Abstract
Abstract 845
Transfusion therapy can effectively treat many complications associated with sickle cell disease (SCD), but iron overload will develop without iron chelation therapy. Despite long-term transfusion requirements, long-term data for iron chelation in SCD are limited. The oral iron chelator, deferasirox, effectively reduced iron burden in SCD patients aged ≥2 years during 1 year of treatment (Vichinsky et al. Br J Haematol 2007). This 5-year follow-up is the first report of long-term deferasirox treatment in SCD patients.
Eligible patients that completed the 1-year core study (randomized to deferasirox [deferasirox cohort] or deferoxamine [crossover cohort]) entered the 4- year extension. All received deferasirox while continuing their regular transfusion regimen. Deferasirox dose in the extension was initially based on serum ferritin (SF) trends in the core study (deferasirox cohort) or transfusion requirements (crossover cohort). Dose adjustments were based on monthly SF and safety assessments (investigator-reported adverse events [AEs] and centrally processed lab parameters). Growth and sexual development (Tanner staging) were assessed annually.
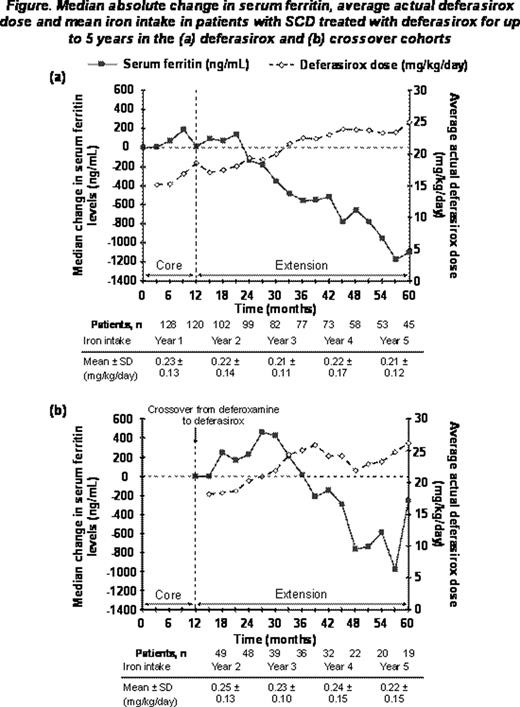
Of the patients in the deferasirox (n=132) and crossover (n=53) cohorts that received ’1 deferasirox dose during the core or extension, 43 (33%) and 19 (36%) patients, respectively, completed the extension. Reasons for discontinuation included withdrawal of consent (n=44, 23.8%), loss to follow-up (n=17, 9.2%) and AEs (n=14, 7.6%). Three deaths occurred, all in the extension (intraventricular hemorrhage, n=2; intracranial bleed post liver transplantation, n=1); none considered by investigators to be deferasirox-related. Mean dose during the study was 18.7 ± 6.5 and 21.2 ± 5.3 mg/kg/day in the deferasirox and crossover cohorts, respectively. Investigator-assessed drug-related AEs (≥5% overall) included nausea (14.6%), diarrhea (10.8%), increased blood creatinine (5.9%) and vomiting (5.4%). Generally, these AEs were manageable and transient, and decreased in frequency over time. Serious AEs were reported in 70.8% of patients overall and were mostly related to the underlying disease. Serious investigator-assessed drug-related AEs were reported in 8 patients (6.1%) in the deferasirox cohort and 1 patient (1.9%) in the crossover cohort. In the deferasirox and crossover cohorts respectively, 9 (6.8%) patients and 1 (1.9%) patient had 2 consecutive serum creatinine level increases >33% above baseline and >upper limit of normal (ULN). Median creatinine clearance remained stable within normal range throughout the study. One patient from each cohort had alanine aminotransferase (ALT) >10 × ULN on 2 consecutive visits; both had ALT values ≤ULN at the start of deferasirox treatment. In 37 patients with data available before and after dose increases to ≥30 mg/kg/day, no clinically relevant differences were observed in AE profile or laboratory parameters before and after dose increase. Deferasirox had no adverse effect on pediatric growth and adolescent sexual development. Overall, median SF levels in patients who received deferasirox treatment for ≥4 years decreased significantly from 3410 to 3108 ng/mL at end of study (median absolute change, –591 ng/mL, P=0.027; n=67). Decreases in SF were more pronounced when mean deferasirox dose increased to >20 mg/kg/day (Figure 1). In the deferasirox cohort, the median absolute change in SF levels from start of deferasirox to end-of-study was greater in patients aged ≥16 than 2–<16 years (–476 vs –156 ng/mL) reflecting low 1st-year deferasirox doses and conservative dose escalation in pediatric patients.
This is the first study to report that deferasirox can significantly decrease SF levels over the long-term in SCD patients without evidence of renal toxicity. Treatment for up to 5 years was associated with a clinically manageable safety profile, without progressive decreases in median creatinine clearance, which remained within normal range. The modest, but statistically significant, decrease in SF is most likely due to under-dosing in the first 2 years and variability in SF related to disease factors. Patients titrated to an appropriate dose had a clinically relevant decrease in SF, highlighting the need to adjust dose, usually to >20 mg/kg/day, to achieve negative iron balance.
Vichinsky:Novartis: Consultancy, Research Funding, Speakers Bureau; Apotex: Consultancy, Research Funding; Hemaquest: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bernaudin:Novartis: Investigator for SCD deferasirox (Exjade). Forni:Novartis: Research Funding. Gardner:Novartis: Research Funding. Hassell:Novartis: Research Funding. Heeney:Novartis: Research Funding. Kutlar:Novartis: Research Funding. Lane:Novartis: Research Funding. Mathias:Novartis: Research Funding. Porter:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Tebbi:Novartis: Speakers Bureau. Wilson:Novartis: Honoraria, Research Funding, Speakers Bureau. Griffel:Novartis: Employment. Deng:Novartis: Employment. Giannone:Novartis: Employment. Coates:Novartis: Research Funding, Speakers Bureau.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal