Abstract
Beyond its role in immunity, complement mediates a wide range of functions in the context of morphogenetic or tissue remodeling processes. Angiogenesis is crucial during tissue remodeling in multiple pathologies; however, the knowledge about the regulation of neovascularization by the complement components is scarce. Here we studied the involvement of complement in pathological angiogenesis. Strikingly, we found that mice deficient in the central complement component C3 displayed increased neovascularization in the model of retinopathy of prematurity (ROP) and in the in vivo Matrigel plug assay. In addition, antibody-mediated blockade of C5, treatment with C5aR antagonist, or C5aR deficiency in mice resulted in enhanced pathological retina angiogenesis. While complement did not directly affect angiogenesis-related endothelial cell functions, we found that macrophages mediated the antiangiogenic activity of complement. In particular, C5a-stimulated macrophages were polarized toward an angiogenesis-inhibitory phenotype, including the up-regulated secretion of the antiangiogenic soluble vascular endothelial growth factor receptor-1. Consistently, macrophage depletion in vivo reversed the increased neovascularization associated with C3- or C5aR deficiency. Taken together, complement and in particular the C5a-C5aR axes are potent inhibitors of angiogenesis.
Introduction
The complement system represents a major component of immunity and host defense to infection bridging the innate with the adaptive immune response. It consists of serum proteins, membrane-bound receptors, and regulatory proteins.1 Upon activation of the complement cascade, the formation of C3 convertases C4bC2a and C3bBb results in the cleavage of the central complement component C3 to C3b and C3a. Deposition of C3b on cell surfaces is important for opsonization and phagocytosis. The released anaphylatoxin C3a as well as C5a that derives from C5 cleavage trigger further immune reactions upon binding to their cellular receptors (C3aR, C5aR, and C5L2).1,2 The conversion of C3 can be triggered by 3 distinct pathways: the classical, alternative, and lectin pathways.1 However, C3 or C5 can also be cleaved by proteases that are abundant in inflamed or injured tissues.1,3 Besides the well-established actions of complement in the elimination of pathogens, the complement system has been recently implicated in a variety of pathophysiological processes, such as ischemia/reperfusion injury, sepsis, and autoimmune and inflammatory disorders.1,2,4 Moreover, recent studies have demonstrated that complement components, through their inflammatory or novel noninflammatory functions, contribute substantially to and modulate several complex tissue remodeling processes relevant to tissue development or regeneration, including liver regeneration or synapse elimination in the CNS system, whereas C5 facilitates remyelination and prevents gliosis in experimental autoimmune encephalomyelitis.5–8
Angiogenesis is crucial to multiple malignant or inflammatory pathologies and to vision-threatening proliferative retinopathies, such as diabetic retinopathy or retinopathy of prematurity.9 The hallmark of vasoproliferative retinopathies is exuberant neovascularization triggered by retina ischemia/hypoxia, which is the result of retina vessel regression.10 Inflammatory cells or the innate immunity have been implicated as regulators of pathological angiogenesis,11 including proliferative retinopathies,12 and can exert both proangiogenic and antiangiogenic actions. For instance, the impact of macrophage subpopulations on angiogenesis may differ substantially. Depending on the environmental stimuli, macrophages can obtain a proangiogenic or an antiangiogenic signature.13,14
Previous studies addressing the influence of complement on neovascularization have revealed contradictory results. For instance, a proangiogenic function of complement component has been implicated in the model of laser-induced choroidal neovascularization, which represents a model for angiogenesis during the wet form of age-related macular degeneration (AMD). A hallmark of this disease is subretinal drusen formation, where deposits of complement components have been detected, as assessed by proteomic analysis.15–18 Moreover, complement factor H and factor B polymorphisms have been associated with neovascular AMD.19 In contrast, another study suggested that complement may act in an antiangiogenic fashion in the context of placental dysfunction in a mouse model of spontaneous miscarriage and intrauterine growth restriction.20 Importantly, the mechanistic insights in regards to the crosstalk between the complement system and neovascularization remain largely unclear. These observations have prompted us to investigate the role of the complement system in postnatal neovascularization by engaging the disease model of proliferative retinopathy of prematurity and the in vivo Matrigel angiogenesis model. By engaging mice deficient in different components of the complement system, we unexpectedly found that complement and the C5a/C5aR axis in particular, can exert antiangiogenic activity. Moreover, we identified that the inhibition of neovascularization by the complement system was predominantly mediated by macrophages.
Methods
Reagents
Recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) was obtained from Endogen. Allophycocyanin (APC)–conjugated antibody against mouse CD11b (clone M1/70) was from BD Biosciences. Enzyme-linked immunosorbent assay (ELISA) kits for mouse and human soluble vascular endothelial growth factor (VEGF) receptor-1 were from R&D Systems. RPMI medium, fetal bovine serum (FBS), Trizol, and 7-aminoactinomycin D (7-AAD) were from Invitrogen/Gibco. To deplete macrophages in vivo, clodronate liposomes or empty control liposomes were used (Encapsula NanoSciences). Matrigel and growth factor–reduced Matrigel were bought from Becton Dickinson Labware. Fluorescein isothiocyanate (FITC)–conjugated Griffonia simplicifolia isolectin-FITC and blocking goat serum were bought from Sigma-Aldrich. For immunodepletion of soluble VEGFR-1 from human monocyte supernatants we used a polyclonal goat anti–human vascular endothelial growth factor receptor-1 (VEGFR-1) antibody (Calbiochem) or goat Immunoglobulin G (IgG) as control (AbD Serotec). Human C3a peptide agonist NYITELRRQHARASHLGLAR-COOH and human C5a peptide agonist (YSFKPMPLaR), which can interact with their respective mouse receptors, were synthesized using Fmoc-based solid phase synthesis as previously described.21–23 C3a and C5a peptide agonists will be referred to throughout the paper as C3a and C5a, respectively. C5aR antagonist AcF[OPdChaWR], which has been shown to specifically block C5a-mediated effects in various rodent disease models,5,24 and control C5aR antagonist AcF[OPdChaAdR] were previously described.25 The monoclonal antibody BB5.1 against C5 was used to block C5 and terminal complement activation (C5a and C5b-9 generation) and has been previously described.26 Rat antibody to mouse C3b, MoAb 2/11 (Hycult Biotech) was previously described.27
Hypoxia-induced retinal vascularization, retinopathy of prematurity model
Mouse experiments were approved by the National Cancer Institute Animal Care and Use Committee. The hypoxia-induced retinal vascularization model, the retinopathy of prematurity (ROP) model, was described by Smith et al28 and was used in previous studies.29,30 C3 deficient mice and their wild-type (WT) littermate controls, C3aR-deficient mice (kindly provided by Dr R. Wetsel, The University of Texas Health Science Center at Houston) and their WT littermate controls as well as C5aR deficient mice (kindly provided by Dr C. Gerard, Harvard Medical School; all mice in the C57BL/6 background) were used, which had been described previously.31–33 C57BL/6 mice were also purchased from The Jackson Laboratory. Briefly, 7-day-old (p7) mice were exposed to 75% oxygen for 5 days in an incubator (BioSpherix) with their nursing mother. At p12, mice were returned to room air. Mice were killed at p17, and eyes were processed for quantification of epiretinal neovascular nuclei.30 Briefly, 6-μm paraffin-embedded sections were stained with periodic acid-Schiff (PAS) and hematoxylin, and 10 intact sections of equal length, each 18 μm apart, were evaluated. All retinal vascular cell nuclei anterior to the internal limiting membrane were counted in each section. The mean of the 10 counted sections represented the average neovascular nuclei per section per eye.
In some experiments, pups were treated with daily intraperitoneal injections of C5a (8 μg/200 μL phosphate-buffered saline [PBS]) or vehicle control, with C5aR antagonist (15 μg/200 μL PBS) or control scrambled antagonist, or with C3a or C3a control (scrambled chicken C3a, EAYKQRYEDRLELRIELIG-COOH; 8 μg/200 μL PBS, each) from p12 until p16 as indicated in figure legends. In other experiments, pups subjected to the ROP model were treated at day p12.5 with an intraperitoneal injection of control IgG or anti-C5 (clone BB5.1, 250 μg per pup). In a further experimental setting, WT or C3−/− mice were treated intraperitoneally with control liposomes or clodronate liposomes (Encapsula NanoSciences) on day p13 to deplete macrophages. Successful macrophage depletion was monitored in spleen single cell suspensions on day p17 by flow cytometric analysis of CD11b-positive cells.
Immunofluorescence analysis
Pups were subjected to the ROP model. The eyes of 17-day-old pups were enucleated and immersed in 4% paraformaldehyde. After a 24-hour fixation, the eyes were frozen and 6-μm-thick sections were processed for immunofluorescence staining. The slides were fixed for 5 minutes with ice-cold acetone and blocked with 3% bovine serum albumin for 1 hour at room temperature. Subsequently the sections were incubated with FITC- conjugated Griffonia simplicifolia isolectin (Sigma-Aldrich, 1:50) overnight at 4°C. After washing with PBS and blocking with 5% goat serum and 3% bovine serum albumin for 1 hour at room temperature, sections were incubated with a rat anti–mouse C3b antibody (clone 2/11, 1:10) overnight at 4°C to detect C3b deposition. After washing with PBS, the sections were then probed with AlexaFluor 568-conjugated goat anti–rat antibody (Invitrogen) as well as with DAPI (4′,6-diamidino-2-phenylindole) to visualize nuclei. The labeled sections were mounted with glass coverslips on slides and imaged using a Zeiss LSM510META laser scanning confocal microscope (Carl Zeiss MicroImaging Inc) equipped with a 63× plan-apochromat (numeric aperture 1.4) objective lens. Confocal images were collected with 0.9-μm optical slice thickness and 0.14-μm x-y pixel sampling.
Cell isolation, culture, stimulation, and ELISA analysis
Mouse bone marrow derived macrophages were isolated and cultured from femurs and tibias as described before.34 Macrophages (1 × 106 per well of a 6-well plate) were stimulated with vehicle control or C5a (100nM) in low serum medium (RPMI, 0.1% FBS) for 4 or 12 hours, supernatant was collected, centrifuged to remove cell debris and analyzed by ELISA (R&D Systems) for murine soluble VEGF-receptor 1 (sVEGFR1). For analysis by real-time polymerase chain reaction (PCR), cells were stimulated for 4 hours and RNA isolation was performed with the RNAeasy kit (QIAGEN) following the manufacturer's instructions.
Elutriated human peripheral blood derived monocytes from healthy donors were provided by the NIH Department of Transfusion Medicine. Human monocytes (1 × 106 per well of a 6-well plate in RPMI, 0.1% FBS) were treated with vehicle control or C5a (100nM) and supernatants were collected after 4 and 12 hours. The supernatant was subjected to analysis of human sVEGFR1 content by ELISA (R&D Systems).
Human umbilical vein endothelial cells (HUVECs) and HUVEC media were purchased from Cambrex and cultivated as described before.30 All endothelial cells were used in low passages. HUVECs were grown on culture dishes precoated with 0.2% gelatin (Sigma-Aldrich).
Cell proliferation
Endothelial cell proliferation was determined by measuring total cell number. Briefly, HUVECs (5 × 103/well) were plated onto 96-well plates and incubated for 6 hours in full medium, after which cells were washed once with PBS and the medium was changed, and cells were incubated in endothelial basal medium containing 0.1% FBS (Gibco), or in full endothelial growth medium for 72 hours, after which cells were quantified.
In vitro Matrigel tube formation assay
HUVECs (2 × 105) were plated on Matrigel-coated tissue culture plates (MatTek) in endothelial culture medium containing 10% FBS. In addition, PBS, C3a (100nM), or C5a (100nM) was added to the medium as indicated in figure legends and cells were incubated for 6 hours.
In another set of experiments, HUVECs (2 × 105) were plated on growth factor-reduced Matrigel-coated tissue culture plates (MatTek) in endothelial culture medium containing 0.2% FBS, supplemented with VEGF 25 ng/mL. In addition, the supernatant of human monocytes that were pretreated with vehicle control or C5a (100nM) obtained as described above was added to the wells. In other experiments, human sVEGFR1 was immunodepleted from the supernatant of human monocytes as previously described.35 After 6 hours, wells were imaged by phase-contrast microscopy using a Zeiss axiovert 200M microscope (Carl Zeiss MicroImaging Inc) equipped with a 20× LD acroplan Ph2 (N.A. 0.4) objective lens and Cool Snap ES monochrome charge-coupled device camera (Photometrics). Images were acquired and analyzed using MetaMorph software (Version 6.1; Molecular Devices). Multiple fields per well were examined and the length of tubular structures was quantified.
Real-time reverse transcriptase–PCR
Preparation of RNA, cDNA, and analysis was basically performed as described previously.36 Total RNA was isolated from mouse retinas or from nonstimulated (vehicle control) or stimulated (C5a 100nM for 4 hours) primary bone marrow–derived mouse macrophages using the RNAeasy RNA extraction kit (QIAGEN) with DNase I treatment (Ambion) following the manufacturer's instructions. To generate cDNA, total RNA (500 ng) was converted into cDNA using random primers and Superscript III reverse transcriptase (Invitrogen).
The cDNA samples were subsequently subjected to real-time PCR amplification using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems) and a QuantiTect SYBR Green PCR kit (QIAGEN). For hypoxanthine-guanine phosphoribosyltransferase, a primer mix (QIAGEN) was used; otherwise the primers used were as follows: actin, 5′-CGTGGGCCGCCCTAGGCACCA-3′ and 5′- TTGGCCTTAGGGTTCAGGGGGG-3′; IL-6, 5′-CACGGCCTTCCCTACTTCAC-3′ and 5′- TGCAAGTGCATCATCGTTGT-3′; IL-10, 5′-aactgcacccacttcccagt-3′ and 5′-aggcttggcaacccaagtaa-3′; TNF-α, 5′-CGGCATGGATCTCAAAGACAT-3′ and 5‘-CTTGACGGCAGAGAGGAGGT-3′; IL12, 5′-acctgtgacacgcctgaaga-3′ and 5′-ccactcacatctgctgctcc-3′; CD11b, 5′-gacgctgatggcaataccaa-3′ and 5′-ttcacgtctcccagcactgt-3′; CD4, 5′-agctccagctgaaggaaacg-3′ and 5′-cccatcacctcacaggtcaa-3′; CD8, 5′-tggcttcatcccacaacaag-3′ and 5′-tttctgaaggactggcacga-3′; sVEGFR-1 5′-ccggtgtgtgtttggattt-3′ and 5′-gttggcatcagagtggaaga-3′ C5a receptor 5′-gaagcggcaacctggggatgt-3′ and 5′-cgtctggctcgaaggctgtcac- 3′. The following PCR cycle was used: 95°C for 15 minutes; 40 cycles, each cycle containing 30 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C. Dissociation curve analysis was performed on all PCR products to ensure that specific PCR products were generated. PCR reactions for each product were performed in duplicate, and data were analyzed using the comparative CT method as described before.36,37
In vivo Matrigel plug assay
The Matrigel assay was generally performed as described previously.30 Two aliquots of Matrigel (0.5 mL, containing heparin, 10 μg/mL) supplemented with VEGF (200 ng/mL) were injected subcutaneously into the mid-abdominal region of mice. In some experiments, 6 hours and 3 days after matigel implantation, C57BL/6 WT mice were injected with control IgG or anti-C5 (clone BB5.1, 750 μg per mouse), whereas in other experiments, 6 hours after matigel implantation, mice were injected with control liposomes or clodronate liposomes (Encapsula NanoSciences) to deplete macrophages. After 7 days, the mice were killed and the Matrigel plugs were fixed with 4% paraformaldehyde, processed for histology (frozen sections) and stained with hematoxylin and eosin staining. Bright-field images were collected using a Zeiss axiovert 200M microscope equipped with a 10× plan-apochromat (NA 0.45) objective lens and axiocam MRc5 charge-coupled device camera using Axiovision image acquisition software (Version 4.6; Carl Zeiss MicroImaging). The resultant 24-bit red-green-blue color model Tagged Image File Format (TIFF) digital images were processed and analyzed using MetaMorph software (Version 6.1; Molecular Devices) and a ratio of the vessel area per Matrigel area was calculated.
Statistical analysis
Comparisons between group means were performed using Student t test or Mann-Whitney U test, as appropriate. P < .05 was considered statistically significant.
Results
C3 deficiency in mice results in increased postnatal angiogenesis
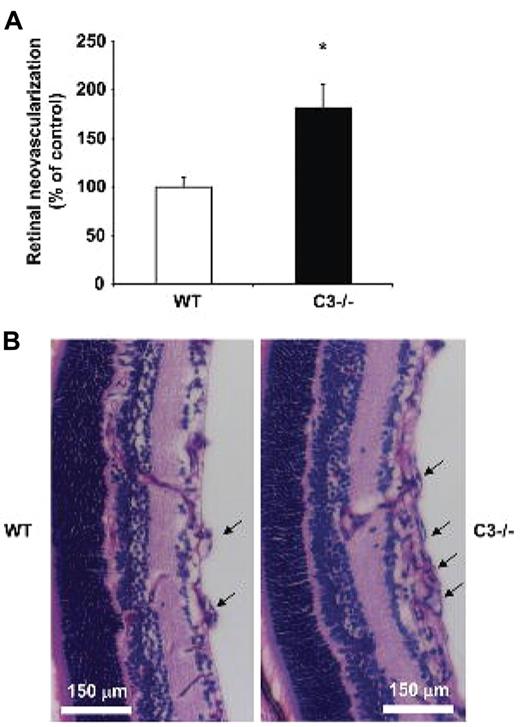
To address the role of the complement system in neovascularization, we engaged the model of ROP.28,30 In the ROP model, high oxygen (75%)–induced obliteration of the developing retina vessels in pups between postnatal day 7 and 12 leads to retinal hypoxia after postnatal day 12 when the pups are returned to normoxia. The subsequent hypoxia-driven proangiogenic response culminates in pathological excessive neovascularization.28,30 In the retinas of WT mice that were subjected to the ROP model, C3 deposition in vessels was observed by immunostaining (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). As expected, the staining for C3 deposition was negative in mice deficient in C3 (supplemental Figure 1). Then we assessed the role of complement in retina neovascularization functionally by comparing WT and C3−/− mice in the ROP model. In contrast to our initial prediction that the pro-inflammatory complement system would act in a proangiogenic direction, we found that the absence of a central component of the complement cascade, C3, resulted in significantly increased pathological retina neovascularization on day 17. In particular, the number of epiretinal pathological neovascular nuclei was significantly increased by approximately 1.7-fold in C3-deficient mice compared with WT mice, as assessed by histological analysis of retina cross-sections (Figure 1A-B).
C3 deficiency results in increased angiogenesis in ROP. WT or C3−/− mice were subjected to the ROP model. (A) Retinal neovascularization was quantified on day p17 in WT and C3−/− mice as described under “Hypoxia-induced retinal vascularization, retinopathy of prematurity model.” Retinal neovascularization is presented as the number of epiretinal neovascular nuclei per section. Data are mean ± SEM (n = 12-16 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in WT mice represents the 100% control. *P < .001. (B) Paraffin-embedded axial sections (6 μm) of the retina were stained with PAS and hematoxylin. Pathological neovascularization invades into the vitreous cavity anterior to the internal limiting membrane. C3−/− mice subjected to the ROP model had more neovascular regions anterior to the internal limiting membrane compared with WT mice. The arrows indicate the neovascular tufts anterior to the internal limiting membrane. Scale bars, 150 μm.
C3 deficiency results in increased angiogenesis in ROP. WT or C3−/− mice were subjected to the ROP model. (A) Retinal neovascularization was quantified on day p17 in WT and C3−/− mice as described under “Hypoxia-induced retinal vascularization, retinopathy of prematurity model.” Retinal neovascularization is presented as the number of epiretinal neovascular nuclei per section. Data are mean ± SEM (n = 12-16 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in WT mice represents the 100% control. *P < .001. (B) Paraffin-embedded axial sections (6 μm) of the retina were stained with PAS and hematoxylin. Pathological neovascularization invades into the vitreous cavity anterior to the internal limiting membrane. C3−/− mice subjected to the ROP model had more neovascular regions anterior to the internal limiting membrane compared with WT mice. The arrows indicate the neovascular tufts anterior to the internal limiting membrane. Scale bars, 150 μm.
The C5a-C5aR axis inhibits pathological retina angiogenesis
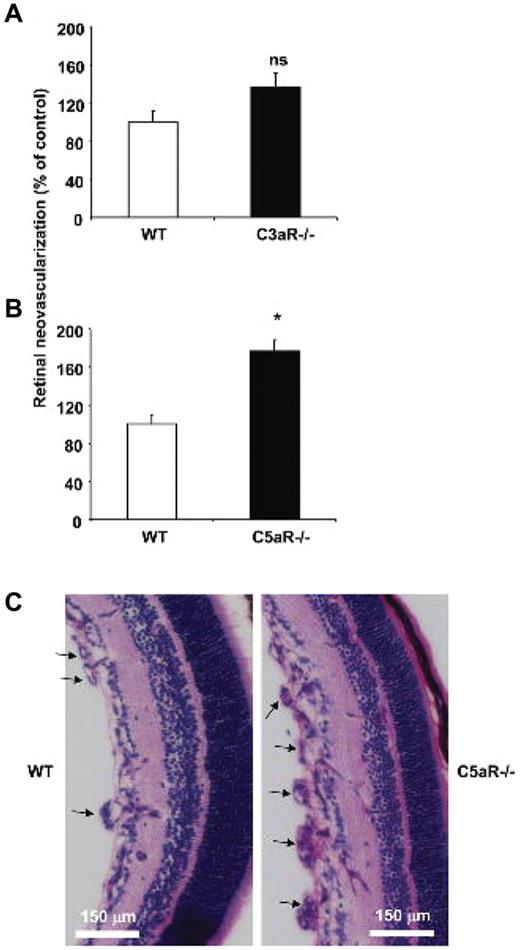
The findings with C3−/− mice pointed to a potential antiangiogenic action of components of the complement system downstream of C3. To provide further evidence for this hypothesis, we assessed neovascularization in mice deficient in the receptors for the active components generated upon C3 cleavage, C3a and C5a. Mice deficient in the receptor for C5a (C5aR) displayed a significant increase in pathological retina angiogenesis, compared with WT mice. Mice deficient in the receptor for C3a (C3aR) showed an increase in pathological retina neovascularization, however the effect of C3aR deficiency was not significant (Figure 2A-B). Representative histological analysis demonstrating higher neovascularization in C5aR−/− mice is shown in Figure 2C. Consistent with a role of C5aR in pathological retina angiogenesis, C5aR expression was found up-regulated in the retinas from mice subjected to the ROP model compared with retinas of mice kept in normal room air (supplemental Figure 2).
C5a receptor deficiency results in increased angiogenesis in ROP. WT, C3aR−/−, or C5aR−/− mice were subjected to the ROP model. (A) Retinal neovascularization was quantified on day p17 in WT and C3aR−/− mice. Retinal neovascularization is presented as the number of epiretinal neovascular nuclei per section. Data are mean ± SEM (n = 12-13 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in WT mice represents the 100% control. There was no significant difference between both groups. (B) Retinal neovascularization was quantified on day p17 in WT and C5aR−/− mice. Data are mean ± SEM (n = 16-17 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in WT mice represents the 100% control. *P < .005. (C) Paraffin-embedded axial sections (6 μm) of the retina were stained with PAS and hematoxylin, and neovascular tufts were observed anterior to the internal limiting membrane (arrows). C5aR−/− mice displayed more neovascular tufts. Scale bars, 150 μm.
C5a receptor deficiency results in increased angiogenesis in ROP. WT, C3aR−/−, or C5aR−/− mice were subjected to the ROP model. (A) Retinal neovascularization was quantified on day p17 in WT and C3aR−/− mice. Retinal neovascularization is presented as the number of epiretinal neovascular nuclei per section. Data are mean ± SEM (n = 12-13 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in WT mice represents the 100% control. There was no significant difference between both groups. (B) Retinal neovascularization was quantified on day p17 in WT and C5aR−/− mice. Data are mean ± SEM (n = 16-17 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in WT mice represents the 100% control. *P < .005. (C) Paraffin-embedded axial sections (6 μm) of the retina were stained with PAS and hematoxylin, and neovascular tufts were observed anterior to the internal limiting membrane (arrows). C5aR−/− mice displayed more neovascular tufts. Scale bars, 150 μm.
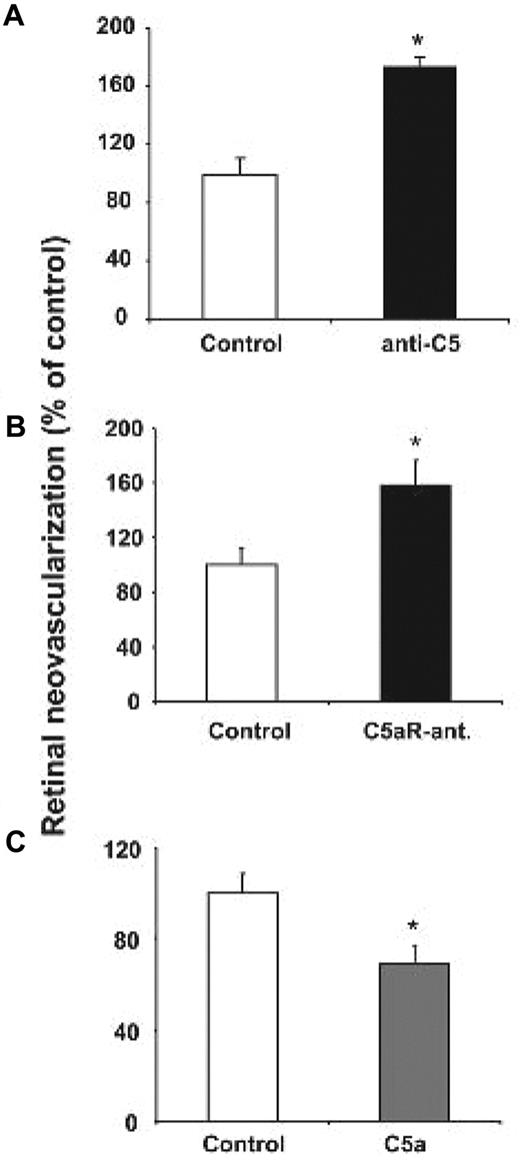
C5a can be generated in C3−/− mice.3 Therefore, to reconcile the findings from the C3−/− mice with the findings from the C5aR−/− mice, we tested the effect of functional C5 deficiency in the ROP model. To do so, we engaged a functional blockade of C5 and of terminal complement activation by the antibody BB5.1.26 C5 blockade resulted in a significant up-regulation of neovascularization in the ROP model (Figure 3A). To further substantiate the findings of increased angiogenesis due to the absence of C3 or C5aR or due to C5 blockade, we engaged the systemic administration of a specific antagonist of C5aR25 as well as of C5a peptide.23 WT mice subjected to the ROP model received every 24 hours on postnatal days 12-16 intraperitoneal injections of C5aR antagonist or control injections. Treatment with 15 μg of C5aR antagonist per dose per mouse resulted in significantly enhanced retina neovascularization on day 17, compared with mice that received a scrambled control peptide (Figure 3B). A slighter nonsignificant increase in retina angiogenesis was observed in mice treated every 36 hours with 8 μg of C5aR antagonist per dose per mouse (data not shown). In the reverse experiment, daily treatments with C5a peptide agonist (8 μg per dose per mouse) from postnatal day 12 to day 16 resulted in a significant reduction in pathological retina neovascularization (Figure 3C). In addition, C5a or C3a treatment reduced the increased angiogenesis observed in C3-deficient mice (supplemental Figure 3). Taken together, these findings from mice deficient in components of the complement cascade or upon C5 or C5aR blockade, as well as upon C5a treatment suggest that the C5a-C5aR axis acts to inhibit pathological hypoxia-driven retina neovascularization.
The role of C5a and C5aR in pathological angiogenesis in ROP. (A) WT mice subjected to the ROP model were treated on day p12.5 with an i.p. injection of anti-C5 or an isotype control antibody. Data are mean ± SEM (n = 6-7 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in control treated mice represents the 100% value. *P < .01. (B) WT mice were subjected to the ROP model and were treated from day p12-16 with daily intraperitoneal injections of C5a receptor antagonist (C5aR-ant.) or control antagonist. Data are mean ± SEM (n = 17-19 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in control treated mice represents the 100% value. *P < .05. (C) WT mice subjected to the ROP model were treated from p12-16 with daily intraperitoneal injections of C5a or vehicle control. Data are mean ± SEM (n = 17 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in control treated mice represents the 100% value. *P < .05.
The role of C5a and C5aR in pathological angiogenesis in ROP. (A) WT mice subjected to the ROP model were treated on day p12.5 with an i.p. injection of anti-C5 or an isotype control antibody. Data are mean ± SEM (n = 6-7 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in control treated mice represents the 100% value. *P < .01. (B) WT mice were subjected to the ROP model and were treated from day p12-16 with daily intraperitoneal injections of C5a receptor antagonist (C5aR-ant.) or control antagonist. Data are mean ± SEM (n = 17-19 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in control treated mice represents the 100% value. *P < .05. (C) WT mice subjected to the ROP model were treated from p12-16 with daily intraperitoneal injections of C5a or vehicle control. Data are mean ± SEM (n = 17 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in control treated mice represents the 100% value. *P < .05.
Macrophages mediate the antiangiogenic effect of complement components
The findings so far suggested an antiangiogenic effect of the complement system and C5a in particular. Since complement receptors have been reported to be expressed on endothelial cells,38 we first addressed whether C5a might affect endothelial cell functions related to angiogenesis. No significant impact of active C5a or active C3a on HUVEC proliferation or capillary–like sprout formation in Matrigels in vitro (supplemental Figure 4A-B) was found, indicating no direct antiangiogenic effect of complement on these endothelial cell functions.
We then analyzed whether monocytes/macrophages might mediate the antiangiogenic effect of C5a, since (1), inflammatory cells and particularly macrophages have been implicated as capable of regulating neovascularization in a positive and negative fashion,13,14,39 and (2), as assessed by real-time PCR analysis, we found that postnatal day p15 retinas from mice subjected to the ROP model had increased levels of the monocyte/macrophage marker CD11b, compared with retinas from mice that were kept in room air since birth (data not shown), whereas the message for lymphoid markers CD4 and CD8 was not altered due to the ROP manipulation (data not shown). In other words, monocytes/macrophages are increasingly abundant in the retina in the course of the ROP model (data not shown), which is also consistent with previous reports showing that macrophages accumulate within the retina in the course of the ROP model.40,41
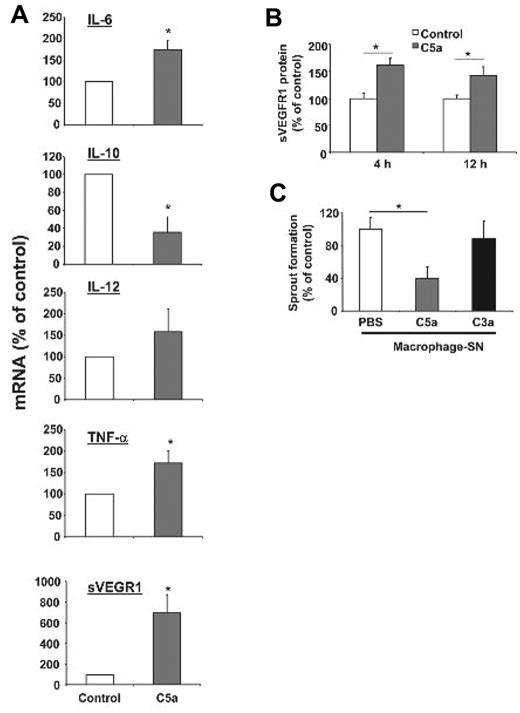
Macrophages can exert pro- or antiangiogenic actions dependent on their polarization.13,14 Inflammatory macrophages characterized by high expression of interleukin-6 (IL-6), IL-12, tumor necrosis factor-α (TNF-α), and low levels of IL-10 are considered antiangiogenic.13,14 Interestingly, this antiangiogenic polarization of macrophages was induced when primary bone marrow–derived macrophages were stimulated with C5a in vitro. In particular, C5a treatment of bone marrow–derived macrophages displayed significantly higher mRNA levels of IL-6 and TNF-α accompanied by decreased levels of IL-10 (Figure 4A).
Macrophages stimulated with C5a display an antiangiogenic phenotype. (A) Isolated primary bone marrow derived mouse macrophages treated with C5a showed a polarization toward an antiangiogenic phenotype as verified by significantly increased mRNA levels of IL-6, TNF-α, and soluble VEGFR-1 and significantly reduced levels of IL-10 mRNA using quantitative real-time PCR. IL-12 mRNA levels were not significantly changed by C5a treatment. The respective mRNA was normalized against actin. Data are mean ± SEM (n = 4) and are shown as percentage of control. mRNA of macrophages treated with vehicle control represents the 100% value. *P < .05. (B) Mouse macrophages were incubated with C5a (100nM) or vehicle control for 4 or 12 hours and supernatants were analyzed for sVEGFR1 by ELISA. Data are mean ± SEM (n = 3-4) and are shown as percentage of control. Detected sVEGFR1 in supernatant of control treated macrophages represents the 100% value. *P < .05. (C) HUVEC were treated with supernatants of human monocytes that were stimulated without (vehicle control) or with C5a (100nM) or C3a (100nM) for 12 hours and analyzed in the in vitro Matrigel tube formation assay. To assess for VEGF-induced tube formation, growth factor-reduced Matrigel supplemented with VEGF was used. The supernatant of C5a-stimulated human monocytes inhibited the VEGF induced tube formation of HUVEC. Length of forming tubes was quantified using Metamorph software. Data are mean ± SEM (n = 3) and are shown as percentage of control. Tube length of endothelial cells incubated with supernatant of control–treated macrophages represents the 100% value. *P < .05.
Macrophages stimulated with C5a display an antiangiogenic phenotype. (A) Isolated primary bone marrow derived mouse macrophages treated with C5a showed a polarization toward an antiangiogenic phenotype as verified by significantly increased mRNA levels of IL-6, TNF-α, and soluble VEGFR-1 and significantly reduced levels of IL-10 mRNA using quantitative real-time PCR. IL-12 mRNA levels were not significantly changed by C5a treatment. The respective mRNA was normalized against actin. Data are mean ± SEM (n = 4) and are shown as percentage of control. mRNA of macrophages treated with vehicle control represents the 100% value. *P < .05. (B) Mouse macrophages were incubated with C5a (100nM) or vehicle control for 4 or 12 hours and supernatants were analyzed for sVEGFR1 by ELISA. Data are mean ± SEM (n = 3-4) and are shown as percentage of control. Detected sVEGFR1 in supernatant of control treated macrophages represents the 100% value. *P < .05. (C) HUVEC were treated with supernatants of human monocytes that were stimulated without (vehicle control) or with C5a (100nM) or C3a (100nM) for 12 hours and analyzed in the in vitro Matrigel tube formation assay. To assess for VEGF-induced tube formation, growth factor-reduced Matrigel supplemented with VEGF was used. The supernatant of C5a-stimulated human monocytes inhibited the VEGF induced tube formation of HUVEC. Length of forming tubes was quantified using Metamorph software. Data are mean ± SEM (n = 3) and are shown as percentage of control. Tube length of endothelial cells incubated with supernatant of control–treated macrophages represents the 100% value. *P < .05.
Consistent with a previous report that C5a can up-regulate sVEGFR1 in macrophages in the context of placental dysfunction,20 we found that C5a-stimulated bone marrow–derived macrophages displayed increased levels of sVEGFR1 mRNA as well as increased secretion of this antiangiogenic factor into their supernatant (Figure 4A-B). Soluble VEGFR1 is a product of alternative splicing of VEGFR-1 and contains the ligand–binding domain but lacks the cytoplasmic and transmembrane domains and it is thereby an inhibitor of VEGF.42 Consistent with the regulation of sVEGFR1 expression by complement, the mRNA levels of sVEGFR1 were reduced in the retinas of C3−/− mice that were subjected to the ROP protocol, compared with WT mice subjected to ROP (data not shown). In addition, active C5a up-regulated the secretion of sVEGFR1 from human peripheral blood isolated monocytes (supplemental Figure 5). Accordingly, the supernatant from C5a-stimulated but not of C3a-stimulated human monocytes inhibited the VEGF-induced capillary–like sprout formation of HUVEC (Figure 4C), suggesting that the supernatant of C5a-stimulated monocytes intervenes with the effects of the VEGF system on proangiogenic endothelial cell functions. To directly demonstrate that the antiangiogenic activity of the supernatant of C5a-treated monocytes was mediated by sVEGFR1, we immunodepleted sVEGFR1 from the monocyte supernatant. Efficient immunodepletion of sVEGFR1 was confirmed by ELISA analysis of the supernatant (supplemental Figure 6A). Whereas sVEGFR1-containing supernatant from C5a-stimulated monocytes (control Ig-immunodepletion) significantly inhibited endothelial capillary–like sprout formation, the inhibitory effect of the monocyte supernatant was completely reversed upon sVEGFR1 immunodepletion (supplemental Figure 6B). Together, these findings indicate that complement stimulated macrophages are polarized to an angiogenesis inhibitory phenotype including the up-regulation of the antiangiogenic sVEGFR1.
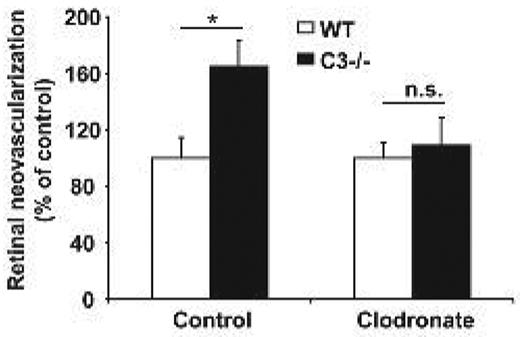
We then sought to provide definitive in vivo evidence for the role of myeloid cells/macrophages in mediating the antiangiogenic effect of complement. To do so, we investigated the effect of macrophage depletion by clodronate liposomes43 on pathological retina angiogenesis in WT and C3−/− mice. WT or C3−/− pups that were subjected to the ROP model received a single injection of clodronate or control liposomes on postnatal day p13. Flow cytometric analysis of the spleen revealed that efficient depletion of CD11b+ cells was achieved (supplemental Figure 7). Macrophage depletion reversed the increased neovascularization in the ROP model due to C3-deficiency. In particular, upon treatment with clodronate liposomes C3−/− mice no longer displayed higher angiogenesis compared with WT mice (Figure 5). In conclusion, the function of complement to inhibit neovascularization in vivo is dependent on the presence of macrophages.
Increased angiogenesis due to C3-deficiency is dependent on macrophages. WT or C3−/− mice were subjected to the ROP model and animals were injected with control liposomes or clodronate liposomes to deplete macrophages. Retinal neovascularization was quantified on day p17. Retinal neovascularization is presented as the number of epiretinal neovascular nuclei per section. Data are mean ± SEM (n = 12-17 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in WT mice treated with control liposomes represents the 100% control. *P < .05; ns, not significant.
Increased angiogenesis due to C3-deficiency is dependent on macrophages. WT or C3−/− mice were subjected to the ROP model and animals were injected with control liposomes or clodronate liposomes to deplete macrophages. Retinal neovascularization was quantified on day p17. Retinal neovascularization is presented as the number of epiretinal neovascular nuclei per section. Data are mean ± SEM (n = 12-17 pups per group) and are shown as percentage of control. Number of epiretinal nuclei in WT mice treated with control liposomes represents the 100% control. *P < .05; ns, not significant.
An inhibitory role of complement in VEGF-induced Matrigel angiogenesis in vivo
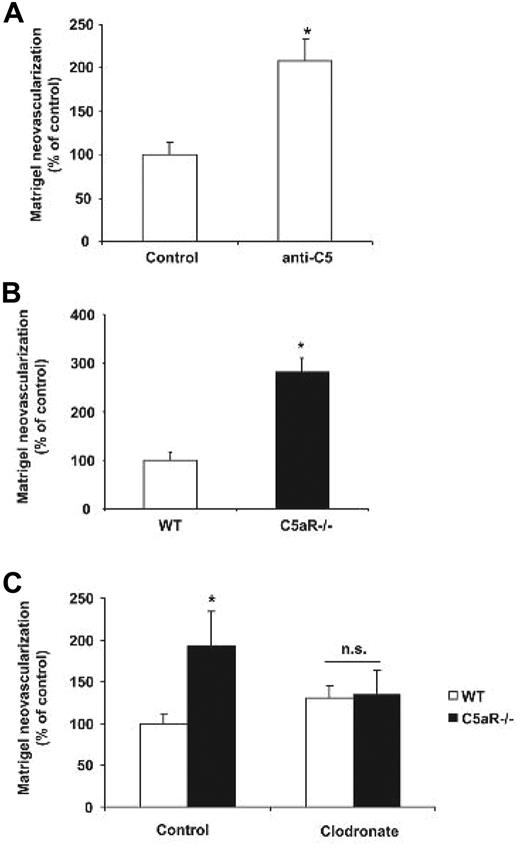
Our findings so far have pointed to a clear neovascularization inhibitory action of complement and in particular C5a, mediated by macrophages in the disease model of vasoproliferative retinopathy. We then went on to address whether the antiangiogenic effect of complement could be observed in a less complex in vivo angiogenesis assay. For this purpose we studied angiogenesis directly induced by VEGF in Matrigel plugs that were implanted into mice. By assessing Matrigel angiogenesis, we found that C3−/− mice had significantly higher levels of new vessel formation compared with WT mice (Figure 6A-B). Similarly, antibody blockade of C5 (clone BB5.1) or C5aR deficiency resulted in significantly higher angiogenesis in the Matrigel plug model (Figure 7A-B). In accordance with the findings from the ROP model, the proangiogenic effect of C3 or C5aR deficiency was mediated by macrophages. Systemic depletion of macrophages by clodronate liposomes reversed the higher levels of Matrigel angiogenesis in C3−/− or C5aR−/− mice (ie, no difference was observed in Matrigel angiogenesis between WT and C3−/− mice or WT and C5aR−/− mice treated with clodronate liposomes; Figures 6A and 7C). Taken together, C3 deficiency and C5aR deficiency are associated with increased angiogenesis in the Matrigel plug assay and macrophages are mediators of the proangiogenic phenotype of C3 and C5aR deficiency.
Matrigel plug angiogenesis in C3-deficient mice. Angiogenesis was assessed using the in vivo Matrigel plug assay as described under “In vivo Matrigel plug assay.” (A) Six hours after Matigel implantation, WT and C3−/− mice were injected with control liposomes or clodronate liposomes to deplete macrophages and after 7 days angiogenesis was assessed within the explanted Matrigels. Data are mean ± SEM (n = 14 Matrigels per group) and are shown as percentage of control. Area of neovascularization in WT mice treated with control liposomes represents the 100% control. *P < .01; ns, not significant. (B) Frozen sections of the explanted Matrigels were stained with H&E. C3−/− mice showed significantly more Matrigel plug neovascularization. Scale bars, 150 μm.
Matrigel plug angiogenesis in C3-deficient mice. Angiogenesis was assessed using the in vivo Matrigel plug assay as described under “In vivo Matrigel plug assay.” (A) Six hours after Matigel implantation, WT and C3−/− mice were injected with control liposomes or clodronate liposomes to deplete macrophages and after 7 days angiogenesis was assessed within the explanted Matrigels. Data are mean ± SEM (n = 14 Matrigels per group) and are shown as percentage of control. Area of neovascularization in WT mice treated with control liposomes represents the 100% control. *P < .01; ns, not significant. (B) Frozen sections of the explanted Matrigels were stained with H&E. C3−/− mice showed significantly more Matrigel plug neovascularization. Scale bars, 150 μm.
C5aR plays an inhibitory role in Matrigel plug angiogenesis. (A) Angiogenesis was assessed using the in vivo Matrigel plug assay. (A) Six hours and 3 days after Matrigel implantation, C57BL/6 WT mice were injected with control IgG or anti-C5 (clone BB5.1, 750 μg per mouse). After 7 days, angiogenesis was assessed within the explanted Matrigels. Data are mean ± SEM (n = 10 Matrigels per group) and are shown as percentage of control. Area of neovascularization in WT mice treated with IgG control represents the 100% value. *P < .005. (B) Matrigel angiogenesis was quantified in WT and C5aR−/− mice. Data are mean ± SEM (n = 5 Matrigels per group) and are shown as percentage of control. The area of neovascularization of WT mice represents the 100% control. *P < .01. (C) Six hours after Matrigel implantation, WT and C5aR−/− mice were injected with control liposomes or clodronate liposomes to deplete macrophages and after 7 days angiogenesis was assessed within the explanted Matrigels. Data are mean ± SEM (n = 8 Matrigels per group) and are shown as percentage of control. Area of neovascularization in WT mice treated with control liposomes represents the 100% control. *P < .05; ns, not significant.
C5aR plays an inhibitory role in Matrigel plug angiogenesis. (A) Angiogenesis was assessed using the in vivo Matrigel plug assay. (A) Six hours and 3 days after Matrigel implantation, C57BL/6 WT mice were injected with control IgG or anti-C5 (clone BB5.1, 750 μg per mouse). After 7 days, angiogenesis was assessed within the explanted Matrigels. Data are mean ± SEM (n = 10 Matrigels per group) and are shown as percentage of control. Area of neovascularization in WT mice treated with IgG control represents the 100% value. *P < .005. (B) Matrigel angiogenesis was quantified in WT and C5aR−/− mice. Data are mean ± SEM (n = 5 Matrigels per group) and are shown as percentage of control. The area of neovascularization of WT mice represents the 100% control. *P < .01. (C) Six hours after Matrigel implantation, WT and C5aR−/− mice were injected with control liposomes or clodronate liposomes to deplete macrophages and after 7 days angiogenesis was assessed within the explanted Matrigels. Data are mean ± SEM (n = 8 Matrigels per group) and are shown as percentage of control. Area of neovascularization in WT mice treated with control liposomes represents the 100% control. *P < .05; ns, not significant.
Discussion
We demonstrated that the complement system is a negative regulator of pathological neovascularization in the context of vasoproliferative retinopathy. C3-deficient and C5aR-deficient mice revealed increased pathological retina angiogenesis. Consistently, antibody blockade of C5 or C5aR antagonism increased and active C5a inhibited pathological retina angiogenesis. Interestingly, the same proangiogenic phenotype of complement deficiency was observed in VEGF-induced angiogenesis in the Matrigel plug assay in vivo. The angiogenesis inhibitory effect of complement was not mediated by a direct antiangiogenic effect of complement components on endothelial cells. In contrast, we found that macrophages acted as the mediators of the antiangiogenic actions of complement and of C5a in particular. C5a polarized macrophages to a pro-inflammatory phenotype with increased expression of IL-6 and TNF-α and decreased expression of IL-10, which is recognized as the antiangiogenic macrophage signature.14 In addition, monocytes/macrophages exposed to C5a in vitro secreted increased amounts of sVEGFR1, an established angiogenesis inhibitor.42 In conclusion, our data make a strong case for an important role of the complement system in antagonizing pathological postnatal angiogenesis.
Our findings provide the first evidence that the complement system may exert an antiangiogenic action in the course of pathological postnatal neovascularization in the retina. However, our present findings from the less complex growth factor–triggered Matrigel angiogenesis assay in vivo imply that the herein described angiogenesis inhibitory function of complement may not necessarily be restricted to the retina but may extend to other tissues and other forms of postnatal and pathological neovascularization. This hypothesis is supported by a previous report that complement triggers release of sVEGFR-1 by monocytes thereby resulting in angiogenic factor imbalance associated with placental dysfunction and fetal growth restriction.20 The sVEGFR1-mediated inhibition of angiogenesis triggered by the complement system might be operative in tissues, where VEGF has been attributed a central function for angiogenesis, such as tumor angiogenesis in the kidney.44
That complement and C5a can polarize macrophages to the M1 proinflammatory phenotype inducing secretion of inflammatory cytokines has been implicated in previous reports1,45–47 and it is increasingly recognized that this macrophage phenotype is associated with antiangiogenic activity.14,48 Consistently, we demonstrated here that the antiangiogenic action of complement was attributed to the presence of macrophages, as macrophage depletion reversed the proangiogenic phenotype of C3 deficiency. Thus, the complement-mediated inhibition of neovascularization in a manner dependent on macrophages may represent a previously not described substantial antiangiogenic mechanism.
How does this hypothesis reconcile with the proangiogenic actions of complement components reported in models of choroidal neovascularization?15,16,49 Whereas complement may polarize macrophages to an antiangiogenic phenotype and this may be a predominant action of complement in some cases, such as in proliferative retinopathy, as described here, in the context of choroidal neovascularization this action of complement may be overridden by the complement-mediated induction of the proangiogenic VEGF in retina pigment epithelial cells.16,50 The retina pigment epithelium plays a unique role in the pathomechanism of choroidal neovascularization in the course of AMD,51,52 whereas these cells are not at all involved in the proliferative retinopathy of prematurity. In addition, intrinsic differences between choroidal and retinal neovascularization may contribute to the opposite actions of the complement system in these 2 vasoproliferative eye pathologies. A major insight from the present study is that the use of complement inhibitors in AMD, which is at present tested in clinical studies,53 requires caution and prior understanding of the underlying pathophysiological role of complement, since our findings imply that inhibition of complement might aggravate proliferative diabetic retinopathy,10 an eye pathology that may be coprevalent with AMD in some patients. Together, the versatility of functions of the complement system make it imperative that the different actions of complement are studied in detail and understood in different tissues/organs and experimental models of disease.
Although the findings in our work point to a role of C5aR mediating the antiangiogenic effects of C5a, it cannot be excluded that the second C5a receptor C5L2,54 which is expressed on macrophages,55 may also participate in mediating the antiangiogenic activity of C5a. The role of C5L2 in angiogenesis merits further investigation. Moreover, our findings add further information regarding the role of inflammatory cells in regulating angiogenesis. Whereas macrophages are often assigned a proangiogenic function, this generalization does not consider how functionally different macrophage subpopulations actually are.48 Proangiogenic M2 macrophages are often found in tumors.56 These cells have rather anti-inflammatory functions and promote tissue remodelling.48 In contrast, proinflammatory M1 macrophages, that can be triggered by interferon-γ, or GM-CSF and display high levels of IL-12, IL-6, or TNF-α and low levels of IL-10 are increasingly recognized as antiangiogenic.14,48 Thus, the presence of macrophages in an angiogenic context, such as neovascular eye diseases, does not always imply a functional link between inflammation and angiogenesis and may actually reveal the opposite (ie, that the angiogenic tissue is dominated by a tilted balance of anti-inflammatory over proinflammatory macrophages).48 For instance, deficiency of the angiogenesis inhibitor thrombospondin-1 was associated with increased M2 polarization and thereby enhanced angiogenesis.57 Whether the herein described antiangiogenic actions of complement are somehow linked to thrombospondin-1 is not clear, which merits investigation, since a potential cooperation between thrombospondin-1 and complement has been suggested in other systems previously.58
In conclusion, our findings emphasize the key role of the complement system as a counterplayer of pathological retina angiogenesis. Besides establishing a direct link between angiogenesis and the innate immunity and the complement cascade in particular, our findings also point to the attractive possibility that components of the complement system or their antagonism may provide new therapeutic tools for inhibiting angiogenesis, for instance in vasoproliferative retinopathies, or for improving insufficient ischemia–driven angiogenesis in peripheral vasoocclusive diseases, respectively.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Apostolia Tzekou for chicken scrambled C3a synthesis.
This work was supported by the NIH Intramural Research Program, National Cancer Institute (T.C.), the German Academy of Sciences Leopoldina (H.F.L), and NIH grants CA112162 and AI-068730 (J.D.L.).
National Institutes of Health
Authorship
Contribution: H.F.L. performed research, analyzed data, and wrote the manuscript; K.-J.C., V.V.O., E.Y.C., and S.K. performed research; M.J.K. analyzed data; M.A. performed research; R.A.D., P.A.R, X.L., and M.E. provided analytical tools; P.M. and S.R. provided vital reagents; and J.D.L. and T.C. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.F.L. is Medizinische Klinik III, Kardiologie und Kreislauferkrankungen, Eberhard Karls-Universität Tübingen, Tübingen, Germany. The current affiliation for T.C. is Division of Vascular Inflammation, Diabetes and Kidney, Department of Medicine III, University Clinic Carl-Gustav-Carus Dresden and Institute of Physiology, Medical Faculty, Dresden University of Technology, Dresden, Germany.
Correspondence: Triantafyllos Chavakis, Division of Vascular Inflammation, Diabetes and Kidney, Department of Medicine III, University Clinic Carl-Gustav-Carus Dresden and Institute of Physiology, Medical Faculty, Dresden University of Technology, Fetscherstrasse 74, 01307 Dresden, Germany; e-mail: triantafyllos.chavakis@uniklinikum-dresden.de; or John D. Lambris, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA; e-mail: lambris@upenn.edu.
References
Author notes
J.D.L. and T.C. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal