Abstract
Abstract LBA-4
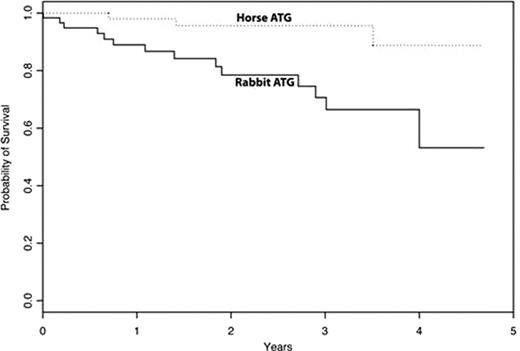
In patients with severe aplastic anemia (SAA) who are not candidates for stem cell transplantation, immunosuppression with horse anti-thymocyte globulin (ATG; ATGAM®) plus cyclosporine (CsA) is standard. In comparison to horse ATG, rabbit ATG (Thymoglobulin®) is more potent and has distinct biological effects on the immune system (Feng × et al Blood 111:3675, 2008). Thymoglobulin is preferred to ATGAM in some clinical settings, such as in the prevention and treatment of kidney allograft rejection. In patients with refractory and relapsed SAA, rabbit ATG improves hematopoiesis as salvage therapy. Here we report the first prospective randomized protocol comparing horse ATG/CsA and rabbit ATG/CsA in treatment-naïve SAA (www.clinicaltrials.govNCT00260689). Consecutive patients with SAA who required initial immunosuppressive therapy were eligible; all were enrolled December 2005-July 2010 and treated at the Hatfield Clinical Research Center of the National Institutes of Health in Bethesda. Randomization was 1:1 in blocks between the two ATGs. The primary endpoint was hematologic response at 6 months, which has been shown consistently to strongly correlate with long-term survival (Rosenfeld S et al. Blood 85: 3058, 1995) The current study was powered to detect a 25% difference between arms at a 5% significance level and with 80% power. Secondary endpoints included hematologic response at 3 months, relapse, clonal evolution to myelodysplasia, and survival. A total of 120 patients (ages 2–78 years) were randomized to receive either horse or rabbit ATG (60 in each arm). The median follow-up for all patients was 850 days from first ATG infusion (range, 2–1757) and for surviving patients 922 days (range, 104–1757). Accrual was completed in early July 2010, and response data were evaluable at 3 months by mid-October in all patients. Demographics and clinical features were well matched between the two ATG groups. The hematologic response rate at 3 months for horse ATG was 62% (95% CI, 49%-74%) and for rabbit ATG 33% (95% CI, 21%-46%; p=0.0017; Table). The hematologic response rate for the horse ATG arm is similar to our previously reported experience with this regimen in SAA (Scheinberg P et al. Br J Haematol 133: 622, 2006; Haematologica 94: 348, 2009). Despite relatively short follow-up, relapse and clonal evolution have occurred with similar frequency; 8 relapses (among 37 responders) and 8 clonal evolutions have been observed in the horse ATG arm, and 2 relapses (among 20 responders) and 4 evolutions in the rabbit ATG group. There have been 3 deaths in the horse ATG arm and 14 in the rabbit ATG arm, resulting in different survival proportions for the two regimens (Figure). We infer from our results that rabbit ATG/CsA is markedly inferior to horse ATG/CsA as first treatment in SAA, as measured by the critical parameter of hematologic response. Horse ATG is presently not available in Europe, Japan and South America, and therefore these data have immediate implications for the treatment of SAA worldwide, as well as to the mechanism of action of polyclonal antisera in general and in particular in this disease.
Hematologic response rates in evaluable patients.
| . | 3 mo response (all patients) . | 6 mo response (evaluable patients) . | P-value* . |
|---|---|---|---|
| Horse ATG | 37/60 (62%) | 37/54 (69%) | 0.0017 |
| Rabbit ATG | 20/60 (33%) | 19/54 (35%) | – |
| . | 3 mo response (all patients) . | 6 mo response (evaluable patients) . | P-value* . |
|---|---|---|---|
| Horse ATG | 37/60 (62%) | 37/54 (69%) | 0.0017 |
| Rabbit ATG | 20/60 (33%) | 19/54 (35%) | – |
The P-value refers to difference between 3-month responses only. No statistics for 6-month responses were conducted since not all patients are evaluable for this time point. For 6-month response, all enrolled patients will be evaluable in early January 2011.
Off Label Use: Rabbit ATG in severe aplastic anemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal