Serial quantitation of BCR-ABL mRNA levels is an important indicator of therapeutic response for patients with chronic myelogenous leukemia and Philadelphia chromosome–positive acute lymphoblastic leukemia, but there is substantial variation in the real-time quantitative polymerase chain reaction methodologies used by different testing laboratories. To help improve the comparability of results between centers we sought to develop accredited reference reagents that are directly linked to the BCR-ABL international scale. After assessment of candidate cell lines, a reference material panel comprising 4 different dilution levels of freeze-dried preparations of K562 cells diluted in HL60 cells was prepared. After performance evaluation, the materials were assigned fixed percent BCR-ABL/control gene values according to the International Scale. A recommendation that the 4 materials be established as the first World Health Organization International Genetic Reference Panel for quantitation of BCR-ABL translocation by real-time quantitative polymerase chain reaction was approved by the Expert Committee on Biological Standardization of the World Health Organization in November 2009. We consider that the development of these reagents is a significant milestone in the standardization of this clinically important test, but because they are a limited resource we suggest that their availability is restricted to manufacturers of secondary reference materials.
Introduction
Reverse-transcription real-time quantitative polymerase chain reaction (RQ-PCR) is used routinely to quantify levels of BCR-ABL mRNA in peripheral blood and bone marrow samples from chronic myelogenous leukemia (CML) patients undergoing therapy. The technique can accurately determine response to treatment and is particularly valuable for patients who have achieved a complete cytogenetic response. The National Comprehensive Cancer Network (NCCN)1 and the European LeukemiaNet (ELN)2 recommend similar monitoring schedules for patients treated with imatinib and the ELN defines an optimal response as the attainment of a major molecular response (MMR) after 18 months of therapy. Monitoring of BCR-ABL mRNA levels is also useful for gauging therapeutic response for patients with Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL).3 Nevertheless, despite efforts to establish standardized protocols for BCR-ABL mRNA quantitation,4 there is still substantial variation in the way in which RQ-PCR for BCR-ABL is performed and how results are reported in different laboratories worldwide.5,–7 Substantial variation is observed even among laboratories that use the same commercially available kit (unpublished data from the College of American Pathologists surveys; Y.L.W., written communication, June 2010).
The CML meeting at the National Institutes of Health in Bethesda in October 2005 made several recommendations for the harmonization of minimal residual disease (MRD) assessment and proposed an international scale (IS) for BCR-ABL RQ-PCR measurements.8 Importantly, the IS is essentially identical to that used in the International Randomized Study of Interferon and STI571 (IRIS) study,9 with the IRIS standardized baseline defined as 100% BCR-ABLIS and MMR (3-log reduction relative to the standardized baseline) defined as 0.1% BCR-ABLIS. The original standards used for the IRIS trial are no longer available, however traceability to the IRIS scale is provided by the extensive quality control data generated by the Adelaide laboratory over a period of several years.10,11
To enable testing centers to gain access to the IS, the Adelaide laboratory initiated a process to develop and validate laboratory-specific conversion factors (CFs) that can be used to convert local values to IS values.11 The strength of this approach is that testing centers can continue to use their existing assay conditions and continue to express results according to local preferences in addition to expressing results on the IS. The concept of the IS is analogous to established procedures for other quantitative assays, for example the International Normalized Ratio (INR) for prothrombin time.12
Many laboratories with validated CFs have established themselves as national or regional reference laboratories and are in the process of propagating CFs to local centers.13 While this process has generally worked well, it is apparent that the establishment of CFs is time-consuming, complex, expensive, and open to only a limited number of laboratories at any given time. Furthermore, it is unclear how frequently any individual CF will need to be revalidated. We sought therefore to develop an alternative means for testing laboratories to access the IS by developing calibrated, accredited primary reference reagents for BCR-ABL RQ-PCR analysis.
Strategy
Ideally, the formulation for primary reference reagents should be as close as possible to the usual analyte, should cover the entire analytical process and should be applicable to methods in use throughout the world.14 However, it is essential that the formulation is stable over a period of several years and that it is physically possible to produce batches of sufficient size to satisfy worldwide demand over a similar period of time. These requirements effectively preclude the use of primary cells and instead we focused on the use of cell lines. It has been shown previously that good quality RNA can be extracted from freeze-dried K562 cells15 and therefore we performed a preliminary assessment of the use of freeze-dried cell line mixtures as universal reference materials.
The basic unit of MRD measurement is the ratio of BCR-ABL transcripts to those of an internal control gene, the latter serving to normalize for variations in cDNA quantity and quality.5,16 In the absence of a universally agreed control gene, the Bethesda group recommended the use of either ABL, BCR, or GUSB as internal control genes, although other genes (eg, G6PD, B2M, PBGD) are still used in some centers. The CF normalization process has proven to be robust with the use of different internal controls11 and we considered that accommodation of the 3 principal control genes would also be highly desirable for any approach that used reference reagents. As an initial step we therefore evaluated the expression of ABL, BCR, and GUSB in a panel of BCR-ABL negative cell lines with the aim of identifying one or more that expressed the 3 genes at similar relative proportions to that seen in normal leukocytes. KG1 and HL60 were identified as the best candidates to take forward for detailed evaluation and, after an international field trial,17 HL60 was selected. For a source of BCR-ABL, K562 was selected based on initial successful analysis of freeze-dried cells.15 The fact that this cell line is known to harbor multiple copies of BCR-ABL and expresses high levels of fusion mRNA is irrelevant since the basic unit of MRD measurement does not incorporate the number of cells analyzed.
As for the number and range of reference materials, we considered that 4 dilutions should enable the construction of a reasonably robust regression line. Clearly it is critical for reference reagents to facilitate the measurement of MMR (0.1%IS) and therefore we considered it essential that the lowest dilution was approximately 10× below this level. As for the highest dilution, the IS in routine practice is valid only below 10% due to control gene-dependent distortions at high disease levels.5 We therefore aimed to generate reference materials that correspond to approximately 10%, 1%, 0.1%, and 0.01% on the IS. The results of an initial pilot study have been reported elsewhere17 ; this paper focuses on the bulk production and performance evaluation of K562/HL60 mixtures.
Methods
Participants
Eleven laboratories were invited to take part in the international collaborative study. The laboratories were chosen because they all have a validated CF that allows them to convert results generated by their own local method (including laboratory protocol, platform, and plasmid standard) to results expressed using the IS.11 Ten laboratories from 9 different countries agreed to participate; these laboratories had CFs for 3 different control genes; ABL (n = 6), BCR (n = 3), and GUSB (n = 3).
Production of cell line mixtures
For bulk preparations of reference materials, the HL60 cell line was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ, Braunschweig, Germany) and the K562 cell line was obtained from the Hammersmith Hospital, (London, United Kingdom). Cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (Sigma-Aldrich); pilot cultures were grown locally and bulk culture growth was outsourced to the European Cell and Culture Collection (ECACC).
HL60 and K562 cultures tested negative for HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), human T-lymphotropic virus type I and II (HTLV-I/II), human herpes virus 8 (HHV-8), and mycoplasma by PCR. Four dilutions of K562 in HL60 that corresponded approximately to 10%IS (designated BCR/ABL 4 08/198), 1%IS (designated BCR/ABL 3 08/196), 0.1%IS (designated BCR/ABL 2 08/194), and 0.01%IS (designated BCR/ABL 1 08/192) were prepared based on %BCR-ABL/ABL values. Cell suspensions in ice-cold 2× phosphate-buffered saline (PBS; Sigma-Aldrich) were transferred to the National Institute for Biological Standards and Control (NIBSC) on ice. Cell suspensions were stored at 4°C overnight with gentle stirring. The next day 3-mL glass ampules were filled with 1.5 × 106 cells (0.5 mL) for freeze-drying. The time interval between cell harvesting and commencement of freeze-drying was approximately 24 hours.
Lyophilization
The freeze-drying process took 99 hours and approximately 3500 vials of each level were produced. Aliquots of 0.5 g of each cell suspension, maintained gently stirred at 5°C-10°C, were dispensed on an automated AVF5090 filling line (Bausch & Strobel) into 3-mL autoclaved DIN glass ampules (Adelphi Tubes). Igloo-style halobutyl closures (13-mm diameter, Adelphi Tubes) were part-inserted and trays of ampules placed on the −50°C precooled shelves of the freeze-dryer (CS-100; Serail), with each batch separately located on a different shelf. The products were frozen for 4 hours at −50°C. Then the shelf temperature was raised to −25°C and a hard vacuum of 30 μbar applied. Primary drying continued for 30 hours, and the shelves were then warmed to 0°C and held for 5 hours, followed by a rise to 25°C, then held at 25°C for secondary drying for another 32 hours. Ampules were back-filled to near atmospheric pressure with boil-off nitrogen of low moisture content and stoppered in the dryer. Products were then removed and flame-sealed on the filling line.
Lyophilized product testing
Residual moisture content was determined by coulometric Karl Fischer titration (Mitsubishi CA100 coulometer; A1-Envirosciences) operated within a dry box environment to prevent atmospheric moisture ingress and checked with a control water solution within defined limits. Ampules were individually broken open at the prescored neck and the lyophilized product reconstituted in anolyte reagent (Mitsubishi Aquamicron A) using a pipette, the anolyte plus product returned to the titration cell and titration begun. Twelve ampules of each batch were tested for residual moisture content expressed as weight/weight percentage based on the determined dry weights of the lyophilized materials.
Residual headspace oxygen was tested using ampules of each batch by an electrochemical method on the Orbisphere Pharmapak 3600 (Hach Ultra). Each ampule was inverted in an ampule holder and a gas-tight seal made to the base of the container. The ampule was then pierced and the headspace gas displaced by injection of 2-3 mL of nitrogen-saturated water. The oxygen concentration of the displaced gas was measured. The unit was calibrated on nitrogen and atmospheric air and 12 ampules of each batch were tested.
Evaluation of reference panel by RQ-PCR
Homogeneity and stability of the reference panel were evaluated in a single center (Salisbury, United Kingdom) using previously described methods for cDNA synthesis18 and RQ-PCR.4,19 For the performance evaluation and assignment of consensus reference values, 3 batches comprising one 3-mL glass ampule of each material (BCR/ABL 4 08/198, BCR/ABL 3 08/196, BCR/ABL 2 08/194, and BCR/ABL 1 08/192) were distributed by courier at ambient temperature to the 10 laboratories that had validated CFs. The materials were analyzed according to a common procedure (see supplemental Methods A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), but each laboratory used its own laboratory protocols that were the same as those used to generate their laboratory-specific conversion factors. RNA was extracted from each batch of vials on different days, using the entire lysate for each RNA extraction. Two RNA extraction methods were used Trizol (n = 8; Invitrogen) and QIAGEN extraction kits (n = 2). 2 cDNA reactions were performed for each of the extracted RNA samples on different days, giving a total of 24 cDNA samples. Samples for BCR-ABL and the control gene(s) were analyzed using the established RQ-PCR methods of the participating laboratories. Eight different types of RQ-PCR machines were used: Applied Biosystems 7500 (n = 3), Applied Biosystems 7000 (n = 1), Applied Biosystems 7700 (n = 1), Applied Biosystems 7900 (n = 1), Applied Biosystems 7300 (n = 1), Corbett RotorGene 6000 (n = 1), Roche LightCycler 2.0 (n = 1), and Roche LightCycler 480 (n = 1). Six of the participants cited Gabert et al4 as one of the references used as their RQ-PCR protocol. Other references cited were Branford et al19 and Emig et al.20 One laboratory used an in-house assay. All laboratories used random hexamers for reverse transcription. The following data were recorded: date of RNA extraction, total micgrograms of RNA, A260/A280, A260/A230, date, slope and gradient of standard curve, BCR-ABL transcript value (cycle threshold [Ct] value and copy number) control gene(s) transcript value (Ct value and copy number), BCR-ABL/control gene (percent) before conversion to IS, BCR-ABL/control gene (percent) converted to the IS.
Results
Cell line mixtures
A total of 44 L of HL60 and 2.5 L of K562 cultures were grown to exponential phase, yielding 4.9 × 1010 and 19 × 108 cells, respectively, after harvesting. Our pilot analysis had indicated a 1:50 mixture of K562:HL60 cells yielded a BCR-ABLIS value of approximately 10% and therefore an initial dilution was made using the same cellular proportions: 1.28 × 108 K562 cells were pelleted and resuspended in 2130 mL of a suspension of HL60 cells at 3 × 106 cells/mL in 2× PBS. Three further 1:10 dilutions were made by sequential dilution of the initial mixture into HL60 cells (3 × 106 cells/mL in 2× PBS) after thorough mixing. For each material the coefficient of variation of the fill, residual moisture after lyophilization, mean dry weight, mean residual oxygen, and number ampules produced are shown in supplemental Table 1.
Stability studies
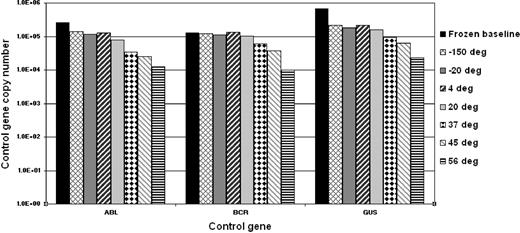
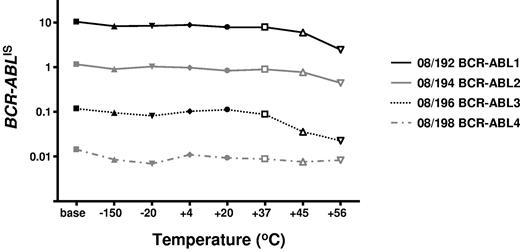
To determine the stability of the freeze-dried cells we performed accelerated degradation studies by RQ-PCR analysis of vials stored continuously at −150°C, −20°C, 4°C, 20°C, 37°C, 45°C, and 56°C. Two sets of data were derived for each material; the absolute copy number of the relevant gene transcripts (BCR-ABL, ABL, BCR, and GUSB) and the ratio between BCR-ABL and the 3 control gene transcripts. Samples were analyzed after storage for 6 and 10 months, respectively, at the designated temperature. Data obtained at both timepoints indicated a reduction in the copy number of all genes for all 4 reference materials when stored at or above 37°C (Figure 1). More importantly, the BCR-ABL/control gene transcript ratio, the critical unit of minimal residual disease measurement, had begun to drop when stored at 45°C and 56°C (Figure 2). Although it is recommended that the materials be stored at −20°C, these results demonstrate that the materials are still acceptable for analysis if stored at or below 37°C for 10 months.
Accelerated degradation study: control genes. The bar chart shows the mean total copy number of ABL, BCR, and GUSB control gene transcripts per fixed volume of cDNA for the 4 reference materials after storage at the designated temperatures for 10 months. Frozen baseline: nonlyophilized material from the same batch stored at −150°C. Data at 6 months, storage showed a similar temperature-dependent decline but with less loss of transcript numbers (data not shown).
Accelerated degradation study: control genes. The bar chart shows the mean total copy number of ABL, BCR, and GUSB control gene transcripts per fixed volume of cDNA for the 4 reference materials after storage at the designated temperatures for 10 months. Frozen baseline: nonlyophilized material from the same batch stored at −150°C. Data at 6 months, storage showed a similar temperature-dependent decline but with less loss of transcript numbers (data not shown).
Accelerated degradation study: control genes: BCR-ABL/control gene ratios. The average BCR-ABL/ABL ratios per fixed volume of cDNA for each of the 4 reference materials are shown after storage at the designated temperatures for 10 months. The data for BCR-ABL/BCR and BCR-ABL/GUSB were very similar, as were the data at 6 months' storage.
Accelerated degradation study: control genes: BCR-ABL/control gene ratios. The average BCR-ABL/ABL ratios per fixed volume of cDNA for each of the 4 reference materials are shown after storage at the designated temperatures for 10 months. The data for BCR-ABL/BCR and BCR-ABL/GUSB were very similar, as were the data at 6 months' storage.
Homogeneity
To assess the homogeneity of the material distributed into the glass ampules, 17 vials of each reference material (BCR-ABL 1 to 4) were picked at random and tested in triplicate in the same RQ-PCR run. To make a comparison with the intra-assay variability, 2 similar samples (nonlyophilized BCR-ABL 1 and BCR-ABL 4) were each tested multiple times (17 triplicate reactions). The coefficients of variation (CVs) for the %BCR-ABL/control gene values are shown in Table 1 and are comparable with those described for the analysis of primary patient samples.7,11 These results suggest that the variability between ampules is no greater than the variability of the assay itself. For BCR and GUSB control genes there was no significant difference in the variabilities shown above (P = .314 and P = .538, respectively). For the ABL control gene there was a significant difference in variabilities (P = .009). This is due to the higher variability observed for 08/192 (CV 23.31%) and no significant difference is found when this data are removed (P = .299).
Coefficient of variation for triplicate analysis of 17 vials of freeze-dried cells of each reference material and 2 samples of nonlyophilized cell line mixtures, each tested 17 times
| . | BCR-ABL/ABL . | BCR-ABL/BCR . | BCR-ABL/GUSB . |
|---|---|---|---|
| 17 ampules tested | |||
| BCR-ABL 1 08/192 | 23.31 | 21.10 | 18.92 |
| BCR-ABL 2 08/194 | 12.84 | 15.74 | 19.57 |
| BCR-ABL 3 08/196 | 9.07 | 13.14 | 13.21 |
| BCR-ABL 4 08/198 | 14.23 | 11.61 | 16.02 |
| 1 sample tested 17 times | |||
| BCR-ABL 1 frozen | 10.31 | 14.52 | 14.52 |
| BCR-ABL 4 frozen | 13.31 | 19.20 | 13.93 |
| . | BCR-ABL/ABL . | BCR-ABL/BCR . | BCR-ABL/GUSB . |
|---|---|---|---|
| 17 ampules tested | |||
| BCR-ABL 1 08/192 | 23.31 | 21.10 | 18.92 |
| BCR-ABL 2 08/194 | 12.84 | 15.74 | 19.57 |
| BCR-ABL 3 08/196 | 9.07 | 13.14 | 13.21 |
| BCR-ABL 4 08/198 | 14.23 | 11.61 | 16.02 |
| 1 sample tested 17 times | |||
| BCR-ABL 1 frozen | 10.31 | 14.52 | 14.52 |
| BCR-ABL 4 frozen | 13.31 | 19.20 | 13.93 |
Values are reported as percent coefficient of variation.
%BCR-ABL/control gene
The mean amount of total RNA extracted from reference materials BCR/ABL 4 08/198, BCR/ABL 3 08/196, BCR/ABL 2 08/194, and BCR/ABL 1 08/192 was 17.42 μg, 14.16 μg, 14.34 μg, and 14.86 μg, respectively, from each vial (n = 30). After reverse transcription, laboratories tested cDNA in their quantitative PCR assay and the resulting Ct values were converted to gene transcript copy number using standard curves. Each of the 4 materials was tested 6 times (extraction on 3 separate days, tested in duplicate). For each of the 4 materials the transcript copy numbers for BCR-ABL and the 3 control genes were used to calculate %BCR-ABL/ABL, %BCR-ABL/BCR, and %BCR-ABL/GUSB. The arithmetic means and CVs of these 6 reactions for each laboratory are shown in supplemental Tables 2-4. The mean percent values for each material were then converted to the IS by multiplying the %BCR-ABL/control gene value by the established CF for each laboratory.11 The fact that the assigned values were slightly different for each control gene reflects the fact that the relative level of expression of these 3 genes in HL60 cells is not identical to that seen in normal peripheral blood leukocytes. The harmonizing effect of the use of individual conversion factors for each laboratory can be seen in Figure 3.
Linear regression plots of the log transformed %BCR-ABL/control gene values obtained from all laboratories for the 4 reference materials (Sample 1, BCR/ABL 1 08/192; Sample 2, BCR/ABL 2 08/194; Sample 3, BCR/ABL 3 08/196; and Sample 4 BCR/ABL 4 08/198). Regression plots are shown for each control gene, shown before and after conversion to the IS.
Linear regression plots of the log transformed %BCR-ABL/control gene values obtained from all laboratories for the 4 reference materials (Sample 1, BCR/ABL 1 08/192; Sample 2, BCR/ABL 2 08/194; Sample 3, BCR/ABL 3 08/196; and Sample 4 BCR/ABL 4 08/198). Regression plots are shown for each control gene, shown before and after conversion to the IS.
Assignment of IS values to reference materials
The mean %BCR-ABL/control gene transcript values obtained after conversion to the IS are shown in Table 2. All laboratories that participated in the study were asked to approve that these IS values be assigned to each material and that the materials should be proposed to the WHO as the 1st International Genetic Reference Panel for the quantitation of BCR-ABL mRNA. All laboratories agreed with the proposal. The study rationale and results were duly submitted to the WHO in July 2009 and a recommendation that the 4 materials be established as the 1st WHO International Genetic Reference Panel quantitation of BCR-ABL translocation by RQ-PCR was approved by the Expert Committee on Biological Standardization in November 2009.
Percent BCR-ABL/control gene values assigned to each reference material
| . | %BCR-ABL/ABL . | %BCR-ABL/BCR . | %BCR-ABL/GUSB . |
|---|---|---|---|
| BCR-ABL 1 08/192 | 0.0118 | 0.0195 | 0.0071 |
| BCR-ABL 2 08/194 | 0.1112 | 0.1753 | 0.0749 |
| BCR-ABL 3 08/196 | 1.1672 | 1.6627 | 0.8295 |
| BCR-ABL 4 08/198 | 10.7469 | 16.3129 | 10.1235 |
| . | %BCR-ABL/ABL . | %BCR-ABL/BCR . | %BCR-ABL/GUSB . |
|---|---|---|---|
| BCR-ABL 1 08/192 | 0.0118 | 0.0195 | 0.0071 |
| BCR-ABL 2 08/194 | 0.1112 | 0.1753 | 0.0749 |
| BCR-ABL 3 08/196 | 1.1672 | 1.6627 | 0.8295 |
| BCR-ABL 4 08/198 | 10.7469 | 16.3129 | 10.1235 |
Discussion
Approximately 98% of CML patients are characterized by BCR-ABL mRNA fusions in which BCR exon 13 and/or BCR exon 14 are spliced to ABL exon 2. Conventionally these are usually referred to as e13a2 (or b2a2) and e14a2 (or b3a2) junctions, respectively, and both encode a chimeric p210 BCR-ABL protein. The remaining 2% of cases have variant fusions that involve different exons of BCR or which lack ABL exon 2. Since BCR exon 14 is only 75 base pairs, the great majority of CML patients can be monitored by RQ-PCR using a single probe/primer pair combination, although some centers prefer to use different assay configurations for b2a2 and b3a2. Sequential analysis by quantitative PCR has proved of value for predicting relapse after stem cell transplantation, evaluating the effectiveness of donor leukocyte infusions as treatment for relapse, gauging response to interferon alpha and defining optimal response to tyrosine kinase inhibitors.2,21,,,–25 Despite the proven clinical value of molecular monitoring and the efforts over many years to improve standardization of laboratory aspects of the test, there is still substantial variation worldwide in the manner that BCR-ABL RQ-PCR results are derived and expressed. Apart from issues relating to basic optimization of the analytic and logistical steps of the process (ie, sample transport, cell preparation, storage, RNA extraction, cDNA synthesis, and real time PCR), the principal reasons for variable results are (1) the fact that there is no single, generally accepted internal control gene and (2) the lack of independent reference materials. As a step toward improving this situation we sought therefore to develop primary, accredited BCR-ABL reference reagents that are linked to the IS and validated for the 3 control genes that are most widely used internationally.
The limiting factor for production of the cell line mixtures was the number of cells required for the BCR-ABL–negative cell line HL60. Starting with a 44-L culture volume of HL60, we were able to prepare approximately 3500 vials at each of the 4 dilutions. It is not known how many laboratories worldwide test for BCR-ABL, but surveys suggest that there are approximately 250 in Europe and more than 100 in North America. Assuming there are 500 testing laboratories worldwide, each performing a median of 3 RQ-PCR runs per week and the reference materials were included on each run, the worldwide demand would be 78 000 vials per annum for each of the 4 dilutions. This would require in excess of 500 L of HL60 culture per annum, a scale of production that is simply not practical. Even if the freeze-dried materials were used by each testing laboratory on a monthly basis they would be depleted in only 7 months. We believe therefore that the principal function of these primary reagents should be limited to the calibration of secondary reference reagents. Such secondary reference reagents may be produced by companies, reference laboratories or other agencies and made available to testing laboratories either on a commercial basis or as part of specific national or regional standardization initiatives.
In principle, these secondary reagents might be formulated in different ways, such as freeze-dried cells, cell lysates, or synthetic RNAs, but they would need to be in a form that enables calibration to the IS (ie, reagents for which a BCR-ABL/control gene ratio can be derived). We have suggested a protocol by which secondary reagents could be calibrated to the primary materials (see supplemental Methods B) designed to capture the intrinsic variation of the assay over time in a similar manner to the process that is performed for the calculation of laboratory-specific CFs.11 The efficacy of the calibration process, however, and the performance of secondary reagents will need to be monitored carefully over time. In addition, the way in which secondary reagents are used will also need to be defined, for example as calibrators for each run (which we believe to be the ideal scenario) or as reagents analyzed less frequently as a more general indicator of assay drift. Finally, previous studies have demonstrated the value of using a common plasmid for harmonization of RQ-PCR results,26 suggesting that the development of accredited traceable plasmid calibrators that accommodate different control genes would also be useful.
The reagents we have produced will not have an immediate impact on testing laboratories who should continue to use established conversion factor processes and other ongoing initiatives to standardize their results. Nevertheless, we believe that the reagents represent an important milestone in the development of robust diagnostic RQ-PCR assays and will facilitate the production of more widely available reagents calibrated to the IS. The availability of these materials should help to make the IS more accessible, as well as provide a more robust framework for the scale itself. Organizations (eg, companies, reference laboratories, and professional bodies) should be encouraged to develop secondary reagents and those that wish to calibrate them to the IS should contact NIBSC to access the primary freeze-dried reagents.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added in proof.
Please note that these materials described in this study have been validated only for BCR-ABL detection in the range 0.01% to 10% on the International Scale using ABL, BCL, or GUSB as internal controls. They have not been validated for other applications, for example, International Scale measurements > 10% or determining assay sensitivity.
Acknowledgments
We thank Chris Morris and Alex Hiscott for assistance with cell culture and harvesting cells, Elaine Gray and Ross Hawkins (NIBSC) for assistance with field trial design, and the team of the Center for Biological Reference Materials (NIBSC) for processing the material.
This work was supported in part by a grant from the United Kingdom Department of Health (H.E.W.).
Authorship
Contribution: H.E.W., P.R., J.G., J.M.G., P. Matejtschuk, P. Metcalfe, and N.C.P.C. designed the study; H.E.W. and F.L. made cell line dilutions; P. Matejtschuk and P. Metcalfe supervised cell line freeze-drying; P.R. provided statistical input; Y.L.W., S.B., M.C.M., N.B., E.B., D.C., D.D., H.E., H.-G.G., H.E.H., D.J., V.K., S.K.-R., D.-W.K., S.L., E.S.K.M., R.D.P., G.R., L.W., K.Z., T.H., G.S., and A.H. undertook or supervised laboratory performance evaluation; and all authors contributed to writing the final manuscript.
Conflict-of-interest disclosure: At the time of the study E.B. was employed by MolecularMD (Portland, OR). D.J. currently provides BCR-ABL testing for Quest Laboratories (Madison, NJ). The remaining authors declare no competing financial interests.
The current affiliation for E.B. is Department of Cancer & Stem Cell Biology, Duke-National University of Singapore Graduate Medical School, Singapore.
The current affiliation for H.E. is Department of Clinical Genetics, University Hospital, Lund, Sweden.
Correspondence: Nicholas C. P. Cross, Wessex Regional Genetics Laboratory, Salisbury District Hospital, Salisbury SP2 8BJ, United Kingdom; e-mail: ncpc@soton.ac.uk; or Paul Metcalfe, National Institute for Biological Standards and Control, Blanche Ln, South Mimms, Potters Bar, Herts EN6 3QG, United Kingdom; e-mail: Paul.Metcalfe@nibsc.hpa.org.uk.