The Gray Platelet Syndrome (GPS) is the paradigm of clinical α-granule deficiency. Yet, its description had been limited to case reports. Gunay-Aygun et al now provide the first comprehensive study of GPS and assign the causative gene to a locus on chromosome 3.1
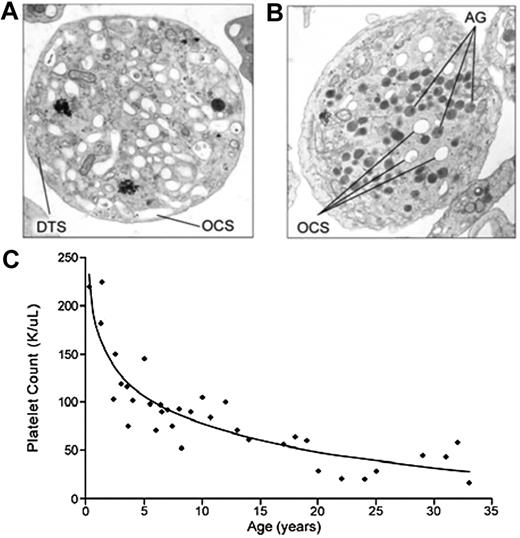
In 1971, Raccuglia described a patient with thrombocytopenia whose platelets included a high percentage of large cells that were nearly devoid of granules, rendering them gray upon Wright staining.2 He termed the disorder the Gray Platelet Syndrome. The sine qua non of GPS is the identification by electron microscopy of a large number of emptyvacuoles appearing as α-granule ghosts (see figure). In contrast, normal numbers of dense granules, lysosomes, peroxisomes, and mitochondria are detected. Features of the clinical syndrome include macrothrombocytopenia, bone marrow fibrosis, and splenomegaly. The bleeding diathesis in GPS has been described as mild to moderate. However, the clinical syndrome has been poorly characterized because of the small number of affected patients.
Electron micrograph of a platelet from (A) a patient with GPS compared with (B) a normal control. (C) Progressive thrombocytopenia of GPS. See complete Figures 2 and 3 in the article beginning on page 4990.
Electron micrograph of a platelet from (A) a patient with GPS compared with (B) a normal control. (C) Progressive thrombocytopenia of GPS. See complete Figures 2 and 3 in the article beginning on page 4990.
Gunay-Aygun and colleagues now report the most systematic and comprehensive evaluation of GPS to date.1 Their study includes 25 GPS patients from 14 independent families from around the world and provides long-term follow-up on many of the patients. The study shows that GPS is a progressive disease characterized by thrombocytopenia and myelofibrosis. The extent of follow-up (up to 32 years) enabled characterization of the progressive nature of GPS. Thrombocytopenia appears to develop early in the course of the disease and worsens with age (see figure). Serial bone marrow biopsies demonstrate progressive reticulin fibrosis, which may be attributable to constitutive release of α-granule contents into the bone marrow. The investigators also identify elevated B12 levels as a novel finding in GPS. Elevated B12 is observed in liver disease and several other hematologic disorders and is not pathognomonic for GPS. However, elevated B12 may serve as a useful marker for determining whether electron microscopy is required in the setting of suspected GPS.
The study shows that the bleeding tendency in GPS is complex, ranging from insignificant to severe. Thrombocytopenia is uniformly observed in affected individuals. In several cases, however, the bleeding tendency is out of proportion with the degree of thrombocytopenia. Platelet aggregation is normal in the majority of patients tested. This observation supports the premise that α-granules do not serve a critical function in ex vivo aggregation under conditions in which adequate fibrinogen is provided in plasma. This observation does not rule out, however, that α-granule contents are important for hemostasis in vivo, as suggested by previous observations of increased bleeding times. The observation that 7 of the 8 GPS patients with a severe bleeding tendency were women with menometrorrhagia may be related to thrombocytopenia and/or factors within α-granules that are required for endometrial hemostasis and perhaps proliferation.
The substantial interest in GPS has been driven by the anticipation that determination of its molecular underpinnings will provide new information about α-granule biogenesis. As an experiment of nature, GPS has been challenging. Although mouse models have been useful in understanding the molecular basis of dense granule release, few rodent models of isolated α-granule deficiency exist. Mice lacking the hematopoietic zinc finger transcription factor and RNA-binding protein, Hzf, produce platelets with empty α-granules and represent a good model of α-granule deficiency.3 However, sequencing of the orthologous Hzf gene in 5 patients with GPS demonstrated no defect.4 The dearth of existing knowledge regarding α-granule formation has limited candidate gene approaches. The small number of affected individuals has hampered previous efforts to identify the putative GPS gene by molecular genetics. Having assembled the majority of known GPS families, Gunay-Aygun et al used genome-wide linkage analysis to map the GPS gene. Although their study falls short of identifying the causative gene, it demonstrates that GPS has an autosomal recessive mode of inheritance in at least 22 of 25 cases and that the causative gene maps to a 9.4-MB interval on chromosome 3p. Thus, it is likely that the majority of cases of GPS have a common genetic etiology. Unfortunately, the linked region on chromosome 3 has a very low rate of recombination and no recombination events were identified to reduce the list of 165 candidate genes. Sequencing of 69% of exons within candidate genes failed to demonstrate pathologic mutations.
Some clues to the underlying mechanism of GPS have been provided by careful analysis of the phenotype. The defect occurs at the level of the megakaryocyte, which contains empty α-granules that are transported to platelets. All other organelles appear normal. The empty α-granules express membrane proteins, contain some endocytosed plasma proteins, and appear to be capable of activation-dependent fusion with surface-connected membranes.1,5,6 Proteomic analyses of α-granule proteins from patients with GPS confirm that soluble endogenous proteins are markedly decreased or undetectable, while endocytosed soluble and membrane-bound proteins are present.6 These observations imply a defect in the production, packing, and/or sorting of endogenous α-granule proteins.
Studies of megakaryocytes from GPS patients indicate a defect in α-granule maturation. Endogenous α-granule proteins are present in the early stages of megakaryopoiesis (days 5 and 6), but are progressively lost (days 9-11).7 In day-12 megakaryocytes from GPS patients, von Willebrand factor is observed in small vesicles near the Golgi, but mature α-granules fail to form.8 Elevation in plasma levels of endogenous α-granule proteins (PF4 and β-thromboglobulin) supports a packing or sorting defect rather than a defect in protein synthesis.5,7 By analogy, mice lacking chondroitin sulfate have α-granules deficient in PF4, β-thromboglobulin, and platelet-derived growth factor because of a granule-packing defect9 and patients lacking the vesicle-sorting protein VPS33B have α-granule deficiency.10 Defects in protein packing, vesicle trafficking, granule acidification, membrane retrieval, or protein processing could prevent normal granule maturation and result in shunting to a default pathway of constitutive secretion. The large number of genes that could potentially contribute to α-granule maturation complicates identification of likely culprits from the long list of candidate genes located in the linked interval on chromosome 3. We can only hope that improvements in sequencing technologies will make identification of the causative gene in GPS more black and white.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal