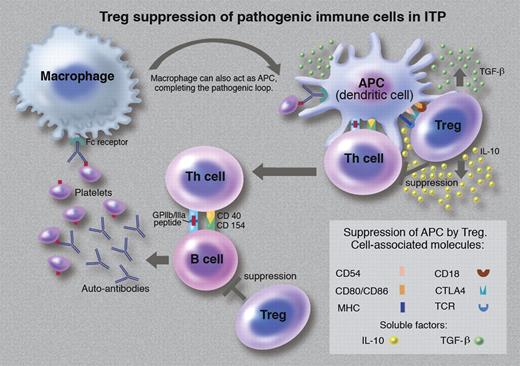
CD4+ T cells are commonly divided in T helper (Th) cells and regulatory T cells (Tregs). Tregs are usually identified by markers such as CD25, CTLA-4, CD127, and Foxp3.2 The main function of Tregs is the prevention of autoimmune diseases by maintaining self-tolerance.2,3 They suppress immune response by a mechanism based on a 3-way interaction between Tregs, Th cells, and antigen-presenting cells (APCs)2,3 (see figure). There is recent evidence that Tregs also suppress B cells.3 These multicellular immunosuppressive activities result in Tregs maintaining self-tolerance and preventing the emergence of autoimmune diseases such as immune primary thrombocytopenia (ITP).
Treg suppression of pathogenic immune cells in ITP. (Professional illustration by Marie Dauenheimer.)
Treg suppression of pathogenic immune cells in ITP. (Professional illustration by Marie Dauenheimer.)
ITP is an autoimmune “bleeding” disorder characterized by low platelet counts.4,5 Similar to other autoimmune diseases, in ITP, APCs, Th cells, and B cells play a crucial role in its pathogenesis.6 Autoreactive Th cells and B cells escape Treg surveillance and thus are allowed to become activated and proliferate. B cells produce auto-antibodies against platelet antigens (GPIIb/IIIa and/or GPIb-IX) on the surface of platelets. Antibody-coated platelets are phagocytosed by splenic and hepatic macrophages. As these antigens are also present on megakaryocytes, the autoantibodies bind to megakaryocytes and induce immune injury, resulting in a reduction in platelet production.6 Splenic macrophages (functioning as APCs) process the phagocytosed platelet antigens and present the antigen-derived peptides to Th cells.6 During this process, APCs and Th cells become activated and produce proinflammatory cytokines. The activated Th cells then interact with B cells inducing these cells to continue producing platelet autoantibodies. The autoantibodies bind to platelet antigens and antibody-coated platelets are phagocytosed by splenic and hepatic macrophages, and thus the pathogenic loop is maintained in chronic ITP.6 These pathogenic immune reactions probably persist because of a deficiency in Treg function.7 If ITP treatment can restore Treg function and suppress autoimmune pathogenic processes, sustained remission of the disease could be expected.
ITP is usually treated medically with immunosuppressive/immunomodulatory agents (such as glucocorticosteroids, intravenous immunoglobulin, anti-CD 20 antibody [rituximab], danazol, azathioprine, and cyclosporine) or surgically with splenectomy.6 Splenectomy gives a long-term remission rate of 66%-70%. With medical treatment, sustained response was until recently uncommon; however, dexamethasone and rituximab treatment in ITP have been reported to produce sustained responses in excess of 40%. Interestingly, improved Treg activity has been observed in ITP patients successfully treated with these 2 drugs.7,8 It is possible that the increased Treg activity may be responsible for the sustained response with these therapies. As these are immunomodulatory drugs, these findings are not totally unexpected.
With the knowledge that the thrombocytopenia in ITP is caused in part by impaired platelet production, TPO-R agonists have recently been used successfully to treat ITP.4,5 Response rates of 70% or higher have been achieved, but only while patients continue on treatment is the effect on their platelets maintained.4,5 As platelet response is driven by TPO-R agonist stimulation of thrombopoiesis, it is not unexpected that drug-induced platelet production thus ceases when the drug is stopped. In this context, the first demonstration of improved Treg activity with TPO-R agonists by Bao and colleagues is surprising and highly significant.1 However, this small cross-sectional study requires confirmation by a much larger longitudinal study, in particular a study which also shows antigen-specific increase in Treg activity (as immunosuppression by Tregs is antigen-dependent2 ). Nevertheless, the finding1 of Bao et al is still interesting and may explain the sustained platelet response experienced by 7% of ITP patients treated with TPO-R agonists in clinical trials. The unexpected remissions were initially attributed to spontaneous remission which can occur occasionally in chronic ITP, although all these patients had failed several ITP treatments before receiving TPO-R agonist therapy.
It is tempting to speculate that TPO-R agonists may have immune-modulating activity and thus induce sustained remission in ITP patients if given to appropriate patients for an adequate duration. However, immune cells do not have TPO-Rs. If this activity exists, TPO-R agonists must exert this effect via a mechanism independent of TPO-R. Tregs and other less well-studied suppressive T cells2 are probably the key to this activity. ITP occurs because APCs, macrophages, stimulatory Th cells, and B cells (which perpetuate the disease) escape the immune surveillance by Tregs. Tregs exert immune control by modifying the functions and numbers of these cells, and consequently return the immune system to homeostasis and health. For example, Tregs can induce apoptosis of the effector cells or can inhibit their activation and functions.2 These Treg actions are mediated by soluble factors (such as transforming growth factor-β [TGF-β], interleukin-10, perforins, etc) and cell-associated molecules (such as cytotoxic T lymphocyte antigen 4, lymphocyte activation gene-3, LFA-1/CD11a, CD18, CD39, etc).2,3 However, how TPO-R agonists improve Treg activity is still unknown. It may be via a sustained increase in antigen load that induces tolerance or via an increase in anti-inflammatory cytokines such as TGF-β as both antigen and TGF-β can induce Tregs.3 There is no good evidence to support either hypothesis and hence further studies are clearly needed.
Conflict-of-interest disclosure: B.H.C. is a consultant for CSL, Australia for development of Intragam 10NF and is a member of the scientific advisory board of GSK and Amgen, Australia. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal