Abstract
Abstract 858
Prior studies have shown that combination chemotherapy using high doses of antimetabolites and alkylating agents over a short duration is effective treatment for Burkitt leukemia and lymphoma. Adults able to tolerate this therapy have had > 50% long term survival, although those with higher risk by the International Prognostic Index (IPI) have had inferior outcomes. Between 5/2002 and 9/2009, we enrolled 105 adults (19-79 yrs old) with untreated Burkitt leukemia/lymphoma onto a phase II study of a high intensity chemo-immunotherapy regimen to assess the benefit of adding rituximab plus growth factor support to the intensive chemotherapy regimen developed in CALGB 9251 and evaluated patterns of relapse when prophylactic cranial irradiation was not given. All subjects were HIV negative and had serum creatinine and bilirubin ≤1.5 × upper limit. Complete data are available on 105 patients for toxicity and 103 patients for efficacy.
Treatment began with cyclophosphamide (CY) 200 mg/m2 × 5 days and prednisone 60 mg/m2 × 7 days. Cycle 2 was started on Day 8 after entry. Cycles 2, 4, and 6 consisted of ifosfamide 800 mg/m2 on days 1–5, methotrexate (MTX) 1.5 g/m2 infused over day 1 with leucovorin rescue, vincristine (VCR) 2 mg day 1, Ara-C 1 gm/m2 days 4 and 5, VP-16 80 mg/m2 days 4 and 5, and dexamethasone 10 mg/m2 on days 1–5. Cycles 3, 5, and 7 included the same doses of MTX, VCR, and dexamethasone, with CY 200 mg/m2 IV on days 1–5 and doxorubicin 25 mg/m2 days 4 and 5. Cycles were delivered every 21 days if blood counts had recovered. Filgrastim was given at 5μg/kg/day SC beginning day 7 of each cycle and continuing until the absolute neutrophil count recovered to > 5000/μL. Rituximab was initiated during cycle 2 on day 8 at 50 mg/m2 and on days 10 and 12 at 375 mg/m2. During cycles 3 through 7, rituximab was infused only on day 8 of each course at 375 mg/m2. Central nervous system (CNS) prophylaxis consisted of triple intrathecal therapy on day 1 of cycles 2–7 (6 total doses).
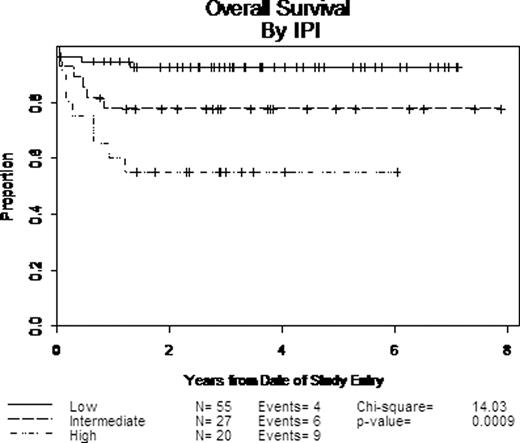
27% of patients were ≥60 years old; 70% were male; 46% had intermediate or high risk disease by the IPI (Table). Overall, 75 of 105 subjects completed all 7 planned courses of therapy. 82% attained a complete response (CR), and 87% of these remain in CR at last follow up. 7% had a partial response. With median follow up of survivors of 3.2 years, 2 year event free survival (EFS) and overall survival (OS) were 77% and 79%, respectively, with a trend favoring those <60 years old (87% and 87%, respectively). There were clear differences in outcome based on IPI score with 2 year EFS and OS for low risk patients of 90% and 92% versus 55% and 55% for high risk patients, respectively (Figure). This protocol did not use prophylactic CNS radiation, and 4 pts had documented CNS relapses; 2 had intermediate and 1 high IPI disease; the 4th was unknown. Relapse after 2 years was rare.
7 subjects (6.8%) died from treatment related causes (1 CNS bleed, 4 infections, 2 respiratory failure). Nearly all subjects experienced the anticipated severe hematologic toxicities. The most common grade 3 and 4 non-hematologic toxicities included stomatitis/upper GI toxicity (∼ 66%), nausea/vomiting (20%), fatigue (26%), rash or erythema multiforme (10%), diarrhea (10%), pulmonary or CNS bleeding (11%), clinically documented infections (72%), neurologic disturbances (8%), and dyspnea (10%). 8 pts (8%) had tumor lysis syndrome (all grade 3).
| . | < 60 years, N= 77 . | ≥ 60 Years, N= 28 . | All, N=105 . |
|---|---|---|---|
| Median age (range) | 36 yrs (19–59) | 64 (60–79) | 43 (19–79) |
| Completed 6 or 7 cycles | 64 (83%) | 16 (57%) | 80 (76%) |
| IPI: Low | 47 (63%) | 8 (30%) | 55 (54%) |
| Intermediate | 17 (23%) | 10 (37%) | 27 (27%) |
| High | 11 (15%) | 9 (33%) | 20 (20) |
| Missing | 2 | 1 | 3 |
| Complete Remissions | 64 (85%) | 21 (75%) | 85 (82%) |
| Continuous CR | 59 (77%) | 15 (54%) | 74 (70%) |
| . | < 60 years, N= 77 . | ≥ 60 Years, N= 28 . | All, N=105 . |
|---|---|---|---|
| Median age (range) | 36 yrs (19–59) | 64 (60–79) | 43 (19–79) |
| Completed 6 or 7 cycles | 64 (83%) | 16 (57%) | 80 (76%) |
| IPI: Low | 47 (63%) | 8 (30%) | 55 (54%) |
| Intermediate | 17 (23%) | 10 (37%) | 27 (27%) |
| High | 11 (15%) | 9 (33%) | 20 (20) |
| Missing | 2 | 1 | 3 |
| Complete Remissions | 64 (85%) | 21 (75%) | 85 (82%) |
| Continuous CR | 59 (77%) | 15 (54%) | 74 (70%) |
This regimen provides a high rate of durable remissions in adult patients with a manageable side effect profile. Chemoimmunotherapy should be the standard for adult patients with Burkitt leukemia/lymphoma.
Off Label Use: Rituximab for use in Burkitt's. Cheson:Genentech: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal