Abstract
Abstract 4766
Erythropoietin concentration in serum spans three orders of magnitude as it regulates erythropoietic rates in basal and stress erythropoiesis. It is not clear how Epo concentration is encoded intracellularly to generate the required erythropoietic rate. Stat5 activation by the Epo receptor (EpoR) is required for both basal and stress erythropoiesis. We asked whether dynamic properties of Stat5 signaling, namely the manner in which the Stat5 signal intensity varies with time or with Epo concentration, might somehow encode downstream responses. To this end, we studied the Stat5 signaling response quantitatively in freshly-isolated single cells in mouse erythropoietic tissue, by flow cytometry. Using wild type mice, as well as mutant EpoR-HM mice expressing a truncated EpoR that lacks cytoplasmic domain tyrosines, we identified two modes of Stat5 signaling, digital and analog, which are specifically required for basal and stress erythropoiesis, respectively.
We found that expression levels of the Stat5 protein are key in shaping the Stat5 signal. Early erythroblasts, ‘S1’ (Subset 1) cells, express high levels of of Stat5, and are able to respond to high Epo with a high intensity graded, or analog, signal. By contrast, mature erythroblasts, labeled ‘S3’ (Subset 3), express lower Stat5 levels, and their response to Epo is limited to a low intensity signal. We found that this low –intensity response is nevertheless decisive, responding to an Epo stimulus with a binary (or digital) ‘on’ or ‘off’ signal. The digital character of this low-intensity signal can be measured quantitatively: we found that its reposnse to increasing levels of Epo may be fitted by a Hill curve with a high Hill coefficient, similar in magnitude to that found for the cooperative binding of oxygen to hemoglobin.
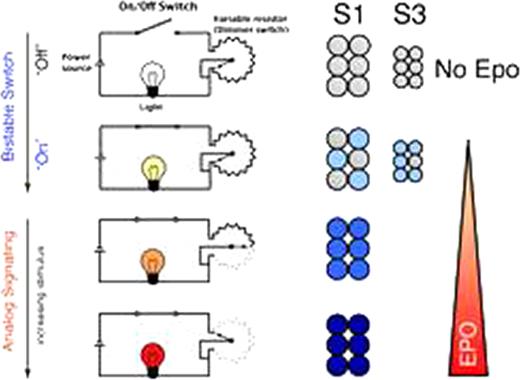
Mechanistically, the digital, low-intensity Stat5 signal is due to bistable Stat5 activation, in turn a result of a positive feedback interaction whereby phosphorylated Stat5 promotes further Stat5 phosphorylation. Following bistable activation, however, and in the presence of high Stat5 expression, Stat5 activation can increase further in a graded fashion, in response to increasing Epo levels. In mature S3 erythroblasts, however, in the absence of high Stat5 levels, no further increase in the signal occurs, giving rise to a digital behavior. Stat5 activation is therefore analogous to that of a dimmer light switch: a toggle-mechanism controls an initial bistable activation, giving rise to a low-level light signal; further graded rotation of a dial lower the circuit's resistance and gives rise to a graded increase in light intensity (Figure 1).
We found that the EpoR-HM erythroblasts lost the ability to signal via the analog Stat5 signaling mode, but retained digital signaling. By comparing genetic models that either completely lack Stat5 function (the Stat5-/- mouse), or that specifically lack the graded, high intensity signal (the EpoR-HM mouse), we found that the digital, low-intensity signal was both necessary and sufficient for erythroblast survival and for maintenance of basal erythropoiesis. By contrast, the high intensity analog p-Stat5 signal was required for the response to erythropoietic stress, as seen from the inability of EpoR-HM mice to respond to stress. Further, we identified a specific gene target of analog Stat5 signaling during stress. We found that the stress-dependent upregulation of CD71 in early erythroblasts was absent in EpoR-HM. By exogenously expressing high levels of Stat5 in EpoR-HM erythroblasts in vitro, we were able to rescue both the analog high-intensity Stat5 signal in these cells, as well as their ability to express high CD71 in response to stress levels of Epo.
We propose that the combined bistable and graded Stat5 signaling responses in early erythroblasts has evolved in order to generate a high fidelity response to Epo over its broad concentration range in basal and stress erythropoiesis. Digital low-intensity signaling transduces a clear signal in response to low-levels of Epo; whereas the ability to respond to higher levels of Epo with a graded, high intensity signal, is retained.
Stat5 activation in response to low, basal Epo is through a bistable switch that generates a low intensity signal. A further increase in Epo results in a graded increase in signal, but only in cells that express high Stat5.
Stat5 activation in response to low, basal Epo is through a bistable switch that generates a low intensity signal. A further increase in Epo results in a graded increase in signal, but only in cells that express high Stat5.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal