Absence of a functional nicotinamide adenine dinucleotide phosphate (NADPH) oxidase predisposes chronic granulomatous disease (CGD) patients to infection, and also to unexplained, exaggerated inflammation. The impaired recognition and removal (efferocytosis) of apoptotic neutrophils by CGD macrophages may contribute to this effect. We hypothesized that peroxisome proliferator-activated receptor γ (PPARγ) activation during CGD inflammation is deficient, leading to altered macrophage programming and decreased efferocytosis, and that PPARγ agonism would enhance resolution. using the gp91phox−/− murine model of X-linked CGD in a well-characterized model of sterile, zymosan-induced peritonitis, it was demonstrated that PPARγ expression and activation in CGD macrophages were significantly deficient at baseline, and acquisition was delayed over the course of inflammation relative to that of wild-type. Efferocytosis by macrophages reflected PPARγ activation during peritonitis and was impaired in CGD mice (versus wild-type), leading to accumulation of apoptotic neutrophils. Importantly, provision of the PPARγ agonist, pioglitazone, either prophylactically or during inflammation, significantly enhanced macrophage PPARγ-mediated programming and efferocytosis, reduced accumulation of apoptotic neutrophils, and normalized the course of peritonitis in CGD mice. As such, PPARγ may be a therapeutic target for CGD, and possibly other inflammatory conditions where aberrant macrophage programming and impaired efferocytosis delay resolution of inflammation.
Introduction
While prophylactic administration of antibiotic and antifungal agents reduce the occurrence of infections in chronic granulomatous disease (CGD) patients, they suffer from exaggerated, often sterile inflammation (eg, poor wound healing, obstructing granuloma, and colitis) and autoimmunity, the mechanisms for which are not fully understood.1,,–4 Among the various inflammatory mechanisms described in CGD, improper or deficient signaling by activated and apoptosing neutrophils has been proposed.5,–7 Impaired degradation of proinflammatory mediators,8,–10 deficient up-regulation of adenosine and cAMP production,11 and tryptophan catabolism12 are also described. Finally, the impaired recognition and clearance (efferocytosis) of dying neutrophils by CGD macrophages is hypothesized.11,13,14 Proper recognition and clearance of apoptotic cells by macrophages is actively anti-inflammatory and required for restoration of tissue function.15,–17 Conversely, dying neutrophils, if not recognized and cleared, release injurious intracellular constituents, which further spur inflammation and autoimmunity.7,18,19
The ability of macrophages to clear dying cells needed for resolving inflammation is dependent on their “programming.” Macrophage programming has been traditionally termed “classical” or M1 when induced with lipopolysaccharide plus interferonγ and associated with Th1 cytokines, and “alternative” or M2 when induced by interleukin-4 (IL-4) plus IL-10, and associated with Th2 cytokines.20,21 While this dichotomy is clearly oversimplified,22 M2 programming is required for efferocytic capability and has been shown to be dependent on macrophage-specific peroxisome proliferator-activated receptor γ (PPARγ).23,–25 We recently demonstrated that macrophages from the naive peritoneum of gp91phox−/− mice (a model of X-linked CGD) were classically activated, had low levels of PPARγ expression, and were poorly programmed to recognize and clear normal (non-CGD) apoptotic cells compared with wild-type (WT) macrophages.13 These observations prompted us to hypothesize that deficient PPARγ signaling, given its various roles in controlling inflammation,26,27 might underlie the heightened inflammation characteristic of CGD. Specifically, deficient PPARγ signaling might lead to impaired clearance of dying neutrophils and loss of suppression of inflammatory mediator production by CGD macrophages. Finally, we hypothesized to the extent that deficient PPARγ signaling was key to exaggerated inflammation in this model, its restoration with a PPARγ agonist would normalize the course of CGD inflammation.
Using a model of acute peritonitis, prolonged inflammation in CGD mice was associated with delayed macrophage PPARγ signaling relative to that of WT mice. Accumulation of apoptotic neutrophils and lower levels of efferocytosis by CGD macrophages in vivo demonstrated impaired apoptotic cell removal in this disorder. As hypothesized, restoration of PPARγ signaling via oral administration of pioglitazone, a potent PPARγ agonist, significantly shortened the course of peritonitis in CGD mice, and the data supported a significant role for enhanced efferocytosis in this effect. Taken together, the data support that PPARγ may be key to regulating inflammation in CGD and point to restoration of PPARγ signaling as a potential therapeutic target in this disorder.
Methods
Animals
Male C57BL/6 and X-CGD (gp91phox−/−) mice were purchased from The Jackson Laboratory or bred in a colony housed at National Jewish Health. All animals received care in accordance with the Institutional Animal Care and Use Committee and were maintained on food and water ad libitum. Mice between the ages of 8 and 16 weeks were age- and sex-matched for all experiments. Mice were euthanized using CO2 inhalation.
Reagents
Zymosan, pioglitazone, GW 9662, and propidium iodide were from Sigma-Aldrich. Antibody to F4/80 (rat immunoglobulin [Ig] G2a) and Alexa Fluor 488–annexinV were from Invitrogen, and anti-CD36 (rat IgG2a) was from eBioscience; macrophage mannose receptor (MMR; goat IgG) was from R&D Systems, and PPARγ (goat IgG) was from SCBT; factor Va antibody and anti–factor Va antibody were from HTI. Carboxylated and noncarboxylated latex beads (5-μm) were from Bangs Laboratories.
Induction of sterile peritonitis and treatment
Mice were injected intraperitoneally with 1 mg/mL zymosan (phosphate-buffered saline; PBS), and peritoneal cells were harvested by lavage with ice-cold sterile Hanks balanced salt solution (with 1mM EDTA [ethylenediaminetetraacetic acid] and 10mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.2) at the times indicated. Absolute cell numbers were obtained using a Coulter counter (Coulter Channalyzer 256). Cytospins of cells were prepared, fixed, and stained with modified Wright Giemsa (Fisher Scientific) and read in a blinded fashion to determine cell differentials and efferocytic indices. Mice were given pioglitazone (10 mg/kg) or vehicle (carboxymethyl cellulose) via oral gavage for 2 days before zymosan and every 24 hours thereafter until they were euthanized. In the delayed treatment protocol, a single dose of pioglitazone (10 mg/kg) or vehicle was given at 24 hours after zymosan, and the mice were euthanized at 48 hours after zymosan treatment.
Flow cytometric analyses of macrophages
Cells were harvested, washed, and resuspended in Cytofix solution for 20 minutes, washed twice in PBS (0.1% bovine serum albumin), blocked with anti–mouse Fc (CD16/32; eBiosciences) for 30 minutes and stained with the following anti–mouse antibodies: Alexa Fluor 488 F4/80 (1:100), phycoerythrin-conjugated CD36 (1:50) and unconjugated MMR (1:50), or after permeabilization, with unconjugated PPARγ (1:50) alongside appropriate isotype controls for 1 hour at 4°C. Secondary conjugated antibodies were used for 30 minutes on ice. Cells were washed twice with PBS and analyzed using a BD FACScan flow cytometer. The percentage of F4/80 positive macrophages staining for each marker and the mean fluorescence intensity of positive cells (above the respective isotype control) was determined.
PS exposure and apoptosis of peritoneal neutrophils
Peritoneal cells were washed and stained immediately after lavage for phosphatidylserine (PS) exposure detected by annexin V binding with propidium iodide staining (for permeability) according to the manufacturer's instructions, and neutrophils were analyzed by flow cytometry. Peritoneal cells were also incubated with factor Va, then stained with anti–factor Va as previously described28 and neutrophils analyzed by flow cytometry. Apoptosis of neutrophils was also determined by nuclear morphology from microscopic examination of cytospins read in a blinded fashion.5
In vivo efferocytosis assays
In vivo, efferocytosis by macrophages was determined by microscopic inspection of cytospins, and efferocytic index was calculated by multiplying the percentage of macrophages that phagocytosed apoptotic cells bythe average number of cells engulfed per macrophage.29 A minimum of 500 macrophages were counted in a blinded fashion. In vivo uptake of beads by peritoneal macrophages was performed by injecting 20 × 106 fluorescent (flash red) 5-μm carboxylated beads intraperitoneally, and 1 hour later, mice were euthanized and lavaged. Peritoneal cells were washed, fixed, stained with anti-F4/80, and analyzed by flow cytometry for the percentage of F4/80-positive macrophages associated with beads. Cytospins from these experiments demonstrated that the beads were almost all inside macrophages. For comparison, 20 × 106 fluorescent (flash red) 5-μm noncarboxylated latex beads were injected intraperitoneally, and cells were analyzed as indicated above.
Ex vivo efferocytosis assays
Inflammatory macrophages (48 hours after zymosan) were plated in 24-well plates (0.3 × 106/well), rested for 2 hours, and treated for 24 hours with either vehicle (dimethyl sulfoxide) or 1μM pioglitazone in the presence or absence of 10μM PPARγ antagonist (GW 9662; added 1 hour before the treatments).13 The cells were washed, cocultured with 0.6 × 106 apoptotic Jurkat T-cells (prepared as described previously13 ) for 1 hour, washed 3 times with PBS, and stained with a modified Wright Giemsa stain (Fisher Scientific). The efferocytic index was calculated as above with a minimum of 200 macrophages counted blindly. Each condition was tested in duplicate and repeated 3 times.
Cytokines
The levels of IL-10, transforming growth factorβ (TGFβ), IL-6, keratinocyte-derived chemokine (KC), and tumor necrosis factorα (TNFα) in lavage fluid or culture supernatants were measured by enzyme-linked immunosorbent assay using antibodies from R&D Systems according to the manu-facturer's instructions. In the case of TGFβ, all assays were performed after acidification for activation and measurement of total TGFβ as described previously.17
Western blotting
Cell lysates (10 μg of protein) were analyzed using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred onto polyvinylidene difluoride membrane, blocked for 1 hour (1% bovine serum albumin), and incubated with antibody (anti–mouse PPARγ, CD36 or MMR, 1:1000) for 20 hours followed by addition of secondary antibody (1:1000) for 30 minutes. Detection was performed using enhanced chemiluminescence substrate (Amersham Biosciences) following the manufacturer's instructions. Western blots were scanned and analyzed using ImageJ (National Institutes of Health). The scans were inverted and the background subtracted before measuring the relative protein amounts. All conditions were expressed as a ratio of test protein to actin.
PPARγ activity
Nuclear extracts (8 μg/condition) were prepared using a kit and activity determined using a TRANSAM assay kit (Activ Motif) according to the manufacturer's instructions.
Statistics
Analysis and P value calculations were conducted using analysis of variance (JMP statistical program 4.0.1; SAS Institute). The Dunnett and Tukey-Kramer tests were used for single and multiple comparisons, respectively. P ≤ .05 were considered significant.
Results
PPARγ expression and activation are deficient in CGD macrophages during acute inflammation
In our earlier investigation, we showed that PPARγ protein levels were significantly lower in resident macrophages from the naive peritoneum of CGD compared with WT mice.13 We therefore asked whether PPARγ levels were also deficient in CGD mice during acute inflammation. After injection of zymosan, peritonea of WT and CGD mice were lavaged at times indicated, and mononuclear phagocyte populations identified by F4/80 staining by flow cytometry. F4/80-positive cells, hereafter referred to as “macrophages,” are known to be a heterogeneous mixture of resident macrophages, recruited monocytes, and differentiating inflammatory macrophages30 (see “Discussion”). The numbers of macrophages lavaged over the course of peritonitis were quite similar between CGD and WT mice (Figure 1A), while significant differences were seen in PPARγ protein levels in macrophages. First, at baseline, the level of intracellular PPARγ (Figure 1B) and percentage of F4/80 macrophages positive for PPARγ (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were lower in CGD compared with WT mice. Second, in the hours immediately following zymosan injection, decreased amounts of PPARγ protein were detected (and fewer macrophages stained positive for PPARγ) relative to resident macrophage populations in both CGD and WT mice. This initial decrease was followed by a time-dependent increase in PPARγ expression over the course of peritonitis in both genotypes (Figure 1B and supplemental Figure 1A), consistent with reports that PPARγ is low in monocytes and is up-regulated in macrophage populations during acute inflammation.27,31 While the percentage of macrophages staining positively for PPARγ was not different between the genotypes during this increase (supplemental Figure 1A), the levels of PPARγ protein per macrophage were significantly lower in CGD than WT mice over the course of the inflammation (Figure 1B). Peritoneal cells negative for F4/80 did not show evidence of PPARγ staining at any time point in either genotype.
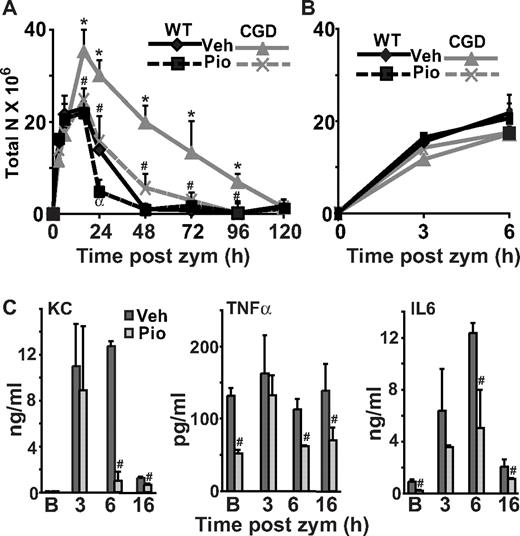
Macrophage PPARγ expression and activity are deficient and acquisition delayed in CGD mice during peritonitis. WT and CGD mice were injected intraperitoneally with zymosan and lavage performed at the indicated times. Peritoneal F4/80 monocytes/macrophages were counted (A) and stained for intracellular PPARγ (following permeablilization) (B), surface CD36 (C), and surface macrophage mannose receptor (MMR; D). Mean fluorescence intensity (MFI) is shown (B-D). Data represent mean ± standard error (SE); N = 8 mice per time point. *P < .02 compared with WT mice at the respective time points; #P < .03 compared with baseline [B] values for each genotype, respectively.
Macrophage PPARγ expression and activity are deficient and acquisition delayed in CGD mice during peritonitis. WT and CGD mice were injected intraperitoneally with zymosan and lavage performed at the indicated times. Peritoneal F4/80 monocytes/macrophages were counted (A) and stained for intracellular PPARγ (following permeablilization) (B), surface CD36 (C), and surface macrophage mannose receptor (MMR; D). Mean fluorescence intensity (MFI) is shown (B-D). Data represent mean ± standard error (SE); N = 8 mice per time point. *P < .02 compared with WT mice at the respective time points; #P < .03 compared with baseline [B] values for each genotype, respectively.
As an indication of PPARγ functional activity, expression of CD36 and MMR, both known to be up-regulated by PPARγ activation and characteristic of M2 macrophage programming,24,32,33 were determined. At baseline, CGD resident peritoneal macrophage surface staining for both markers was lower compared with those of WT mice, both in amounts (Figure 1C-D) and percentage of macrophages staining positively (supplemental Figure 1B-C). After zymosan instillation, macrophages from both genotypes demonstrated a significant decrease in staining for the markers followed by a progressive time-dependent increase during peritonitis (Figure 1C-D and supplemental Figure 1B-C). Comparatively, CGD macrophages demonstrated less CD36 and MMR, both in terms of surface levels and the percentage staining positive, at each time point. Notably, the expression of these markers in each instance paralleled PPARγ expression patterns in both WT and CGD mice.
Lower macrophage PPARγ expression and activation are associated with enhanced inflammation in CGD mice
Given that PPARγ is known to inhibit activator protein 1, specificity protein-1, and nuclear factor-κB driven proinflammatory cytokine transcription,24,26,34 it was hypothesized that elevated levels of mediators might reflect delayed or deficient acquisition of PPARγ expression and activity in CGD mice. Proinflammatory mediators, directly or indirectly associated with leukocyte recruitment, were measured in peritoneal lavage. Baseline levels of IL-6 and TNFα were found to be elevated in CGD (versus WT) mice, and following zymosan installation, IL-6, TNFα, and KC levels were higher and their elevation prolonged in CGD mice (Figure 2). In keeping with these findings, neutrophil numbers during peritonitis were increased and sustained in CGD mice (versus WT; Figure 3A) as shown in previous reports of exaggerated neutrophilic inflammation in this model,5,9,11 while the numbers of other inflammatory cells were very similar (Figure 1A and supplemental Figure 2). Neutrophilia was ultimately self-limited in both WT and CGD mice, and resolution indices described by Bannenberg et al35 were determined (Table 1): the maximal numbers of neutrophils (ψmax) accumulating in the peritoneum and the time for resolution of neutrophilia (T50 and Ri) were greater in CGD compared with WT mice while the time (Tmax) to ψmax was not different. In addition, the time to achieve equal numbers of neutrophils to macrophages (Ipmn = mono) was longer in CGD mice (Table 1). Thus, both inflammatory mediators and neu-trophilic inflammation were significantly exaggerated in CGD mice and associated with delayed acquisition of PPARγ expression and activity.
Proinflammatory cytokines are elevated and prolonged during peritonitis in CGD mice. Peritonitis was induced as in Figure 1, and cytokines were measured in peritoneal lavage supernatants at the times indicated. [B] indicates baseline without zymosan. Data represent mean ± SE; N = 6 mice per time point investigated; *P < .03 compared with WT mice at the respective time points.
Proinflammatory cytokines are elevated and prolonged during peritonitis in CGD mice. Peritonitis was induced as in Figure 1, and cytokines were measured in peritoneal lavage supernatants at the times indicated. [B] indicates baseline without zymosan. Data represent mean ± SE; N = 6 mice per time point investigated; *P < .03 compared with WT mice at the respective time points.
Exaggerated and prolonged neutrophilia characterizing zymosan peritonitis in CGD mice is accompanied by impaired in vivo efferocytosis by macrophages. Peritonitis was induced as in Figure 1 and peritoneal cells analyzed at the times indicated (see “Methods”). (A) Total neutrophils [N] were counted. (B) Percent of neutrophils exposing PS (annexin V–positive staining), and (C) absolute numbers of PS exposing neutrophils were determined. (D) Apoptotic neutrophils identified by nuclear morphology and the ratio of apoptotic neutrophils to macrophages were determined microscopically. (E) Efferocytic Index was determined by microscopic examination of macrophages lavaged from the inflamed peritonea. (F) Carboxylated beads were injected into the peritonea to measure in vivo efferocytic capability, and 1 hour later, the percentage of lavaged peritoneal macrophages positive for beads was determined by flow cytometry. [B] indicates baseline without zymosan. Data represent mean ± SE; N = 8 mice per time point. *P < .02 compared with WT mice at the respective time points. #P < .02 compared with WT mice at baseline.
Exaggerated and prolonged neutrophilia characterizing zymosan peritonitis in CGD mice is accompanied by impaired in vivo efferocytosis by macrophages. Peritonitis was induced as in Figure 1 and peritoneal cells analyzed at the times indicated (see “Methods”). (A) Total neutrophils [N] were counted. (B) Percent of neutrophils exposing PS (annexin V–positive staining), and (C) absolute numbers of PS exposing neutrophils were determined. (D) Apoptotic neutrophils identified by nuclear morphology and the ratio of apoptotic neutrophils to macrophages were determined microscopically. (E) Efferocytic Index was determined by microscopic examination of macrophages lavaged from the inflamed peritonea. (F) Carboxylated beads were injected into the peritonea to measure in vivo efferocytic capability, and 1 hour later, the percentage of lavaged peritoneal macrophages positive for beads was determined by flow cytometry. [B] indicates baseline without zymosan. Data represent mean ± SE; N = 8 mice per time point. *P < .02 compared with WT mice at the respective time points. #P < .02 compared with WT mice at baseline.
Neutrophilia is prolonged and exaggerated in CGD
| Mouse* . | ψmax, ×106 . | Tmax, h . | T50, h . | Ri, h . | Ipmn=mono, h . |
|---|---|---|---|---|---|
| WT | 22.8 ± 1.3 | 16 ± 3.5 | 29 ± 2.1 | 13.0 ± 1.6 | 31.4 ± 1.5 |
| CGD | 32.5 ± 5.5* | 18.8 ± 1.1 | 58.3 ± 1.4* | 39.5 ± 0.5* | 78.3 ± 1.4* |
| Mouse* . | ψmax, ×106 . | Tmax, h . | T50, h . | Ri, h . | Ipmn=mono, h . |
|---|---|---|---|---|---|
| WT | 22.8 ± 1.3 | 16 ± 3.5 | 29 ± 2.1 | 13.0 ± 1.6 | 31.4 ± 1.5 |
| CGD | 32.5 ± 5.5* | 18.8 ± 1.1 | 58.3 ± 1.4* | 39.5 ± 0.5* | 78.3 ± 1.4* |
Data from Figures 1A and 3A were analyzed for ψmax (maximal numbers of neutrophils recovered), Tmax (time to ψmax), T50 (time when neutrophil numbers reach half maximum), Ri (Tmax − T50) and Ipmn = mono (time when there are equal numbers of macrophages and neutrophils) as previously described (Bannenberg et al35 ).
P ≤ .04 compared to WT.
Aside from altered macrophage programming in CGD, increased neutrophil longevity has also been hypothesized to contribute to exaggerated neutrophilia in CGD. Defects and/or delays in the apoptosis of both human and murine CGD neutrophils have been described, although mostly following in vitro culture.5,–7 Thus, we investigated neutrophils for evidence of apoptosis immediately after lavage from inflamed peritonea. PS exposure, which has been demonstrated during both neutrophil activation and apoptosis, was assessed by binding of Factor Va (data not shown) and annexinV (Figure 3B). Over the course of peritonitis, 7%-10% of neutrophils exposed PS regardless of genotype or time point and no differences were observed between CGD and WT (Figure 3B), confirming a recent report by Rajakariar et al.11 Similarly, extrapolation of data demonstrated that the absolute numbers of PS exposing neutrophils were similar for CGD compared with WT mice at 16 and 24 hours (at 48 hours, very few neutrophils remained in peritonea of WT mice; Figure 3C). Because of our earlier demonstration that activated neutrophils expose PS5,28 (and may thereby initiate their removal by efferocytosis), we also evaluated neutrophil apoptosis by nuclear morphology. Significantly, using this criterion, the number of apoptotic neutrophils at each time point was higher in CGD than WT mice, as was the ratio of apoptotic neutrophils per macrophage present in the peritonea (Figure 3D).
Given that apoptotic cells are rarely seen in tissues in the absence of defects in clearance,15,19,36,37 these data suggested that impaired clearance of neutrophils contributed to their accumulation during CGD inflammation. Accordingly, macrophages lavaged from peritonea were examined microscopically for evidence of in vivo efferocytosis of apoptotic cells. Detectible efferocytosis was found to be significantly lower for CGD compared with WT macrophages (Figure 3E) further supporting impaired clearance of apoptotic cells in CGD. Finally, to formally test this hypothesis, fluorescent carboxylated beads (surrogates for apoptotic cells33 ) were injected into inflamed peritonea, and 60 minutes later their uptake by F4/80 macrophages was quantified by flow cytometry. As shown, efferocytosis by CGD macrophages was impaired relative to WT macrophages (Figure 3F) for resident populations at baseline (as reported previously13 ) and inflammatory macrophages throughout the course of peritonitis. Nonefferocytic phagocytic capacity of the macrophages was also tested in vivo using noncarboxylated beads. No differences in uptake of these beads was observed between CGD and WT macrophages at baseline or during peritonitis (data not shown) demonstrating that impairment in CGD macrophages was specific for efferocytosis. Interestingly, WT macrophages demonstrated a decrease in the uptake of carboxylated beads at 6 hours following zymosan injection relative to baseline (see the Discussion), and in both WT and CGD macrophages, efferocytic capability increased thereafter in a time-dependent fashion mirroring PPARγ expression and activation. Thus, these data suggested that despite possible delays in apoptosis, apoptotic CGD neutrophils accumulate in association with delayed or deficient macrophage PPARγ expression/activation and impaired efferocytic function. In turn, these events are hypothesized to contribute to prolonged neutrophilic inflammation characteristic of CGD.
Treatment with pioglitazone restores macrophage PPARγ activation and efferocytosis and enhances resolution of neutrophilia in CGD mice
It was hypothesized that therapeutic intervention aimed at restoring PPARγ signaling would ameliorate inflammation in CGD mice. The PPARγ agonist, pioglitazone (10 mg/kg/d)38 versus vehicle was administered orally to both WT and CGD mice for 2 days before the instillation of zymosan and every 24 hours thereafter. Macrophage PPARγ expression and activity were assessed following treatment. Pioglitazone treatment for 2 days enhanced PPARγ protein levels in both WT and CGD macrophages at baseline (Figure 4A). Notably, PPARγ agonism has been shown to provide positive feedback for its own expression in other settings39 (and see below). In CGD mice, the percentage of resident peritoneal macrophages positive for PPARγ was also enhanced (supplemental Figure 3 baseline). After instillation of zymosan, pioglitazone treatment of CGD mice increased the expression levels of PPARγ in the macrophages (Figure 4A), nearly normalizing them to levels seen in WT cells. In WT mice, pioglitazone treatment did not further enhance time-dependent macrophage PPARγ expression during peritonitis (Figure 4A and supplemental Figure 3A). As before, the only cells in peritonea of either genotype that expressed PPARγ were F4/80 positive macrophages.
Pioglitazone treatment enhances macrophage PPARγ expression and activity in CGD mice. Mice were gavaged with pioglitazone or vehicle for 2 days before zymosan injection and every 24 hours thereafter. F4/80-positive macrophages from lavage were analyzed as in Figure 1 for intracellular PPARγ (A), and surface CD36 (B), and MMR (C) expressed as MFI. Data represent mean ± SE; N = 8 mice per time point; #P ≤ .01 compared with vehicle-treated CGD mice at the respective time points; α,*P ≤ .01 compared with WT mice treated with vehicle, at the respective time points. [B] indicates baseline after 2 days of treatment without zymosan. Symbols for significant changes in values between baseline [B] and the early time points following zymosan for either vehicle or pioglitazone-treated mice of each genotype were as shown in Figure 1, but omitted here for simplicity.
Pioglitazone treatment enhances macrophage PPARγ expression and activity in CGD mice. Mice were gavaged with pioglitazone or vehicle for 2 days before zymosan injection and every 24 hours thereafter. F4/80-positive macrophages from lavage were analyzed as in Figure 1 for intracellular PPARγ (A), and surface CD36 (B), and MMR (C) expressed as MFI. Data represent mean ± SE; N = 8 mice per time point; #P ≤ .01 compared with vehicle-treated CGD mice at the respective time points; α,*P ≤ .01 compared with WT mice treated with vehicle, at the respective time points. [B] indicates baseline after 2 days of treatment without zymosan. Symbols for significant changes in values between baseline [B] and the early time points following zymosan for either vehicle or pioglitazone-treated mice of each genotype were as shown in Figure 1, but omitted here for simplicity.
As measures of PPARγ activity, macrophage surface expression of the induced markers CD36 and MMR was investigated. On CGD macrophages, pioglitazone treatment enhanced surface expression of these markers and the percentage of macrophages staining positive, both at baseline and during peritonitis (Figure 4B-C and supplemental Figure 3B-C). Furthermore, their increased expression was reflective of levels of PPARγ expression: where pioglitazone enhanced levels of PPARγ expression in CGD macrophages, surface levels of these proteins were also enhanced. For WT macrophages, pioglitazone treatment similarly enhanced levels of these markers (Figure 4B-C) and the percentage of macrophages staining positively for them (supplemental Figure 3B-C) suggesting that PPARγ agonism accelerated M2 programming of WT macrophages during inflammation, as well as restoring this property in the CGD animals.
To further investigate the differential PPARγ expression and activity between genotypes and to determine the effects of pioglitazone treatment, experiments were conducted ex vivo. Inflammatory macrophages from CGD and WT mice were harvested (48 hours after zymosan) and stimulated ex vivo with pioglitazone (or vehicle) for 24 hours. PPARγ expression and activation were measured, the latter both directly (in nuclear extracts) and by assessment of expression of downstream targets. As shown in supplemental Figure 4A, the expression of PPARγ in CGD macrophages was diminished compared with WT macrophages mirroring the levels in freshly lavaged macrophages (Figure 1B). Baseline PPARγ activity was also reduced in CGD macrophages relative to WT (supplemental Figure 4B). Importantly, treatment of cultured CGD macrophages with pioglitazone enhanced PPARγ expression (supplemental Figure 4A), its activity (supplemental Figure 4B), and downstream markers (supplemental Figure 4C-D) to levels at or above those observed in vehicle-treated WT macrophages. The restoration of PPARγ expression and activity in CGD macrophages was blocked by the PPARγ antagonist (supplemental Figure 4A-D). In WT macrophages, ex vivo pioglitazone treatment enhanced PPARγ activity as assessed by both direct and downstream measures (supplemental Figure 4B-D; while having no effect on its expression). Similarly, the antagonist diminished baseline and pioglitazone stimulated PPARγ activity and reduced the expression of the markers, but had no effect on the expression of PPARγ in WT macrophages (supplemental Figure 4). Collectively, these data confirmed the in vivo findings and supported the hypothesis that PPARγ is deficient in CGD macrophages and that treatment with pioglitazone restored PPARγ activity and consequent macrophage programming in CGD mice.
Given these findings, it was hypothesized that restoration of PPARγ signaling would ameliorate neutrophilic inflammation in CGD mice. As shown in Figure 5A, treatment of CGD mice with pioglitazone restored the time course of neutrophilia during peritonitis to that seen in vehicle-treated WT mice. In addition, in WT mice, pioglitazone treatment diminished the course of neutrophilia. Analyses demonstrated that for CGD mice, the maximum numbers of neutrophils (ψmax) was significantly reduced, and for both CGD and WT mice, resolution indices (T50, Ri and Ipmn = mono) were shortened following treatment with pioglitazone compared with vehicle treatment (Table 2). Since the numbers of macrophages lavaged from peritonea were not altered in either of the treatment groups (data not shown), the experiments support a restoration of macrophage function rather than alteration of their numbers.
Pioglitazone treatment enhances resolution of neutrophilia and ameliorates inflammation in CGD mice. Mice were treated as described in Figure 4. The time course of neutrophilia was determined (A, with a blowup of the early time points shown in B), and cytokines were measured in lavage supernatants (CGD mice shown) (C). Data represent mean ± SE; N = 8 mice per time point. #P ≤ .02 compared with vehicle-treated CGD mice at the respective time points, and α,*P ≤ .01 compared with WT mice treated with vehicle, at the respective time points.
Pioglitazone treatment enhances resolution of neutrophilia and ameliorates inflammation in CGD mice. Mice were treated as described in Figure 4. The time course of neutrophilia was determined (A, with a blowup of the early time points shown in B), and cytokines were measured in lavage supernatants (CGD mice shown) (C). Data represent mean ± SE; N = 8 mice per time point. #P ≤ .02 compared with vehicle-treated CGD mice at the respective time points, and α,*P ≤ .01 compared with WT mice treated with vehicle, at the respective time points.
Pioglitazone treatment results in faster resolution of neutrophilia in CGD and WT mice
| Condition* . | ψmax, ×106 . | Tmax, h . | T50, h . | Ri, h . | Ipmn=mono, h . |
|---|---|---|---|---|---|
| WT-Veh | 22.9 ± 4.3 | 18 ± 2.5 | 30 ± 2.2 | 12 ± 0.8 | 32 ± 2.2 |
| WT-Pio | 21.9 ± 2.5 | 16 ± 2.2 | 24 ± 1.5† | 8 ± 2.2† | 24 ± 2.5† |
| CGD-Veh | 35.2 ± 4.7* | 22 ± 2.18 | 60 ± 1.7‡ | 36 ± 1.8‡ | 78 ± 1.8‡ |
| CGD-Pio | 24.7 ± 2.5§ | 20 ± 1.1 | 36 ± 2.3§ | 16 ± 1.9§ | 40 ± 1.9§ |
| Condition* . | ψmax, ×106 . | Tmax, h . | T50, h . | Ri, h . | Ipmn=mono, h . |
|---|---|---|---|---|---|
| WT-Veh | 22.9 ± 4.3 | 18 ± 2.5 | 30 ± 2.2 | 12 ± 0.8 | 32 ± 2.2 |
| WT-Pio | 21.9 ± 2.5 | 16 ± 2.2 | 24 ± 1.5† | 8 ± 2.2† | 24 ± 2.5† |
| CGD-Veh | 35.2 ± 4.7* | 22 ± 2.18 | 60 ± 1.7‡ | 36 ± 1.8‡ | 78 ± 1.8‡ |
| CGD-Pio | 24.7 ± 2.5§ | 20 ± 1.1 | 36 ± 2.3§ | 16 ± 1.9§ | 40 ± 1.9§ |
P ≤ .03 compared to WT vehicle-treated mice.
P ≤ .04 compared to CGD vehicle-treated mice.
Notably, early accumulation of neutrophils during peritonitis (up to 6 hours following zymosan instillation) was not affected by pioglitazone treatment in either CGD or WT mice (Figure 5B) suggesting that PPARγ activation did not suppress early neutrophil recruitment in this model, but rather altered subsequent events. Along these lines, levels of proinflammatory cytokines associated with leukocyte recruitment (eg, KC) were measured in lavage following pioglitazone treatment. At 3 hours following zymosan instillation, levels of these mediators were not altered, regardless of genotype or treatment (Figure 5C, data shown for CGD mice only and compare with Figure 2), showing that early inflammatory signals, like early neutrophil recruitment were not affected by PPARγ agonism. These observations aside, baseline elevations of TNFα and IL-6 in CGD peritonea, and their prolonged elevation during peritonitis in CGD mice were significantly diminished by pioglitazone treatment (Figure 5C) and comparable with untreated WT mice (compare to Figure 2). These data suggest that in CGD mice, deficient PPARγ mediated suppression of proinflammatory mediator production contributes to baseline inflammation and to the protracted course of neutrophilia.
PPARγ agonism enhances efferocytosis in CGD mice
We hypothesized that restoration of PPARγ activation and consequent M2 programming would restore efferocytosis in CGD. As predicted, pioglitazone treatment normalized in vivo efferocytosis by CGD macrophages during peritonitis to levels seen in WT macrophages (Figure 6A). Reflective of heightened clearance, the ratio of apoptotic neutrophils to macrophages during peritonitis was also reduced with pioglitazone treatment in CGD mice compared with vehicle treatment (Figure 6B). Finally, in vivo capacity for efferocytosis was formally tested by challenge with carboxylated beads injected into peritonea. Pioglitazone treatment significantly enhanced bead uptake by CGD macrophages at baseline compared with vehicle, and importantly, normalized efferocytosis to levels seen in WT mice over the course of inflammation (Figure 6C). In WT mice, pioglitazone treatment did not enhance in vivo macrophage efferocytosis and had no detectible effect on accumulation of apoptotic neutrophils (Figure 6A-B). Finally, while pioglitazone treatment did not significantly increase the percentage of WT macrophages that took up beads (Figure 6C), bead fluorescence was higher in WT macrophages at all time points, suggesting that PPARγ agonism increased the uptake of targets per macrophage (data not shown).
Pioglitazone enhances efferocytosis and reduces accumulation of apoptotic neutrophils in CGD mice. Mice were treated as described in Figure 4. Efferocytosis by peritoneal macrophages (A) and accumulation of apoptotic neutrophils (identified by nuclear morphology) (B) were determined microscopically. In vivo uptake of carboxylated beads by macrophages was determined by flow cytometry (C) as in Figure 3. Data represent mean ± SE; N = 8 mice per time point; #P ≤ .01 compared with vehicle-treated CGD mice at the respective time points; *P ≤ .01 compared with WT mice treated with vehicle, at the respective time points. αP < .02 between baseline [B] and 6-hour after zymosan for the corresponding genotype and treatment condition, respectively.
Pioglitazone enhances efferocytosis and reduces accumulation of apoptotic neutrophils in CGD mice. Mice were treated as described in Figure 4. Efferocytosis by peritoneal macrophages (A) and accumulation of apoptotic neutrophils (identified by nuclear morphology) (B) were determined microscopically. In vivo uptake of carboxylated beads by macrophages was determined by flow cytometry (C) as in Figure 3. Data represent mean ± SE; N = 8 mice per time point; #P ≤ .01 compared with vehicle-treated CGD mice at the respective time points; *P ≤ .01 compared with WT mice treated with vehicle, at the respective time points. αP < .02 between baseline [B] and 6-hour after zymosan for the corresponding genotype and treatment condition, respectively.
These effects of pioglitazone treatment on efferocytosis were further investigated ex vivo. Baseline differences in efferocytosis between the genotypes were also observed in inflammatory macrophages lavaged from peritonea and cultured overnight (vehicle treated; supplemental Figure 5A). Ex vivo treatment of macrophages of both genotypes with pioglitazone (versus vehicle) resulted in enhanced efferocytosis. The PPARγ antagonist inhibited these responses to pioglitazone, and reduced baseline levels of efferocytosis by vehicle-treated WT macrophages to levels resembling CGD (supplemental Figure 5A). Importantly, these data mirrored PPARγ activity and expression investigated for both genotypes (supplemental Figure 4) and further supported the hypothesis that restoration of macrophage programming restores efferocytosis of CGD macrophages in a PPARγ-dependent manner. Since the proresolution cytokines IL-10 and TGFβ have been shown to be associated with macrophage recognition and clearance of apoptotic cells,15,–17 these cytokines were determined in the culture supernatants. After coculture with apoptotic cells for 1 hour, unengulfed cells were washed out and the macrophages incubated for an additional 18 hours. Associated with their deficient efferocytosis, vehicle-treated CGD inflammatory macrophages produced less IL-10 and TGFβ than WT following coculture with apoptotic cells (supplemental Figure 5). Ex vivo pioglitazone treatment in addition to restoring efferocytosis in CGD macrophages, restored production of these cytokines made in response to apoptotic cells to levels seen in vehicle-treated WT macrophages. Treatment with the PPARγ antagonist reversed these effects of pioglitazone on CGD macrophages and had suppressive effects on WT macrophage cytokine production both at baseline and following pioglitazone treatment (supplemental Figure 5B-C). Collectively, these data suggest that PPARγ activation of CGD macrophages by pioglitazone normalizes their ability to respond appropriately to apoptotic cells with the production of anti-inflammatory cytokines and to clear the apoptotic cells, both key for resolving inflammation.
Pioglitazone enhances resolution of neutrophilia in CGD mice via macrophage programming and efferocytosis even when administered after onset of inflammation
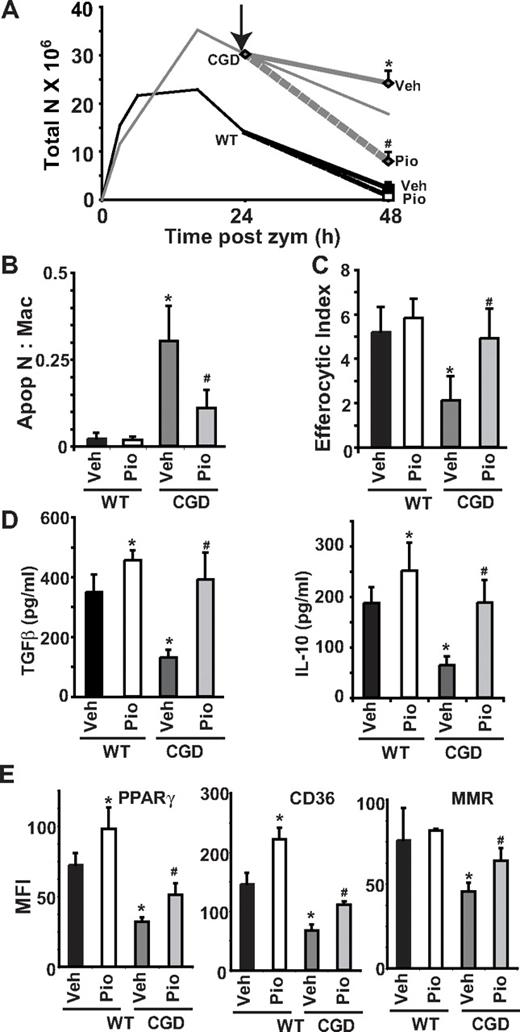
The in vivo experiments above demonstrated that prophylactic PPARγ agonism ameliorated baseline differences in macro-phage programming and cytokines as well as suppressed exaggerated inflammatory responses in CGD animals (Figures 4, 5C, and 6). Thus, it was important to further sort out the effects of PPARγ agonism on the baseline milieu versus its alteration of events in the propagation of inflammation or its resolution in CGD mice. In addition, these experiments would indicate whether PPARγ activation might be beneficial in the treatment of ongoing inflammation. Therefore, we asked whether pioglitazone treatment begun after zymosan instillation would alter the course of subsequent inflammation. Mice were treated with a single oral dose of pioglitazone or vehicle 24 hours after zymosan injection and peritoneal lavage was performed 24 hours later (48 hours after zymosan). As such, pioglitazone treatment was begun well after acute inflammatory mediators had subsided (Figure 2) and after maximal neutrophil numbers had been achieved in both genotypes and coincident with resolving peritonitis in WT. Analysis of peritoneal cells from CGD mice at 48 hours demonstrated that neutrophil numbers were significantly reduced by delayed pioglitazone treatment compared with vehicle (Figure 7A). No effect on neutrophilia of WT mice was observed, though peritonitis was largely resolved in these mice at this point even without treatment. Significantly, the ratio of apoptotic neutrophils to macrophages was lower in CGD peritonitis following pioglitazone treatment (Figure 7B) and was associated with enhanced in vivo efferocytosis by macrophages approximating levels seen in WT (Figure 7C). Paralleling the effect on efferocytosis, the levels of TGFβ as well as IL-10 in peritoneal lavage were increased following pioglitazone in the CGD mice and also WT mice (Figure 7D). In addition, delayed pioglitazone treatment of CGD mice resulted in enhanced PPARγ expression and activity as indicated by an increase in CD36 and MMR staining (Figure 7E), although delayed treatment did not completely restore levels to those seen in WT mice or following prophylactic/concurrent treatment in CGD. These observations during delayed treatment collectively support the hypothesis that PPARγ agonism significantly attenuates neutrophilic inflammation in CGD via normalization of inflammatory macrophage programming and efferocytosis.
Resolution of neutrophilia and macrophage reprogramming are enhanced by pioglitazone even when administered after onset of inflammation in CGD. Twenty-four hours after zymosan injection, mice were treated by oral gavage with a single dose of either vehicle or pioglitazone. At 48 hours after zymosan, peritonea were lavaged, and cells were analyzed as before. The course of neutrophilia is shown in panel A: solid lines represent time course of zymosan-induced peritoneal neutrophilia without treatment derived from data shown in Figure 3A; arrow shows the time at which pioglitazone or vehicle was administered; dashed lines show changes in neutrophilia following treatment. Accumulation of apoptotic neutrophils (B) and efferocytosis by macrophages (C) in peritonea at 48 hours are shown. (D) Cytokines were measured in lavage supernatants by enzyme-linked immunosorbent assay. (E) F4/80 positive macrophages were analyzed for PPARγ, CD36, and MMR by flow cytometric analysis as in Figure 1. Data represent mean ± SE; N = 8 mice per treatment group. #P ≤ .02 compared with vehicle-treated CGD mice, and *P ≤ .01 compared with vehicle-treated WT mice.
Resolution of neutrophilia and macrophage reprogramming are enhanced by pioglitazone even when administered after onset of inflammation in CGD. Twenty-four hours after zymosan injection, mice were treated by oral gavage with a single dose of either vehicle or pioglitazone. At 48 hours after zymosan, peritonea were lavaged, and cells were analyzed as before. The course of neutrophilia is shown in panel A: solid lines represent time course of zymosan-induced peritoneal neutrophilia without treatment derived from data shown in Figure 3A; arrow shows the time at which pioglitazone or vehicle was administered; dashed lines show changes in neutrophilia following treatment. Accumulation of apoptotic neutrophils (B) and efferocytosis by macrophages (C) in peritonea at 48 hours are shown. (D) Cytokines were measured in lavage supernatants by enzyme-linked immunosorbent assay. (E) F4/80 positive macrophages were analyzed for PPARγ, CD36, and MMR by flow cytometric analysis as in Figure 1. Data represent mean ± SE; N = 8 mice per treatment group. #P ≤ .02 compared with vehicle-treated CGD mice, and *P ≤ .01 compared with vehicle-treated WT mice.
Discussion
PPARγ expression is markedly up-regulated in macrophages during acute inflammation,27,31 and its activation plays various roles in resolution including suppression of inflammatory mediator production24,26,34 and altering macrophage phenotype and functions.23,–25 In this model of acute inflammation, increased expression of PPARγ and evidence of its activation (ie, CD36 and MMR) were demonstrated in WT macrophage populations by 24 hours following zymosan and were temporally associated with resolution of neutrophilia. Although CGD macrophages also showed up-regulation of PPARγ expression and activation, significant delay was demonstrated, and peritoneal inflammation was exaggerated and extended. In addition to deficiency of PPARγ expression shown here, insufficient ligands for PPARγ activation are also hypothesized due to the loss of the functioning nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.40 Although physiologically relevant PPARγ ligands remain elusive, H2O2/MPO oxidized fatty acids are among the diverse lipids that bind the large ligand-binding site of PPARγ. These data and noted similarities between inflammation in CGD and PPARγ blockade/knockdown models support the hypothesis that deficiencies in PPARγ signaling contribute to inflammation and autoimmunity of this disorder.27,41
This investigation shows that pioglitazone treatment administered either prophylactically or during zymosan-induced inflammation significantly shortened the duration of neutrophilia in CGD mice. Exaggerated neutrophilic inflammation is prominent in human CGD (eg, characteristic pyogranulomata).1,3,4,42 Tissue neutrophilia is the net result of simultaneous neutrophil recruitment and removal, and data support that both are dysregulated in CGD. Notably, with prophylactic pioglitazone treatment, neither initial mediator production (Figure 5C) nor early neutrophil recruitment (up to 6 hours after zymosan; Figure 5B) were altered in CGD or WT mice. These early events were associated temporally with very low levels of PPARγ expression in F4/80 positive populations, characteristic of recruited blood monocytes from which inflammatory macrophages differentiate.31 These macrophages were also functionally distinct in their impaired efferocytosis (Figures 1 and 3) and, given their low expression of PPARγ, would be predicted to be unresponsive to pioglitazone treatment. Only as macrophage PPARγ protein increased, were subsequent inflammatory events suppressed by pioglitazone treatment. Accordingly, the prolonged elevations of inflammatory mediators (Figure 5C) and consequent neutrophil recruitment (Figure 5A) characteristic of CGD mice were reduced by PPARγ activation.
Although the effect of PPARγ activation may be mediated via its transrepression of proinflammatory mediator transcription,24,26,34 we hypothesize an even more significant indirect effect via enhanced apoptotic cell recognition that then leads to the production of suppressive mediators such as IL-10 and TGFβ (Figure 7D and supplemental Figure 5).17,43,44 Further, we hypothesized that efficient removal of dying neutrophils prevented disintegration and release of phlogistic intracellular constituents that prolong production of inflammatory mediators.7,18 PPARγ agonism normalized efferocytosis in CGD mice (Figures 6A,C and 7C and supplemental Figure 5A); enhanced uptake was demonstrated in vivo for bead mimics and, importantly, for CGD neutrophils that reportedly signal suboptimally for uptake.5,–7 Further insights are provided by the delayed administration model. Here, pioglitazone enhanced resolution of neutrophilia when administered after inflammatory mediators had subsided, and maximal neutrophil accumulation was achieved. These data strongly support the hypothesis that impaired efferocytosis significantly contributes to CGD inflammation. The mechanisms by which PPARγ activation mediate efferocytic capacity are to be further investigated, but likely involve ligand-dependent transactivation. After ligand binding, PPARγ heterodimerizes with the retinoid X receptor or other nuclear receptors (eg, liver X receptor [LXR] or glucocorticoid receptor) and with associated coactivators binds to PPAR-response elements in promoter regions altering transcription.26,27 PPARγ transactivation is known to up-regulate proteins involved in both apoptotic cell recognition (eg, CD36 and MMR) and digestion (eg, ATP-binding cassette transporter 1).24,32,33,45
Administration of PPARγ agonists in vivo significantly ameliorates arthritis, colitis, and atherosclerosis in animal models,27 and these agents are currently in trial in humans with metabolic syndrome, obesity, end-stage renal disease, ulcerative colitis, rheumatoid arthritis, allergic asthma, and chronic obstructive pulmonary disease (many of which are also associated with aberrant macrophage programming and impaired efferocytosis46 ). Based on this preclinical model, PPARγ agonism may be effective in the treatment of CGD inflammation. Notably, in this peritonitis model, pioglitazone was effective even when started after the induction of inflammation, raising the possibility of intermittent, as needed therapy rather than prophylactic. Thiazolidinedione treatment is attractive in that these agents are not globally immunosuppressive like glucocorticoids currently used to suppress CGD inflammation, although to date, their use during active bacterial or fungal infections has not been investigated. Studies to investigate the prophylactic or delayed effect of pioglitazone administration (and after initiating treatment with antimicrobials) in CGD mice are underway. In this regard, it is relevant that PPARγ agonism has been reported to enhance phagocytosis of Candida47 and Fc-mediated phagocytosis by macrophages.48
Gene therapy for restoration of the NADPH oxidase is clearly the ultimate goal in the treatment of CGD, although the precise means of accomplishing this safely and effectively are yet to be established.49 Importantly, transduction of even modest numbers of neutrophils is curative of immunodeficiency and may diminish inflammation as well.50 Whether gene therapy will restore proper CGD macrophage programming is entirely unknown. Nonetheless, under conditions where nontransduced neutrophils are recruited to tissues in great numbers, their delayed apoptosis, and deficient PS exposure and/or oxidative modification may overwhelm CGD macrophages poorly programmed for efferocytosis. The data presented here suggest that restoration of PPARγ signaling and macrophage reprogramming downstream of aberrant CGD neutrophil signaling may be key to resolution of inflammation in CGD. As such, these preclinical data suggest that treatment with PPARγ agonists may be of use until such time as gene therapy becomes available or as an adjunct to that therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Funding sources for this investigation included A1058228, HL34303, HL81151, HL68864, GM61031, the Chronic Granulomatous Disorder Research Trust (United Kingdom), and the Eugene F. and Easton M. Crawford Charitable Lead Unitrust.
National Institutes of Health
Authorship
Contribution: R.F.B. designed and performed the research, analyzed data, and wrote the article; S.C.F. designed the research and helped write the paper; D.W.R. helped design the research and write the paper; R.W.V. helped write the paper; P.M.H. helped write the paper; and D.L.B. helped design the research, analyze data, and write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Donna L. Bratton, Department of Pediatrics, National Jewish Health, Rm A540, 1400 Jackson St, Denver, CO 80206; e-mail address: brattond@njhealth.org.

![Figure 1. Macrophage PPARγ expression and activity are deficient and acquisition delayed in CGD mice during peritonitis. WT and CGD mice were injected intraperitoneally with zymosan and lavage performed at the indicated times. Peritoneal F4/80 monocytes/macrophages were counted (A) and stained for intracellular PPARγ (following permeablilization) (B), surface CD36 (C), and surface macrophage mannose receptor (MMR; D). Mean fluorescence intensity (MFI) is shown (B-D). Data represent mean ± standard error (SE); N = 8 mice per time point. *P < .02 compared with WT mice at the respective time points; #P < .03 compared with baseline [B] values for each genotype, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-02-272005/6/m_zh89991060140001.jpeg?Expires=1766032161&Signature=Pqvi73IbN6e34AEHABhEPJ6GjZrfh~gb-0c079oH1YyVLFZ0esqumsfVNj5ViQ0M3D9HnvEqqp26gk3Z9CSl0u5JWFI~-AeGNkTyGRqqOrIpRFITl34zmXzHRC2MJvQxJfVj6v~kNSVmdXFrRrwiTRMewL2yZWp7dPAXJc8PL1xlTGZ1Nz88BzpHru~wVxZVIGptBxSKizmJQdQLSdMQNGWH0f9mhk3rHOgQvGEVsd3g7FEGTcUoq8eaHkPHF5U4IiQxKJ1mz~jF2CBM-ES9wFURNuiR6s7Q4eLwGcL1-8wb8LK~rIsVoPYelqL60ujk3RjcZWTkyxaOmFpzggnVcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Proinflammatory cytokines are elevated and prolonged during peritonitis in CGD mice. Peritonitis was induced as in Figure 1, and cytokines were measured in peritoneal lavage supernatants at the times indicated. [B] indicates baseline without zymosan. Data represent mean ± SE; N = 6 mice per time point investigated; *P < .03 compared with WT mice at the respective time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-02-272005/6/m_zh89991060140002.jpeg?Expires=1766032161&Signature=xOs7PVKdM0Ap92vdkfaA8QG4VmM2DWYj9kUK35JUtouaMase0JTFysNDOjALJmfzmbtq3U3Nqrxjr1NdrxB3d3B~43OAFe-AcRkaQG7AP3BKTo5fED37PI9OiYhWP9VT65Sl9Tz1yVdfWZIBaODKyUWRnzcU~pOZYOIaok2Gwk-PWDK9Fyb0JZAVl7otTduqPTRMerpcaoeRjXKbkiMYO3aAiVTwlNnH2WYkUnreqK2lFF9oE9vPBE8s5uPcz4lNnOI4rLlIGQ7gUr-~EpjjhlT8jyRqd5aAwgU3LLYbIIeCjcJ~MiflelVBfTvdcngfZBbNAlUyTdXFyzh3IKNEXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Exaggerated and prolonged neutrophilia characterizing zymosan peritonitis in CGD mice is accompanied by impaired in vivo efferocytosis by macrophages. Peritonitis was induced as in Figure 1 and peritoneal cells analyzed at the times indicated (see “Methods”). (A) Total neutrophils [N] were counted. (B) Percent of neutrophils exposing PS (annexin V–positive staining), and (C) absolute numbers of PS exposing neutrophils were determined. (D) Apoptotic neutrophils identified by nuclear morphology and the ratio of apoptotic neutrophils to macrophages were determined microscopically. (E) Efferocytic Index was determined by microscopic examination of macrophages lavaged from the inflamed peritonea. (F) Carboxylated beads were injected into the peritonea to measure in vivo efferocytic capability, and 1 hour later, the percentage of lavaged peritoneal macrophages positive for beads was determined by flow cytometry. [B] indicates baseline without zymosan. Data represent mean ± SE; N = 8 mice per time point. *P < .02 compared with WT mice at the respective time points. #P < .02 compared with WT mice at baseline.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-02-272005/6/m_zh89991060140003.jpeg?Expires=1766032162&Signature=Oa2iD8go44HI7BPaeui4Ye1DLiZVS13HMg8P1v4WaCRxa1R-VLJSw6UK5WmNYhhSoMvo398S46RoOXFXSs2itMoYuPs4XSgqT1P3crk7CUnzuGU5MhnRIxWnr1EeMbHv4XcNOIDI50Uc7ZsyeZaGb-5l7InTAdxiH9T4r-FHXsjcrwN-A8UH0TViRGwaOBKUb3BLkyOfcs6muLGhnnTx8~plsQByyVB-wV3bRIYfrwOgtPEUlor2wUlNYIh2LFZHS8VYYYUuYPlHui~se243sQrAHnk3n07g4x4sX5dKSPQWjVCs1NP093skAUElWCoNofIwy-z7GPg4PTMJuWbF0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Pioglitazone treatment enhances macrophage PPARγ expression and activity in CGD mice. Mice were gavaged with pioglitazone or vehicle for 2 days before zymosan injection and every 24 hours thereafter. F4/80-positive macrophages from lavage were analyzed as in Figure 1 for intracellular PPARγ (A), and surface CD36 (B), and MMR (C) expressed as MFI. Data represent mean ± SE; N = 8 mice per time point; #P ≤ .01 compared with vehicle-treated CGD mice at the respective time points; α,*P ≤ .01 compared with WT mice treated with vehicle, at the respective time points. [B] indicates baseline after 2 days of treatment without zymosan. Symbols for significant changes in values between baseline [B] and the early time points following zymosan for either vehicle or pioglitazone-treated mice of each genotype were as shown in Figure 1, but omitted here for simplicity.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-02-272005/6/m_zh89991060140004.jpeg?Expires=1766032162&Signature=ZLNoA1jSzBTudElZT4oMtnGnxqmEU3D~Cae4K~DOC6sLY~ynakyy2IqISM0loz8QOlxmi3HznZPy7Y4mG7CwEcjwqiPbjo-cmemZL8jSAdDxPPcmetoAWBFzmgfi0z3EAieD1A9I0M74sEi9gBU3UkTdbsP-fRX2Bk21UcA~fvLDnRrbnTeNsQxTUxhh1HF-s6n8yB-uQRftAZiON8tODkkwaIc98hw9jmamNi3wJf~afMakEAki~0shEhbvo-g31bvTTpSVM0Tfev~X~qQsN41MBHL3qDqnthXES3Kp8VJ8RC8aNI-N7dRAh1KaiLqKaisvwpWB7lHPv~GbaNCq1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Pioglitazone enhances efferocytosis and reduces accumulation of apoptotic neutrophils in CGD mice. Mice were treated as described in Figure 4. Efferocytosis by peritoneal macrophages (A) and accumulation of apoptotic neutrophils (identified by nuclear morphology) (B) were determined microscopically. In vivo uptake of carboxylated beads by macrophages was determined by flow cytometry (C) as in Figure 3. Data represent mean ± SE; N = 8 mice per time point; #P ≤ .01 compared with vehicle-treated CGD mice at the respective time points; *P ≤ .01 compared with WT mice treated with vehicle, at the respective time points. αP < .02 between baseline [B] and 6-hour after zymosan for the corresponding genotype and treatment condition, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-02-272005/6/m_zh89991060140006.jpeg?Expires=1766032162&Signature=HOc-RJ7DcvYUqYER0ZaJxMaSV3pDLgd3V-~M16ROQGb2A7eJpwl~J6edZkQSLCH6qO-NxEDAAdE5kL~STfrtY42em4qhGYxUX-XNUugsLHNXIQupAgzVZeC7ekKJM0EeNIyqzc~u1QkV3QYFQFE1j9zidKNBF1PBBXYGq5qOxU2zn7RWSjDyVvtianq66ubJGVtUNSU1EBZkcqFF1KmLnT8CWry2QItvHIAPLuxTilEqI2pQglVtrmxZ7v21bPMvOVKgTXHg20Gct8Qs2G1grzrkoG3jsTY2DioxoxLXm2gvs6I7HZKH3~Y7Wr8nvWP5Urzaebd8P56KT5FVZLsWPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal