Abstract
Abstract 71
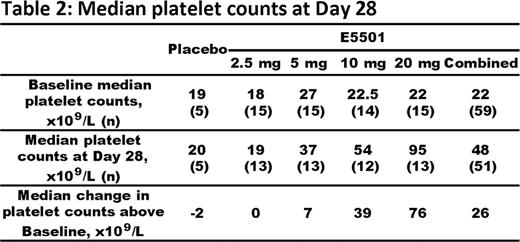
Chronic immune thrombocytopenia (ITP) is a condition of low platelet counts due to increased autoimmune-mediated platelet destruction and suboptimal platelet production. Thrombopoietin (TPO) receptor agonists are a novel class of agents which increase platelet counts by mimicking the principal physiologic regulator of platelet production, TPO. TPO agonists have demonstrated efficacy in randomized controlled trials in patients with ITP who are refractory to 1st and 2nd line agents. E5501 (previously AKR501) is a novel, orally-active, once-a-day TPO agonist which increased platelet counts in healthy volunteers. Here, we report data from a Phase II, multicenter, randomized, double-blind, placebo-controlled, dose-ranging, parallel group 4-week study (501-CL-003) of E5501 in subjects with ITP whose disease was refractory to, or had relapsed after, at least one prior ITP therapy. Subjects were enrolled if they had a baseline platelet count of <30 x109/L, or if they had a baseline platelet count of <50 x109/L and were on stable corticosteroid therapy. Sixty-four subjects were randomized to E5501 (2.5, 5, 10 or 20 mg) or placebo in a 3:3:3:3:1 randomization ratio, respectively. E5501 or placebo was administered orally, once daily for 28 days. Response to E5501 was based on weekly platelet counts. The primary endpoint was responder rate at Day 28. Responders were defined as subjects whose platelet count was ≥50 x109/L and had risen by a minimum of 20 x109/L above baseline. Responder rate increased in subjects receiving E5501 in a dose-dependent manner (Table 1). At Day 28, the responder rate in the E5501 20 mg group was 80% (12 of 15 subjects) vs 0% in the placebo group (p=0.0036). The responder rate was also significantly higher with E5501 20 mg than with E5501 2.5 mg (80% vs 13.3%; p=0.0007). In non-splenectomized subjects, the responder rate at Day 28 was 51.2% in the combined E5501 group and 88.9% in the E5501 20 mg group, compared with 44.4% and 66.7% respectively in splenectomized subjects. Median platelet counts at Day 28, and change in platelet counts above baseline, increased in subjects receiving E5501 in a dose-dependent manner (Table 2). The majority (57.6%) of subjects responded to a dose of ≥5 mg E5501 by Day 7. Subjects treated with E5501 20 mg achieved a 93.3% response rate on Day 7. None of the 5 placebo-treated subjects responded at any time during the study. E5501 was well tolerated, with a similar proportion of subjects showing treatment-emergent adverse events (TEAEs) across all dose groups. Most TEAEs were mild, transient, and resolved completely. TEAEs occurring in ≥10% of E5501-treated subjects were fatigue (20.3%), headache (20.3%) and epistaxis (15.3%). There were no clinically relevant changes in vital signs or physical examination findings. Three subjects (2 in the 2.5 mg and 1 in the 10 mg E5501 group) reported serious TEAEs. Of the two subjects in the 2.5 mg E5501 group, one reported thrombocytopenia and one reported a GI bleed; both had platelet counts <10 x109/L. The one subject in the 10 mg E5501 group, a 72-year-old Hispanic male with a significant history of cardiovascular disease (including myocardial infarction [MI], coronary artery vein bypass graft, 3 prior transient ischemic attacks [TIAs], chronic obstructive pulmonary disease, hypertension, systolic ejection murmur, angioplasty, stent placement, hyperlipidemia, and small vessel disease), had TIA and MI on Day 20 and a retinal artery occlusion 14 days after E5501 was discontinued. At the time of the events his platelet counts were 40–47 x109/L. Three other E5501-treated subjects (6.8% in total) experienced TEAEs leading to study drug withdrawal: 1 receiving 5 mg E5501 had Grade 2 musculoskeletal chest pain; 2 receiving 20 mg E5501 had excessively increased platelet counts with no clinical sequellae. In conclusion, E5501 was effective in increasing platelet counts in subjects with ITP. At 20 mg E5501, 80% of patients had responded at Day 28, with a median platelet count of 95 x109/L, and >90% of patients had responded by Day 7. E5501 was generally well tolerated and had a favorable safety profile. These data support continued development of E5501 as a potentially effective treatment with an acceptable safety profile in non-splenectomized and splenectomized patients with ITP.
Bussel:Amgen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; GlaxoSmithKline: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Research Funding; Immunomedics: Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sysmex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Portola: Consultancy. Zhang:Eisai: Employment. Tang:Eisai: Employment. McIntosh:Eisai: Employment. Kuter:Amgen: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; ONO: Consultancy; Shionogi: Consultancy, Research Funding; Pfizer: Consultancy; Protalix: Consultancy, Research Funding; Risk Managment Foundation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal