Abstract
Churg-Strauss syndrome (CSS) is characterized by systemic vasculitis and blood and tissue eosinophilia. Blood eosinophilia correlates with disease activity, and activated T cells from CSS patients are predominantly T helper 2 (Th2). Interleukin (IL)-25 has been shown to link innate and adaptive immunity by enhancing Th2 cytokine production. We sought to determine the involvement of IL-25 and its receptor IL-17RB in the pathogenesis of CSS. We found increased levels of IL-25 in the serum of active CSS patients (952 ± 697 vs 75 ± 49 pg/mL in inactive patients and 47 ± 6 pg/mL in healthy donors). IL-25 was correlated with disease activity and eosinophil level. Eosinophils were the main source of IL-25, whereas activated CD4+ memory T cells were the IL-17RB–expressing cells in CSS. IL-25 enhanced the production of IL-4, IL-5, and IL-13 by activated peripheral blood mononuclear cells. IL-25 and IL-17RB were observed within the vasculitic lesions of patients with CSS, and IL-17RB colocalized with T cells. Increased expression of IL-17RB, tumor necrosis factor receptor–associated factor 6, and JunB in vasculitic lesions of CSS underscored the IL-25–mediated activation, whereas up-regulation of GATA3 and IL-10 supported Th2 differentiation. Our findings suggest that eosinophils, through the production of IL-25, exert a critical role in promoting Th2 responses in target tissues of CSS.
Introduction
Churg-Strauss syndrome (CSS) is a rare disorder characterized by systemic vasculitis, blood and tissue eosinophilia, and a long-term history of asthma.1–4 Systemic vasculitis mainly affects the lower respiratory tract, kidneys, skin, and peripheral nervous system.2 Infiltrating eosinophils are frequently found in vasculitic lesions,5 and peripheral blood eosinophils correlate with the course of the disease, suggesting that eosinophils play a role in the pathogenesis of CSS.5,6 However, T cells have been shown to be involved in CSS. Cellular immunity has been inferred from various findings, including the presence of memory T cells in vasculitic lesions,7 increased T-cell activation as reflected by cellular and soluble T-cell activation markers,8 and responsiveness of disease activity to treatment with suppressors of cellular immunity.9 Activated T cells from CSS patients are predominantly Th2, with increased production of interleukin-4 (IL-4), IL-5 and IL-13.10,11 Th2-mediated immune response in CSS might be the consequence of an abnormal eosinophil response, causing T-cell activation and Th2 cytokine production. Alternatively, T-cell dysregulation could cause eosinophilia and eosinophil activation.
IL-25 (also known as IL-17E), produced by epithelial cells and other innate cells such as eosinophils, basophils, and mast cells, has been shown to license innate and adaptive immunity by enhancing Th2 cytokine production.12–14 Because treatment with exogenous IL-25 elicited a granulocyte infiltration into the lungs, increased mucus production, and airway constriction, it was suggested that IL-25 played a role in the promotion of Th2 cytokine-mediated allergic airway inflammation.14 Elevated expression of IL-25 and IL-17RB (also known as IL-25R) was observed in tissues from patients with chronic asthma and atopic dermatitis.15 These findings suggest that IL-25 plays important role in evoking Th2 cell–mediated inflammation that features the infiltration of eosinophils and Th2 memory cells, and it may increase allergic inflammation by enhancing the maintenance and functions of adaptive Th2 memory cells.15
In the present study, we sought to determine the involvement of IL-25 and its receptor IL-17RB in the pathogenesis of CSS as a possible link between eosinophils and adaptive immunity. We found increased levels of IL-25 in the serum of a patient with active CSS. IL-25 correlates with disease activity and eosinophil level. Eosinophils are the main source of IL-25, whereas activated CD4+ memory T cells are the IL-17RB–expressing cells in CSS. IL-25 and IL-17RB have been observed within the vasculitic lesions of patients with CSS and IL-17RB is colocalized with T cells within the perivascular infiltrate. Increased expression of IL-17RB, TRAF6, and JunB in vasculitic lesions of CSS underscores the IL-25–mediated activation, whereas up-regulation of GATA3 and IL-10 supports Th2 differentiation. Taken together, our findings suggest that eosinophils, through the production of IL-25, may have a critical role in promoting Th2 responses in target tissues of CSS.
Methods
Patients
The study population consisted of 15 CSS patients (sex ratio men:women, 7:8; mean age, 60 years; range, 18-76 years; perinuclear anti–neutrophil cytoplasmic antibody anti-myeloperoxidase (MPO) positive in 9/15; median eosinophil count, 2395/mm3), with serum samples available in the active phase (n = 6) and/or inactive phase (n = 13), 8 atopic patients, 15 patients with undefined hypereosinophilic syndrome (HES), and 20 healthy blood donors. Undefined HES were characterized as (1) blood eosinophilia of greater than 1500/mm3 on at least 2 occasions or evidence of prominent tissue eosinophilia associated with symptoms and marked blood eosinophilia; (2) exclusion of secondary causes of eosinophilia, such as parasitic or viral infections, allergic diseases, drug-induced or chemical-induced eosinophilia, hypoadrenalism, and neoplasms; and (3) without features of myeloproliferative or lymphocytic forms, as previously described.16 The Birmingham Vasculitis Activity Score was used to assess the disease activity of CSS.17 Active CSS patients were untreated. The main patient characteristics are summarized in Table 1.
Main characteristics of active and inactive CSS, atopic, and HES patients
| . | Active CSS (n = 6) . | Inactive CSS (n = 13) . | Atopic patients (n = 8) . | Undefined HES patients (n = 15) . |
|---|---|---|---|---|
| Demography | ||||
| Median age, years (range) | 63 (54-74) | 61 (18-76) | 44 (31-63) | 46 (23-79) |
| Female sex, n (%) | 1 (17) | 10 (77) | 5 (63) | 6 (40) |
| Clinical features | ||||
| General signs, n (%) | 6 (100) | 0 (0) | 0 (0) | 2 (13) |
| Asthma, n (%) | 6 (100) | 0 (0) | 5 (74) | 1 (7) |
| Arthralgias, n (%) | 1 (17) | 0 (0) | 0 (0) | 1 (7) |
| Myalgias, n (%) | 2 (33) | 0 (0) | 0 (0) | 0 (0) |
| Rhinitis/Sinusitis, n (%) | 4 (67) | 0 (0) | 3 (26) | 2 (13) |
| Active neuropathy, n (%) | 6 (100) | 0 (0) | 0 (0) | 1 (7) |
| Pulmonary infiltrates, n (%) | 1 (17 | 0 (0) | 0 (0) | 2 (15) |
| Cardiopathy, n (%) | 2 (33) | 0 (0) | 0 (0) | 0 (0) |
| Cutaneous, n (%) | 0 (0) | 0 (0) | 0 (0) | 5 (33) |
| Gastrointestinal, n (%) | 1 (17) | 0 (0) | 0 (0) | 4 (27) |
| BVAS, median (range) | 17 (14-27) | 0 (0) | NR | NR |
| Laboratory features | ||||
| Eosinophil count, per mm3, median | 9250 | 175 | 130 | 2550 |
| Range | (530-19 350) | (0-790) | (33-908) | (1500-13 800) |
| pANCA*, n (%) | 6 (100) | 8 (62) | NR | NR |
| Treatments | ||||
| Prednisone, n (%) | 0 (0) | 13 (100) | 0 (0) | 0 (0) |
| Prednisone dosage, median (range), mg/d | NR | 20 (5-50) | NR | NR |
| Immunosuppressant agents, n (%) | 0 (0) | 5 (38) | NR | 0 (0) |
| . | Active CSS (n = 6) . | Inactive CSS (n = 13) . | Atopic patients (n = 8) . | Undefined HES patients (n = 15) . |
|---|---|---|---|---|
| Demography | ||||
| Median age, years (range) | 63 (54-74) | 61 (18-76) | 44 (31-63) | 46 (23-79) |
| Female sex, n (%) | 1 (17) | 10 (77) | 5 (63) | 6 (40) |
| Clinical features | ||||
| General signs, n (%) | 6 (100) | 0 (0) | 0 (0) | 2 (13) |
| Asthma, n (%) | 6 (100) | 0 (0) | 5 (74) | 1 (7) |
| Arthralgias, n (%) | 1 (17) | 0 (0) | 0 (0) | 1 (7) |
| Myalgias, n (%) | 2 (33) | 0 (0) | 0 (0) | 0 (0) |
| Rhinitis/Sinusitis, n (%) | 4 (67) | 0 (0) | 3 (26) | 2 (13) |
| Active neuropathy, n (%) | 6 (100) | 0 (0) | 0 (0) | 1 (7) |
| Pulmonary infiltrates, n (%) | 1 (17 | 0 (0) | 0 (0) | 2 (15) |
| Cardiopathy, n (%) | 2 (33) | 0 (0) | 0 (0) | 0 (0) |
| Cutaneous, n (%) | 0 (0) | 0 (0) | 0 (0) | 5 (33) |
| Gastrointestinal, n (%) | 1 (17) | 0 (0) | 0 (0) | 4 (27) |
| BVAS, median (range) | 17 (14-27) | 0 (0) | NR | NR |
| Laboratory features | ||||
| Eosinophil count, per mm3, median | 9250 | 175 | 130 | 2550 |
| Range | (530-19 350) | (0-790) | (33-908) | (1500-13 800) |
| pANCA*, n (%) | 6 (100) | 8 (62) | NR | NR |
| Treatments | ||||
| Prednisone, n (%) | 0 (0) | 13 (100) | 0 (0) | 0 (0) |
| Prednisone dosage, median (range), mg/d | NR | 20 (5-50) | NR | NR |
| Immunosuppressant agents, n (%) | 0 (0) | 5 (38) | NR | 0 (0) |
Perinuclear anti-neutrophil cytoplasmic antibody with anti-myeloperoxydase specificity.
General signs are defined as weight loss > 10%, fever and/or night sweats. NR indicates not relevant.
Immunohistochemical analysis and gene quantification with mRNA on peripheral nerve biopsy samples included active CSS patients, patients with microscopic polyangiitis (MPA; disease controls), and controls with noninflammatory axonopathy. All patients had severe axonal degeneration and biopsy evidence of an inflammatory process involving nerves. Necrotizing arteritis was present in all vasculitis patients.
The study was approved by the institutional review board of Institut National de la Santé et de la Recherche Médicale, and written informed consent was obtained from all patients. The study conformed to the ethical guidelines of the Declaration of Helsinki (1975). The type of vasculitis was defined according to the Chapel Hill criteria.18
Immunoassay for quantitative measurement of serum human IL-25
Quantitative measurement of serum human IL-25 was performed with Human IL-17E ELISA Development Kit (Peprotech), a quantitative sandwich enzyme immunoassay using a purified rabbit antibody against human IL-25 precoated onto an enzyme-linked immunosorbent assay (ELISA) plate. Human recombinant IL-25 protein at serial concentrations and 2-fold diluted serum samples were incubated into wells. After washing, a biotinylated purified rabbit anti-human IL-25 antibody, then an avidin-horseradish peroxidase conjugate were added to the wells. After further washings, 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) liquid substrate (Sigma-Aldrich) was added and color developed in proportion to the amounts of bound IL-25. Development kit standards were used for construction of standard curves. The IL-25 ELISA had a threshold sensitivity of 46 pg/mL.
Analysis of cell-surface markers and cytokine production
Whole blood was collected in heparinized tubes and analyzed within 6 hours following collection. Lysis solution was added and cells were resuspended in phosphate-buffered saline and stained with anti–cluster of differentiation 15 (CD15)-fluorescein isothiocyanate, anti-CD14-PE, anti-CD45-ECD and anti-CD16-PC7 (Beckman Coulter). Cells were fixed and permeabilized using Cytofix/Cytoperm solution (BD Biosciences), and stained with anti–IL-25-APC (R&D Systems). Cells were washed, resuspended in phosphate-buffered saline/1% paraformaldehyde solution, and then analyzed by flow cytometry. Neutrophils were identified in the granulocytes gate using side scatter and forward scatter as CD15+ CD16+ cells, and eosinophils as CD15+ CD16− cells. Monocytes were identified in the monocytes gate as CD14+ cells.
Peripheral blood mononuclear cells (PBMCs) were purified from whole blood using Ficoll-hypaque density gradient solution. For the detection of IL-17RB surface expression, PBMCs were stimulated with anti-CD3/anti-CD28 dynabeads (Invitrogen) whether or not in the presence of IL-25 (100 ng/mL, R&D Systems) for 48 hours, and then stained with the following antibodies: anti–CD45RA-FITC, anti–CD3-ECD, anti–CD8-APC, anti–CD4-PC7 (Beckman Coulter), and anti–IL-17RB-PE (R&D Systems). Culture supernatants were collected assessed by Luminex (Invitrogen) for IL-4, IL-5, IL-13, and IFN-γ.
Immunohistochemical analysis
Patients underwent full-thickness open biopsy of the superficial peroneal nerve and peroneus brevis muscle in the most affected limb. Specimens were immediately snap-frozen in liquid nitrogen and stored at −80°C. Part of the specimen was paraffin-embedded, and both transversal and longitudinal sections were stained with hematoxylin and eosin (H&E), periodic acid–Schiff, or Congo red. Only the superficial peroneal nerve samples were analyzed for mRNA quantification. For immunohistochemistry studies, all consecutive serial sections with inflammatory vascular lesions were analyzed.
Detection of IL-25+ and IL-17RB+ cells was performed on fixed, paraffin-embedded samples from 3 CSS patients, 3 MPA patients, and 2 controls with noninflammatory axonopathy. Dewaxed slides were submitted to antigen retrieval by heating in citrate buffer pH 6.0. Before incubation with primary antibodies, the slides were treated with avidin/biotin blocker (Dako) and Fc receptor was blocked with 2% bovine serum albumin. Slides were incubated for 1 hour with polyclonal rabbit anti–human IL-17RB (working dilution 1:40; Sigma-Aldrich) and monoclonal mouse anti–human IL-25 (clone 68C1039.2; LifeSpan Biosciences). Antibody binding was visualized with diaminobenzidine tetrahydrochloride (Dako). For negative control, the primary antibody was omitted.
Detection of CD3+ IL-17RB+ cells was performed on a fixed, frozen sample from one CSS patient. Tissue was cut into 5-μm sections, dried at room temperature for 1 hour, and fixed in acetone at −20°C. Slides were incubated overnight at 4°C with polyclonal rabbit anti–human IL-17RB (working dilution 1:20) and monoclonal FITC-conjugated mouse anti-human CD3 antibody (Miltenyi Biotec). Isotype-matched antibodies (Dako) were used for control staining. Slides were then incubated for 1 hour at room temperature with cyanine 3–conjugated goat anti–rabbit antibody (working dilution 1:200, Invitrogen), mounted in Fluoromount (Amersham), and evaluated under fluorescence microscopy. For negative control, the primary antibody was omitted.
Gene-expression quantification at the mRNA level in peripheral nerve samples
mRNA expression quantification was performed on frozen samples from CSS patients and controls with noninflammatory axonopathy. Twenty-one genes involved in IL-25/IL-17RB pathway, Th2 lineage and main eosinophil and lymphocytes chemokines were analyzed (Table 2). The theoretical and practical aspects of real-time quantitative reverse transcription polymerase chain reaction (PCR), using the ABI Prism 7900 Sequence Detection System (PerkinElmer Applied Biosystems) have been described in detail elsewhere.19,20 Briefly, total RNA was reverse-transcribed before real-time PCR amplification. Quantitative values were obtained from the threshold cycle (Ct) number at which the increase in the signal associated with exponential growth of PCR products began to be detected using analysis software, according to the recommendations of the manufacturer (PerkinElmer Applied Biosystems). The precise amount of total RNA added to each reaction mixture (based on optical density) and its quality (ie, lack of extensive degradation) were both difficult to assess. We therefore also quantified transcripts of the Thymine Adenine Thymine Adenine Box Binding Protein (TBP) gene, which encodes the TATA box-binding protein (a component of the DNA binding protein complex transcription factor IID), as the endogenous RNA control, and each sample was normalized on the basis of its TBP content. Results, expressed as fold differences in target gene expression relative to the TBP gene (termed Ntarget), were determined using the formula Ntarget = 2ΔCtsample. The ΔCt value of the sample was determined by subtracting the Ct value of the target gene from the Ct value of the TBP gene. The Ntarget values of the samples were subsequently normalized as the median value of the noninflammatory control neuropathies; the Ntarget values for the noninflammatory controls were set at 1. Genes were considered markedly overexpressed when they were ≥ 5-fold the value of noninflammatory idiopathic controls. Primers for TBP and the 21 target genes were chosen using Oligo, version 5.0 (National Biosciences). To avoid amplification of contaminating genomic DNA, 1 of the 2 primers was placed at the junction between 2 exons. The thermal cycling conditions comprised an initial denaturation step at 95°C for 10 minutes, followed by 50 cycles at 95°C for 15 seconds and 65°C for 1 minute.
The 21 genes selected for the gene-expression study of Churg-Strauss syndrome–associated vasculitic lesions
| Gene . | Alternate name . | Gene definition . |
|---|---|---|
| IL-25/IL-17RB pathway (n = 6) | ||
| IL-25 | IL-17E, IL-25, IL17E | interleukin 25 |
| IL-17RB | CRL4, EVI27, IL17BR, IL17RH1, MGC5245 | interleukin 17 receptor B |
| TRAF6 | MGC:3310, RNF85 | TNF receptor-associated factor 6 |
| SOCS3 | ATOD4, CIS3, Cish3, MGC71791, SOCS-3, SSI-3, SSI3 | suppressor of cytokine signaling 3 |
| MAPK14 | RP1–179N16.5, CSBP1, CSBP2, CSPB1, EXIP, Mxi2, PRKM14, PRKM15, RK, SAPK2A, p38, p38ALPHA | mitogen-activated protein kinase 14 |
| JunB | AP-1 | jun B proto-oncogene |
| Th2 lineage (n = 5) | ||
| GATA-3 | HDR, MGC2346, MGC5199, MGC5445 | GATA binding protein 3 |
| IL-4 | BCGF-1, BCGF1, BSF1 | interleukin 4 |
| IL-5 | EDF, IL-5, TRF | interleukin 5 |
| IL-10 | CSIF, IL-10, IL10A, MGC126450, MGC126451, TGIF | interleukin 10 |
| IL-13 | ALRH, BHR1, IL-13, MGC116786, MGC116788 | interleukin 13 |
| Eosinophil and lymphocytes chemokines (n = 10) | ||
| CXCL12 | PBSF, SCYB12, SDF-1a, SDF-1b, SDF1, SDF1A, SDF1B, TLSF-a, TLSF-b, TPAR1 | chemokine (C-X-C motif) ligand 12 |
| CXCR4 | CD184, D2S201E, FB22, HM89, HSY3RR, LAP3, LCR1, LESTR, NPY3R, NPYR, NPYRL, NPYY3R, WHIM | chemokine (C-X-C motif) receptor 4 |
| CCL8 | HC14, MCP-2, MCP2, SCYA10, SCYA8 | chemokine (C-C motif) ligand 8 |
| CCR2 | CC-CKR-2, CCR2A, CCR2B, CD192, CKR2, CKR2A, CKR2B, CMKBR2, FLJ78302, MCP-1-R | chemokine (C-C motif) receptor 2 |
| IL-8 | CXCL8, GCP-1, GCP1, LECT, LUCT, LYNAP, MDNCF, MONAP, NAF, NAP-1 | interleukin 8 |
| CXCR2 | CD182, CDw128b, CMKAR2, IL8R2, IL8RA, IL8RB | chemokine (C-X-C motif) receptor 2 |
| CCL4 | ACT2, AT744.1, G-26, LAG1, MIP-1-beta, MIP1B, MIP1B1, SCYA2, SCYA4 | chemokine (C-C motif) ligand 4 |
| CCR5 | CC-CKR-5, CCCKR5, CD195, CKR-5, CKR5, CMKBR5, FLJ78003, IDDM22 | chemokine (C-C motif) receptor 5 |
| CCL2 | GDCF-2, HC11, HSMCR30, MCAF, MCP-1, MCP1 | chemokine (C-C motif) ligand 2 |
| CCR3 | CC-CKR-3, CD193, CKR3, CMKBR3, MGC102841 | chemokine (C-C motif) receptor 3 |
| Gene . | Alternate name . | Gene definition . |
|---|---|---|
| IL-25/IL-17RB pathway (n = 6) | ||
| IL-25 | IL-17E, IL-25, IL17E | interleukin 25 |
| IL-17RB | CRL4, EVI27, IL17BR, IL17RH1, MGC5245 | interleukin 17 receptor B |
| TRAF6 | MGC:3310, RNF85 | TNF receptor-associated factor 6 |
| SOCS3 | ATOD4, CIS3, Cish3, MGC71791, SOCS-3, SSI-3, SSI3 | suppressor of cytokine signaling 3 |
| MAPK14 | RP1–179N16.5, CSBP1, CSBP2, CSPB1, EXIP, Mxi2, PRKM14, PRKM15, RK, SAPK2A, p38, p38ALPHA | mitogen-activated protein kinase 14 |
| JunB | AP-1 | jun B proto-oncogene |
| Th2 lineage (n = 5) | ||
| GATA-3 | HDR, MGC2346, MGC5199, MGC5445 | GATA binding protein 3 |
| IL-4 | BCGF-1, BCGF1, BSF1 | interleukin 4 |
| IL-5 | EDF, IL-5, TRF | interleukin 5 |
| IL-10 | CSIF, IL-10, IL10A, MGC126450, MGC126451, TGIF | interleukin 10 |
| IL-13 | ALRH, BHR1, IL-13, MGC116786, MGC116788 | interleukin 13 |
| Eosinophil and lymphocytes chemokines (n = 10) | ||
| CXCL12 | PBSF, SCYB12, SDF-1a, SDF-1b, SDF1, SDF1A, SDF1B, TLSF-a, TLSF-b, TPAR1 | chemokine (C-X-C motif) ligand 12 |
| CXCR4 | CD184, D2S201E, FB22, HM89, HSY3RR, LAP3, LCR1, LESTR, NPY3R, NPYR, NPYRL, NPYY3R, WHIM | chemokine (C-X-C motif) receptor 4 |
| CCL8 | HC14, MCP-2, MCP2, SCYA10, SCYA8 | chemokine (C-C motif) ligand 8 |
| CCR2 | CC-CKR-2, CCR2A, CCR2B, CD192, CKR2, CKR2A, CKR2B, CMKBR2, FLJ78302, MCP-1-R | chemokine (C-C motif) receptor 2 |
| IL-8 | CXCL8, GCP-1, GCP1, LECT, LUCT, LYNAP, MDNCF, MONAP, NAF, NAP-1 | interleukin 8 |
| CXCR2 | CD182, CDw128b, CMKAR2, IL8R2, IL8RA, IL8RB | chemokine (C-X-C motif) receptor 2 |
| CCL4 | ACT2, AT744.1, G-26, LAG1, MIP-1-beta, MIP1B, MIP1B1, SCYA2, SCYA4 | chemokine (C-C motif) ligand 4 |
| CCR5 | CC-CKR-5, CCCKR5, CD195, CKR-5, CKR5, CMKBR5, FLJ78003, IDDM22 | chemokine (C-C motif) receptor 5 |
| CCL2 | GDCF-2, HC11, HSMCR30, MCAF, MCP-1, MCP1 | chemokine (C-C motif) ligand 2 |
| CCR3 | CC-CKR-3, CD193, CKR3, CMKBR3, MGC102841 | chemokine (C-C motif) receptor 3 |
Statistical analysis
Data are presented as median (interquartile ranges [IQRs], 1 and 3) for continuous variables and percentage for qualitative variables. The Fisher exact test was used to compare qualitative variables, and the nonparametric Mann-Whitney U test compared continuous variables. Paired continuous variables were compared using the Wilcoxon test. P < .05 was considered significant. Statistical analyses were performed using GraphPad Prism version 4.0 and Instat version 3.0 for Windows.
Results
Increased level of serum human IL-25 in CSS patients
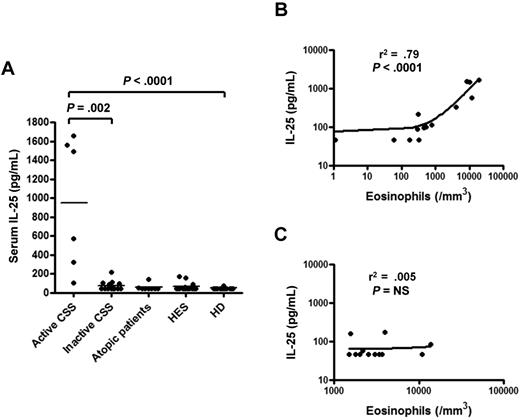
Serum levels of human IL-25 were measured in active CSS patients, in atopic patients, in patients with undefined HES and in healthy donors. Serum IL-25 was significantly higher in active CSS patients (1033 [386-1544] pg/mL) compared with atopic patients (< 46 [< 46-47] pg/mL), patients with undefined HES (< 46 [< 46-49] pg/mL) and healthy donors (< 46 [< 46- < 46] pg/mL; P < .0001) (Figure 1A). In addition, active CSS patients had higher serum levels of IL-25 compared with inactive CSS patients [1033 (386-1544) vs. 46 (46-95) pg/mL, respectively; P = .002] (Figure 1A). Last, serum IL-25 was strongly correlated with the level of eosinophilia in CSS patients (r2 = 0.79, P < .0001) (Figure 1B). In contrast, IL-25 and eosinophil count were not correlated in undefined HES patients (r2 = 0.005, P = not significant [NS]) (Figure 1C).
IL-25 is increased in CSS patients and correlates with disease activity and eosinophil count. (A) Quantitative measurement of IL-25 in the serum of active CSS, inactive CSS, atopic, and HES patients and healthy donors. (B) Correlation between serum IL-25 level and eosinophil count in CSS patients. (C) Correlation between serum IL-25 and eosinophil count in undefined HES patients.
IL-25 is increased in CSS patients and correlates with disease activity and eosinophil count. (A) Quantitative measurement of IL-25 in the serum of active CSS, inactive CSS, atopic, and HES patients and healthy donors. (B) Correlation between serum IL-25 level and eosinophil count in CSS patients. (C) Correlation between serum IL-25 and eosinophil count in undefined HES patients.
Eosinophils are the IL-25–producing cells in the blood
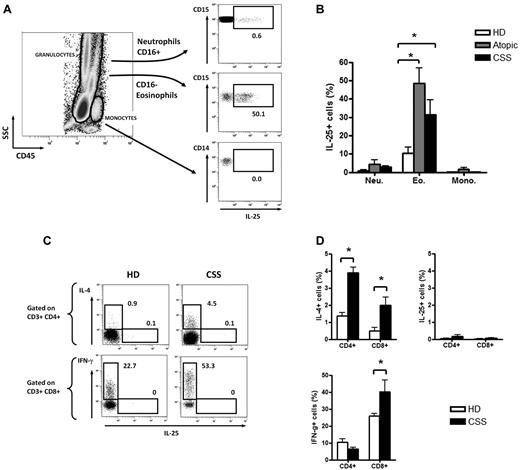
Flow cytometric analysis of whole blood revealed that eosinophils were the main IL-25–producing cells at steady state in CSS patients (IL-25+ cells among eosinophils 35.4% [31.9%-42.8%]; P < .05) but also in atopic patients (45.3% [40.3%-54.9%]; P < .05) compared with healthy donors (11.6% [6.3%-15.6%]), whereas neutrophils and monocytes did not produce significant levels of IL-25 (Figure 2A-B).
Eosinophils are the main IL-25–producing cells in the blood. (A-B) Flow cytometric analysis of intracellular production of IL-25 by eosinophils, neutrophils, and monocytes in an active CSS patient (A) and in all tested patients (B). (C-D) Flow cytometric analysis of intracellular production of IL-25, IL-4, and IFN-γ by T cells in an active CSS patient and a healthy donor (C) and in all tested patients (D). * P < .05; ** P< .01; *** P < .001. Histograms in each panel are representative of 3 to 5 patients in at least 3 independent experiments.
Eosinophils are the main IL-25–producing cells in the blood. (A-B) Flow cytometric analysis of intracellular production of IL-25 by eosinophils, neutrophils, and monocytes in an active CSS patient (A) and in all tested patients (B). (C-D) Flow cytometric analysis of intracellular production of IL-25, IL-4, and IFN-γ by T cells in an active CSS patient and a healthy donor (C) and in all tested patients (D). * P < .05; ** P< .01; *** P < .001. Histograms in each panel are representative of 3 to 5 patients in at least 3 independent experiments.
No intracellular expression of IL-25 was found on CD4+ and CD8+ T cells after stimulation with PMA and ionomycin for 6 hours. T-cell polarization was mainly Th2 in CSS patients. CD4+ T cells from CSS patients showed higher IL-4 production than healthy donors [3.7% (3.6%-4.1%) vs. 1.4% (1.2%-1.6%); P < .05], whereas CD8+ T cells from CSS patients showed higher IFN-γ production (37.3 [33.8-45.3] vs 25.9 [24.3-27.6; P < .05]) (Figure 2C-D).
Activated CD4+ memory T cells from CSS patients expressed IL-17RB
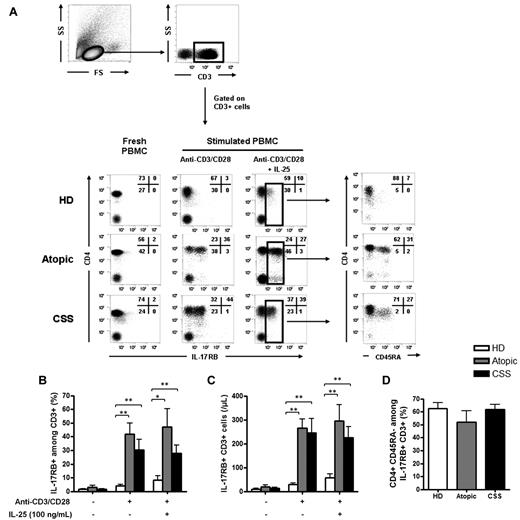
For IL-17RB expression, no significant expression was found on fresh and unstimulated T cells, but significant expression was observed after TCR stimulation with anti-CD3/anti-CD28 in CSS patients (IL-17RB+ among CD3+ T cells 35.2% [19.0%-39.1%]; P < .01) and atopic patients (49.7% [37.9%-49.8%]; P < .01), compared with healthy donors (3.5% [2.3%-5.6%]) (Figure 3A-B). IL-25 slightly increased the expression of IL-17RB on T cells in healthy donors (5.6% [3.1%-9.8%]) but not in CSS (33.7% [18.1%-38.2%]) and atopic patients (45.2% [35.4%-58%]) (Figure 3A-B). The absolute number of IL-17RB+ CD3+ T cells was also significantly higher in CSS and atopic patients than in healthy donors after TCR stimulation with anti-CD3/anti-CD28, with or without IL-25 (Figure 3C). After gating on CD3+ IL-17RB+ cells, 59.2% (54.5%-66.3%) of the IL-17RB+ cells were CD4+ CD45RA− in CSS patients, 55.7% (45.8%-60.3%) in atopic patients and 63.5% (57.1%-70.7%) in healthy donors (Figure 3D).
Activated CD4+ memory T cells express IL-17 RB. (A) Expression of IL-17RB by CD3+ T cells on resting cells and after a 48-hour stimulation with anti-CD3/anti-CD28, with or without IL-25, in an active CSS patient, an atopic patient, and a healthy donor. After gating on IL-17RB+ CD3+ cells, analysis of CD4 and CD45RA expression revealed that IL-17RB+ cells were mainly CD4+ CD45RA− memory T cells in an active CSS patient, an atopic patient, and a healthy donor. (B-C) Expression of IL-17RB by CD3+ T cells in all tested patients in proportion (B) and in absolute number (C). (D) After gating on IL-17RB+ CD3+ T cells, analysis of CD4 and CD45RA expression showed that IL-17RB+ cells were mainly CD4+ CD45RA− memory T cells in CSS patients, atopic patients, and healthy donors. * P < .05; ** P < .01; *** P < .001. Histograms in each panel are representative of 4 to 8 patients in at least 3 independent experiments.
Activated CD4+ memory T cells express IL-17 RB. (A) Expression of IL-17RB by CD3+ T cells on resting cells and after a 48-hour stimulation with anti-CD3/anti-CD28, with or without IL-25, in an active CSS patient, an atopic patient, and a healthy donor. After gating on IL-17RB+ CD3+ cells, analysis of CD4 and CD45RA expression revealed that IL-17RB+ cells were mainly CD4+ CD45RA− memory T cells in an active CSS patient, an atopic patient, and a healthy donor. (B-C) Expression of IL-17RB by CD3+ T cells in all tested patients in proportion (B) and in absolute number (C). (D) After gating on IL-17RB+ CD3+ T cells, analysis of CD4 and CD45RA expression showed that IL-17RB+ cells were mainly CD4+ CD45RA− memory T cells in CSS patients, atopic patients, and healthy donors. * P < .05; ** P < .01; *** P < .001. Histograms in each panel are representative of 4 to 8 patients in at least 3 independent experiments.
IL-25 enhances Th2 cytokine production by PBMCs from CSS patients
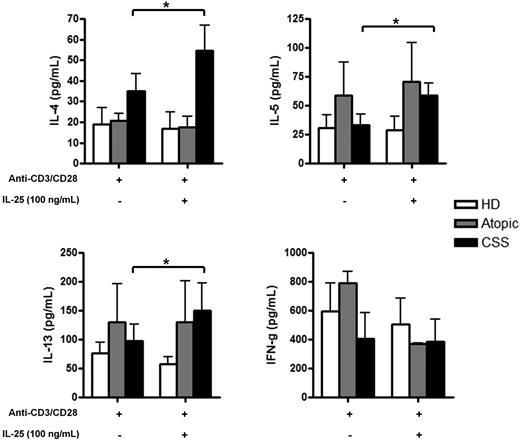
Freshly isolated PBMCs were cultured with anti-CD3/CD28 with or without IL-25 stimulation for 48 hours, and culture supernatants were harvested and examined for cytokine production by Luminex analysis. Although activated PBMCs from CSS patients were capable of producing moderate amounts of IL-4, IL-5, and IL-13 (35.5 [20.7-48.3], 42 [16.8-46.8], and 91.0 [66.6-104.2] pg/mL, respectively), IL-25 stimulation led to a 1.4- to 1.5-fold increase of Th2 cytokine production (53.0 [30.7-76.3], 62.5 [44.6-73.2], and 124.7 [109.5-150.1] pg/mL, respectively), but not of IFN-γ. In contrast, IL-25 seems to have no effect on PBMCs from atopic patients and healthy donors, except for a slight increase in IL-5 production in atopic patients (29.1 [18.0-102.9]pg/mL with IL-25 vs 17.6 [13.7-83.6] pg/mL without IL-25) (Figure 4).
IL-25 enhances the Th2 cytokine production in CSS patients. Th2 (IL-4, IL-5, IL-13) and Th1 (IFN-γ) cytokines production by PBMCs from active CSS patients, atopic patients, and healthy donors after stimulation with anti-CD3/CD28 with or without IL-25 (100 ng/mL) assessed by Luminex in culture supernatants. * P < .05; ** P < .01; *** P < .001. Histograms in each panel are representative of 3 to 4 patients in at least 3 independent experiments.
IL-25 enhances the Th2 cytokine production in CSS patients. Th2 (IL-4, IL-5, IL-13) and Th1 (IFN-γ) cytokines production by PBMCs from active CSS patients, atopic patients, and healthy donors after stimulation with anti-CD3/CD28 with or without IL-25 (100 ng/mL) assessed by Luminex in culture supernatants. * P < .05; ** P < .01; *** P < .001. Histograms in each panel are representative of 3 to 4 patients in at least 3 independent experiments.
IL-25 and IL-17RB expression in vasculitis nerve lesions of CSS patients
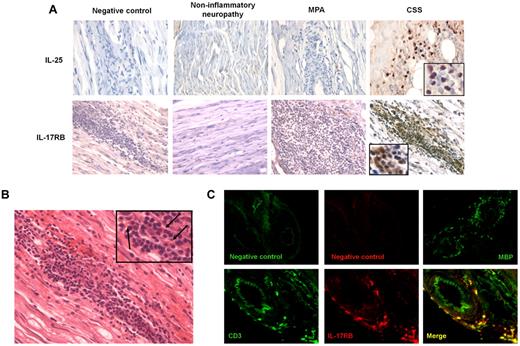
Immunohistochemical analyses of nerve tissue specimens from patients with CSS, MPA and noninflammatory neuropathy were used to investigate the pattern of expression of IL-25 and IL-17RB. H&E staining showed necrotizing vasculitis in samples from CSS and MPA patients. IL-25 was expressed within the inflammatory perivascular infiltrates in patients with CSS compared with patients with MPA and those with noninflammatory idiopathic neuropathy (Figure 5A). Higher expression of IL-17RB was also observed in mononuclear cells within the inflammatory perivascular infiltrates in patients with CSS but not in patients with MPA or those with noninflammatory idiopathic neuropathy (Figure 5A).
IL-17RB+ T cells and IL-25 are expressed in vasculitic lesions of CSS patients. (A) Immunohistochemical analyses of nerve tissue specimens from patients with CSS, MPA, and noninflammatory neuropathy. IL-25 and IL-17RB were expressed within the perivascular infiltrates in patients with CSS, compared with that in patients with MPA and those with noninflammatory idiopathic neuropathy (original magnification ×540; × 1080 for the inserts in CSS panels). (B) H&E staining. Mononuclear cells and eosinophils (black arrows) were observed within the perivascular inflammatory infiltrate (original magnification ×540; ×1080 for the insert). (C) Immunofluorescence microscopy analysis of frozen nerve tissue specimen from CSS patients revealed expression of the eosinophil granule Major Basic Protein (MBP) and colocalization of IL-17RB with CD3 within the perivascular infiltrates, indicating that infiltrating IL-17RB–expressing cells were T cells (original magnification ×270 for negative control and MBP; ×540 for CD3 and IL-17RB staining). Immunohistochemistry and immunofluorescence images were acquired using a Leica DMI6000B microscope (Leica) and analyzed using the Metaview software.
IL-17RB+ T cells and IL-25 are expressed in vasculitic lesions of CSS patients. (A) Immunohistochemical analyses of nerve tissue specimens from patients with CSS, MPA, and noninflammatory neuropathy. IL-25 and IL-17RB were expressed within the perivascular infiltrates in patients with CSS, compared with that in patients with MPA and those with noninflammatory idiopathic neuropathy (original magnification ×540; × 1080 for the inserts in CSS panels). (B) H&E staining. Mononuclear cells and eosinophils (black arrows) were observed within the perivascular inflammatory infiltrate (original magnification ×540; ×1080 for the insert). (C) Immunofluorescence microscopy analysis of frozen nerve tissue specimen from CSS patients revealed expression of the eosinophil granule Major Basic Protein (MBP) and colocalization of IL-17RB with CD3 within the perivascular infiltrates, indicating that infiltrating IL-17RB–expressing cells were T cells (original magnification ×270 for negative control and MBP; ×540 for CD3 and IL-17RB staining). Immunohistochemistry and immunofluorescence images were acquired using a Leica DMI6000B microscope (Leica) and analyzed using the Metaview software.
H&E staining revealed the presence of eosinophils within the inflammatory infiltrate (Figure 5B). Immunofluorescence microscopy analysis of frozen nerve tissue specimens showed expression of the eosinophil granule Major Basic Protein within the perivascular infiltrate (Figure 5C). We also observed colocalization of IL-17RB with CD3 within the perivascular infiltrates (Figure 5C), indicating that infiltrating IL-17RB–expressing cells were T cells.
Gene-expression profile in vasculitis nerve lesions of CSS patients
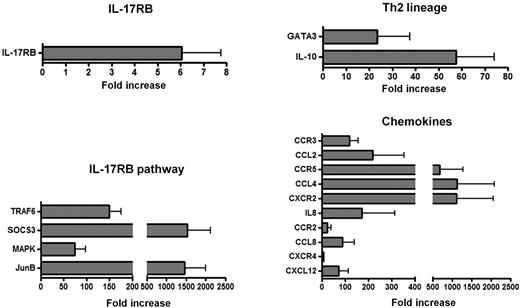
Gene quantification at the mRNA level was performed in peripheral nerve tissue samples from patients with CSS and noninflammatory neuropathy to assess the overexpression of 21 genes reported to be involved in the IL-25/IL-17RB pathway, Th2 lineage, and main eosinophil and lymphocyte chemokines/receptors.
Differential expression higher than 5 times the value of noninflammatory neuropathy controls was observed for IL-17RB (fold increase 6.0 ± 1.6), TRAF6 (150 ± 25), SOCS3 (1527 ± 588), MAPK (74 ± 23), and JunB (1464 ± 535) (Figure 6). Two genes involved in Th2 lineage were overexpressed in the nerve biopsy specimens from patients with CSS, ie, GATA-3 (38 ± 14) and IL-10 (58 ± 17), suggesting predominant Th2 responses within mononuclear infiltrates of CSS patients (Figure 6). IL-4, IL-5, IL-13, and IL-25 transcripts were not expressed in nerve biopsy specimens from patients with CSS and noninflammatory neuropathy.
Gene-expression profile in vasculitis nerve lesions of CSS patients. Gene-expression quantification at the mRNA level in peripheral nerve tissue samples from patients with CSS showed up-regulation of genes involved in IL-25/IL-17RB pathway, Th2 lineage and main eosinophil and lymphocyte chemokines/receptors.
Gene-expression profile in vasculitis nerve lesions of CSS patients. Gene-expression quantification at the mRNA level in peripheral nerve tissue samples from patients with CSS showed up-regulation of genes involved in IL-25/IL-17RB pathway, Th2 lineage and main eosinophil and lymphocyte chemokines/receptors.
For a better analysis of cellular involvement in the pathogenesis of CSS vasculitis neuropathy, we also analyzed a set of 10 chemokines and receptors. The mRNA expression for IL-8 and CXCR2 (IL-8RB) (171 ± 143 and 1108 ± 946, respectively), which are involved in the migration of neutrophils, was higher in CSS vasculitis patients compared with noninflammatory neuropathy controls. Moreover, CSS patients had overexpression of CCL2 (219 ± 136), CCR3 (118 ± 38), CCL4 (1123 ± 962), CCR5 (675 ± 600), CCL8 (89 ± 51), CCR2 (24 ± 15), CXCL12 (71 ± 42), and CXCR4 (6.2 ± 1.4), which are involved in the migration of eosinophils, basophils, and lymphocytes (in particular Th2 cells), compared with the noninflammatory neuropathy controls (Figure 6).
Discussion
The pathogenesis of CSS is complex and is likely to involve many mechanisms, primarily related to eosinophils toxicity and adaptive immunity through the production of inflammatory cytokines by T cells. Increase in blood and tissue eosinophilia is commonly considered to be the consequence of the production of Th2 cytokines by CD4+ T cells,11 in particular IL-5, which is the most potent stimulator of eosinophil production and functional activation of mature eosinophils.21,22 However, the mechanisms by which eosinophils could elicit a Th2-mediated immune response in CSS remains unclear.
Recently, IL-25 has been shown to play a critical role in evoking Th2 cell–mediated inflammation that features the infiltration of eosinophils and Th2 memory cells.12,13
We found higher levels of IL-25 in the serum of active CSS patients compared with atopic and HES patients and with healthy donors. IL-25 serum level was correlated with disease activity of CSS and with the eosinophil count and was more than 10 times higher in CSS patients than that observed in undefined HES and atopy. Although the eosinophil count was elevated in HES patients (median 2550/mm3), their serum IL-25 level was low. Thus, increased levels of IL-25 observed in CSS patients may depend on a specific priming and differentiation of activated eosinophils. Although our understanding of the biology of IL-25 is increasing, the means by which IL-25 is regulated is still poorly understood. Exposure to allergens and air pollutants has been reported to increase IL-25/IL-17RB in the lung.13,23 Eosinophils may release preformed cationic proteins exhibiting cytotoxic activity, and a variety of proinflammatory cytokines and chemokines.24,25 In CSS, the release of eosinophil cationic proteins was shown to be correlated with disease activity,26 and eosinophils were found to show a polyclonal pattern, in contrast to HES, in which patients exhibit a clonal pattern.27
We observed that IL-25 was mainly produced in the peripheral blood of CSS patients by eosinophils. In contrast, neutrophils, monocytes, and T cells did not produce IL-25. These findings are consistent with previous reports showing that resting and activated eosinophils were the main producers of IL-25.15 However, activated CD4+ CD45RA− memory T cells were the IL-25 receptor (IL-17RB)–expressing cells in CSS patients, and IL-25 slightly enhanced the IL-17RB expression on memory CD4+ T cells.
The analysis of T-cell differentiation in CSS patients pointed toward a polarized type 2 immune response with increased production of IL-4. Interestingly, we found in CSS patients, but not in healthy donors, that IL-25 enhanced the production of IL-4, IL-5, and IL-13, but not of IFN-γ, supporting its role in promoting Th2 cell differentiation.12,13 Taken together, these findings suggest that under T-cell receptor stimulation (using anti-CD3/CD28), T cells from CSS patients overexpress IL-17RB and could be exquisitely sensitive to IL-25 produced by eosinophils.
To further explore the interaction between IL-25 produced by eosinophils and Th2 cells, we studied vasculitic nerve lesions of CSS patients in which infiltration by eosinophils and memory T cells was demonstrated.7 We found an increased expression of IL-25 and IL-17RB in vasculitic nerve infiltrates of CSS, which further support the idea that IL-25/IL-17RB may be a key molecular pair in the maintenance of inflammation and therefore could be a potential target for the development of novel therapies. We observed that IL-17RB–expressing cells were mostly CD3+ T cells. Along this line, we found in peripheral blood that IL-17RB–expressing cells were mainly CD4+ memory T cells.
Gene-expression profile analysis revealed the overexpression of genes involved in the IL-25/IL-17RB signaling pathway and the predominant Th2 lineage commitment in target tissues. Moreover, we found an overexpression of chemokines and receptors involved in the recruitment of eosinophils and mononuclear cells within the inflamed tissues, consistent with local production of IL-25 in the nerves and the enhancement of Th2 cell differentiation, and the recruitment of inflammatory cells, which could amplify the inflammatory loop in tissue. IL-25 has been shown to regulate adaptive immunity by enhancing Th2 cytokine production, and by potentiating expression of the nuclear factor of activated T cells c1 and JunB transcription factors; this may result in increased levels of initial IL-4 production, up-regulation of GATA-3 expression, and enhanced Th2 cell differentiation.12,13
Recently, the involvement of IL-25 and its receptor, IL-17RB, was clearly shown in mice models of airway hyperreactivity, which represent animal models for human asthma.28–30 Interestingly, asthma is almost always present at the onset of CSS. In a model of sensitization and challenge with ovalbumin to induce airway inflammation, it was demonstrated that IL-25 mRNA expression was up-regulated after ovalbumin challenge and that neutralization of IL-25 with a soluble IL-17RB fusion protein reduced eosinophil and CD4+ T-cell recruitment into the lungs.23 These findings support the potential interest of blocking IL-25 during CSS.
In conclusion, our study is the first to demonstrate the involvement of IL-25 in the pathogenesis of CSS and its correlation with disease activity. Our findings suggest that eosinophils, through the production of IL-25, may have a critical role in promoting Th2 responses in peripheral blood and target tissues in CSS, and are a potential target for novel therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Valérie Thuries, Nathalie Ferry, and Véronique Bon-Durand for technical assistance and to Janet Ratziu for editorial assistance.
B.T. was supported by Fondation pour la Recherche Médicale.
Authorship
Contribution: B.T. and D.S. designed research; B.T., I.B., I.L., and D.S. performed research; B.T., T.M., M.R., J.E.K., M.-C.D., L.M., M.V., D.S., N.C.-C., D.L.T.-H., Z.A., D.K., P.C., and D.S. collected data; B.T., P.C., and D.S. analyzed and interpreted data; B.T. and D.S. performed statistical analysis; and B.T., P.C., and D.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr David Saadoun, Service de Médecine Interne and Laboratoire I3: Immunologie-Immunopathologie-Immunothérapeutique, UMR 7211 (UPMC/CNRS), U 959 (Inserm), Université Pierre Marie Curie, Paris 6, Hôpital Pitié-Salpêtrière, 83 Bd de l'hôpital, 75013 Paris, France; e-mail: david.saadoun@psl.aphp.fr.
References
Author notes
P.C. and D.S. are co-senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal