Abstract
Abstract 428
Galiximab is a primatized chimeric monoclonal antibody directed against CD80, an immunoregulatory protein normally expressed on antigen presenting cells and T cells, as well as in B-cell NHL, Hodgkin lymphoma, multiple myeloma, and certain leukemias. Galiximab directly mediates antibody-dependent cell-mediated cytotoxicity against tumor cells in vitro. In ex vivo assays, galiximab can act on non-malignant cells to modulate immune signaling within the tumor microenvironment.
Subjects with relapsed or refractory, Grade I-IIIa, follicular NHL in relapse following treatment with at least 1 chemotherapy regimen, and who were not refractory to rituximab were randomized to rituximab (375 mg/m2) plus galiximab (500 mg/m2; R+G) or rituximab plus placebo (R+P) and treated on Days 1, 8, 15, and 22. Randomization and primary efficacy analyses were stratified by age (≤60 vs >60), rituximab exposure (rituximab naïve vs non-naïve), and baseline tumor bulk (diameter of largest lesion ≤7 cm vs >7 cm). Primary endpoint of progression-free survival (PFS) was analyzed using a stratified log-rank test.
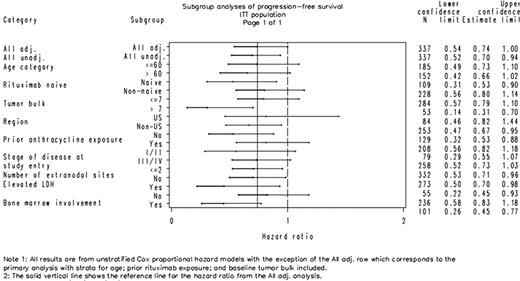
This study, originally planned as a Phase 3 confirmatory study, was terminated early due to changes in standard of care and converted to a Phase 2 study. Therefore, interpretation of p-values was focused on assessing the potential of these data to support subsequent Phase 2 and Phase 3 studies. At study termination, 337 subjects were randomized (175 R+G and 162 R+P) with median follow-up of 13.8 months. Demographics and disease characteristics were well balanced across the 2 treatment groups (Table 1). The addition of galiximab to rituximab reduced the hazard for disease progression or death by 26% (hazard ratio [HR] = 0.738; 95% confidence interval [CI] [0.543, 1.002]; p = 0.050) compared to the R+P group. Kaplan-Meier median PFS was 12.0 months (95% CI [9.0, 14.7]) for R+G and 9.0 months (95% CI [8.9, 10.5]) for R+P. Overall response rate was 51% for R+G vs 48% for R+P (p = 0.455) and complete response was 20% for R+G and 15% for R+P (p = 0.251). Consistency in treatment effect was seen across patient subgroups (Figure 1). A trend toward a larger PFS effect was observed in patients who were rituximab-naïve, had bulky tumor (largest lesion >7 cm), had lactate dehydrogenase (LDH) >1 × upper limit of normal, or had bone marrow involvement at study entry. There were 10 deaths in R+G vs 17 deaths in R+P; HR = 0.549 based on a stratified log-rank analysis (95% CI [0.248, 1.217]; p = 0.135). No substantial difference was observed between groups for Grade 3/4 adverse events (AEs) or serious AEs, and there were no treatment-related deaths in either group. Incidence of AEs was ≥3% higher in the R+G vs R+P group for the following: pyrexia (18% vs 11%), headache (13% vs 7%), cough (10% vs 6%), upper respiratory infection (8% vs 4%), insomnia (8% vs 4%), neutropenia (6% vs 3%), muscle spasms (5% vs <1%), and oropharyngeal pain (4% vs 1%). Anti-galiximab antibodies were not detected in 169 subjects treated with galiximab who were tested while on study.
Galiximab in combination with rituximab demonstrated a trend toward an improved PFS compared with rituximab alone and was well tolerated in subjects with relapsed or refractory follicular NHL.
Demographics and Baseline Disease Characteristics, Galiximab Clinical Study
| . | R+G Group(N = 175) . | R+P Group(N = 162) . | Total(N = 337) . |
|---|---|---|---|
| Age: | |||
| ≤60 years | 53% | 57% | 55% |
| Median | 59.0 y | 59.0 y | 59.0 y |
| Min, Max | 27, 91 y | 33, 88 y | 27, 91 y |
| Male | 46% | 52% | 49% |
| Time since diagnosis: | |||
| Median | 5.05 y | 5.26 y | 5.10 y |
| Min, Max | 0.6, 24.0 y | 0.2, 24.8 y | 0.2, 24.8 y |
| Disease stage at study entry: | |||
| Stage III/IV | 76% | 77% | 77% |
| FLIPI risk group: | |||
| Low/Intermediate (0-2) | 67% | 63% | 65% |
| High (3-5) | 32% | 33% | 32% |
| Missing | <1% | 4% | 2% |
| Prior lymphoma therapies: | |||
| ≤2 | 62% | 59% | 61% |
| >2 | 38% | 41% | 39% |
| Tumor bulk: | |||
| Largest lesion >7 cm | 15% | 15% | 15% |
| Rituximab naive | 35% | 30% | 32% |
| . | R+G Group(N = 175) . | R+P Group(N = 162) . | Total(N = 337) . |
|---|---|---|---|
| Age: | |||
| ≤60 years | 53% | 57% | 55% |
| Median | 59.0 y | 59.0 y | 59.0 y |
| Min, Max | 27, 91 y | 33, 88 y | 27, 91 y |
| Male | 46% | 52% | 49% |
| Time since diagnosis: | |||
| Median | 5.05 y | 5.26 y | 5.10 y |
| Min, Max | 0.6, 24.0 y | 0.2, 24.8 y | 0.2, 24.8 y |
| Disease stage at study entry: | |||
| Stage III/IV | 76% | 77% | 77% |
| FLIPI risk group: | |||
| Low/Intermediate (0-2) | 67% | 63% | 65% |
| High (3-5) | 32% | 33% | 32% |
| Missing | <1% | 4% | 2% |
| Prior lymphoma therapies: | |||
| ≤2 | 62% | 59% | 61% |
| >2 | 38% | 41% | 39% |
| Tumor bulk: | |||
| Largest lesion >7 cm | 15% | 15% | 15% |
| Rituximab naive | 35% | 30% | 32% |
Progression-Free Survival by Patient Subgroup, Galiximab Clinical Study Solid line represents the hazard ratio observed in the primary analysis.
Progression-Free Survival by Patient Subgroup, Galiximab Clinical Study Solid line represents the hazard ratio observed in the primary analysis.
McKinney:Biogen Idec: Employment. Ruffner:Biogen Idec: Employment. Wilson:Biogen Idec: Employment. Whiteley:Biogen Idec: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal