Abstract
Abstract 669
The prognostic relevance of major molecular remission (MMR, <0.1% BCR-ABL according International Scale, IS) for survival has remained uncertain. Gold standard for the evaluation of treatment response is the achievement of complete cytogenetic remission in spite of its limited sensitivity and the requirement of bone marrow puncture. The standardization of PCR methods and the introduction of conversion factors to account for differences among European laboratories, has resulted in a uniform reporting system allowing comparable BCR-ABL expression levels derived from peripheral blood samples. We sought to evaluate an association of the degree of molecular response and survival.
We have analyzed 848 patients within the CML-Study IV (randomized comparison of imatinib 800 mg vs 400 mg vs 400 mg + IFN). BCR-ABL (IS) was determined by quantitative RT-PCR. Patients with atypical BCR-ABL transcripts were excluded from the analysis. Median observation time was 40 months (minimum 12). Landmark analyses have been performed at 12 months for overall and progression-free survival using 3 groups of response (<0.1%, 0.1%-1%, >1% BCR-ABL IS).
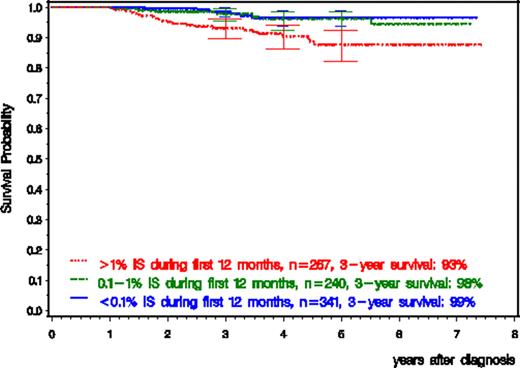
341 patients achieved a BCR-ABL expression <0.1% (MMR), 240 patients between 0.1% and 1% and 267 patients >1% by 12 months. Independent of treatment approach, the groups of patients achieving MMR and 0.1%-<1% at 12 months showed significantly higher progression free survival (PFS) (p=0.0023; 99% [95% CI: 97–100%] vs 97% [95% CI: 94–99%] vs 94% [95% CI: 90–97%] at 3 years) and better overall survival (p=0.0011; 99% [95% CI: 97–100%] vs 98% [95% CI: 95–100%] vs 93% [95% CI: 90–96%] at 3 years) compared to the group with >1% BCR-ABL by 12 months (Figure).
Faster and deeper response to imatinib-based treatment by 12 months revealed to be associated with improved PFS and overall survival. The critical cutoff level seems to be 1% BCR-ABL IS which has been shown to closely correlate with complete cytogenetic remission.
Müller:Novartis: Honoraria, Research Funding. Schnittger:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. German CML-Study Group:Deutsche Krebshilfe: Research Funding; Novartis: Research Funding; Roche: Research Funding; BMBF: Research Funding; Essex: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal