I | 21 (17.36) | 40 (9.48) | |
| II | 25 (20.66) | 49 (11.61) | |
| III | 22 (18.18) | 62 (14.69) | |
| IV | 34 (28.1) | 203 (48.1) | |
| Not applicable | 19 (15.7) | 68 (16.11) | |
| Index VTE site [N(%)] | <0.001 | ||
| Deep vein thrombosis (lower limb) | 41 (32.71) | 167 (39.57) | |
| Pulmonary embolism | 50 (17.82) | 91 (21.56) | |
| Deep vein thrombosis and pulmonary embolism | 19 (15.7) | 48 (11.37) | |
| Deep vein thrombosis (upper limb) | 8 (6.61) | 98 (23.22) | |
| Unusual site | 3 (2.48) | 18 (4.27) | |
| Anticoagulant treatment [N(%)] | 0.531 | ||
| Low molecular weight heparin | 73 (60.33) | 270 (63.98) | |
| Warfarin | 48 (39.67) | 152 (36.02) | |
| VTE£ recurrence [N(%)] | 17 (14.05) | 61 (14.45) | 0.972 |
| Characteristics . | Surgery (N=121) . | No surgery (N=422) . | P . |
|---|---|---|---|
| Age in years [mean (SD)] | 62.7 (13.5) | 63.1 (13.6) | 0.774 |
| Male gender [N(%)] | 55 (45.45) | 184 (43.6) | 0.796 |
| Primary Tumor Site [N(%)] | <0.0001 | ||
| Hematological | 4 (3.31) | 54 (12.8) | |
| Thorax# | 11 (9.09) | 88 (20.85) | |
| Gastrointestinal | 50 (41.32) | 99 (23.46) | |
| Breast | 12 (9.92) | 73 (17.3) | |
| Central nervous system | 15 (12.4) | 15 (3.55) | |
| Genito-urinary | 11 (9.09) | 36 (8.53) | |
| Gynecological | 12 (9.92) | 38 (9) | |
| Musculo-skeletal | 6 (4.96) | 14 (3.32) | |
| Mouth/salivary glands | 0 (0) | 5 (1.18) | |
| Histology [N(%)] | 0.017 | ||
| Adenocarcinoma | 75 (61.98) | 231 (54.74) | |
| Non adenocarcinoma | 42 (34.71) | 140 (33.18) | |
| Not applicable | 4 (3.31) | 51 (12.09) | |
| TNM Stage [N(%)] | <0.001 | ||
| I | 21 (17.36) | 40 (9.48) | |
| II | 25 (20.66) | 49 (11.61) | |
| III | 22 (18.18) | 62 (14.69) | |
| IV | 34 (28.1) | 203 (48.1) | |
| Not applicable | 19 (15.7) | 68 (16.11) | |
| Index VTE site [N(%)] | <0.001 | ||
| Deep vein thrombosis (lower limb) | 41 (32.71) | 167 (39.57) | |
| Pulmonary embolism | 50 (17.82) | 91 (21.56) | |
| Deep vein thrombosis and pulmonary embolism | 19 (15.7) | 48 (11.37) | |
| Deep vein thrombosis (upper limb) | 8 (6.61) | 98 (23.22) | |
| Unusual site | 3 (2.48) | 18 (4.27) | |
| Anticoagulant treatment [N(%)] | 0.531 | ||
| Low molecular weight heparin | 73 (60.33) | 270 (63.98) | |
| Warfarin | 48 (39.67) | 152 (36.02) | |
| VTE£ recurrence [N(%)] | 17 (14.05) | 61 (14.45) | 0.972 |
Thorax includes patients with lung cancer and thymoma;
VTE: Venous thromboembolism.
Kaplan-Meier estimates of the cumulative probability of VTE recurrence among patients with cancer, according to previous surgery exposure.
Kaplan-Meier estimates of the cumulative probability of VTE recurrence among patients with cancer, according to previous surgery exposure.
Rodger:Pfizer: Research Funding; Leo Pharma: Research Funding; Sanofi Aventis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Canadian Institutes of Health Research: Research Funding; Heart and Stroke Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal