In this issue of Blood, Barker et al demonstrate that third-party non-neonatal T cells specific for Epstein-Barr virus (EBV) can be safely used to treat EBV-associated disease after allogeneic umbilical cord blood (UCB) transplantation (UCBT).1 This is made possible by the a priori generation of cryopreserved banks of EBV-specific T cells from peripheral blood.
The clinical benefits of infusing third-party T cells is predicted from extensive nonhuman and human experiences demonstrating that the adoptive transfer of antigen-specific T cells from a donor can successfully augment an immune response protecting and treating a designated recipient against pathogens and tumors after allogeneic hematopoietic stem cell transplantation (HSCT). What is only recently becoming apparent is that one donor can be used to generate antigen-specific T cells that can be infused into multiple recipients.
The report in this issue joins a growing list of clinical trials in which previously generated cryopreserved third-party EBV-specific T cells have been adoptively transferred.2-4 Multiple infusions were administered to achieve a clinical response with thawed T cells given every week, every 2 weeks, or every 3 weeks, at intravenous doses of 106/kg/infusion to 2 × 106/kg/infusion based on recipient weight. These data describe clinical responses to opportunistic EBV infection and associated disease that were apparently long-lasting and significantly were not associated with severe adverse effects. The anonymity, small size, and functional naiveté of the UCB graft typically preclude isolation of clinical-grade T cells with desired specificity and suitable for adoptive immunotherapy. Thus, the testing of third-party EBV-specific T cells from non-neonatal donors is particularly compelling in this clinical context. In aggregate, Barker's data highlight that third-partycryopreserved T cells can be successfully infused and that anti-EBV responses occur with sufficient rapidity despite the likely elimination of the infused T cells due to immune recognition by the recipient of mismatched major histocompatibility complex (MHC) molecules.
What is particularly heartening is that all published reports to date demonstrate an absence of clinically significant graft-versus-host-disease (GVHD) after infusion of off-the-shelf EBV-specific T cells that are at best only partially MHC matched. This is somewhat surprising considering that only up to ∼ 104/kg of donor-derived lymphocytes can be infused and reinfused after haploidentical HSCT without causing severe GVHD.5 Alloreactive T cells are described as having intrinsic affinity for the surface of disparate MHC molecules as well as maintaining conventional recognition for cognate peptides presented in the context of MHC.6 As shown by Amir et al,7 populations of T cells bearing αβ T-cell receptors with either unknown specificity or defined specificity for viral antigens both demonstrate alloreactivity in vitro. The absence of GVHD after the infusions of EBV-specific T cells perhaps reflects that the tissue-culturing process (coculture of T cells with recurrent additions of autologous γ-irradiated EBV-transformed B cells) to obtain EBV-specific T cells reduces the potential for alloreactivity. The apparent lack of alloreactivity in clinical practice was recently confirmed in recipients of allogeneic HLA-matched and HLA-mismatched HSCT who received donor-derived viral-specific T cells, despite in vitro data to the contrary.8
The future clinical impact regarding infusion of third-party EBV-specific T cells after UCBT will be interpreted regarding competing approaches and technologies. To generate T-cell effectors capable of long-lived immunosurveillance, investigators are developing methodologies to generate viral-specific T cells derived from the infused UCB allograft.9 For example, populations of UCB-derived “trivirus”-specific T cells can be generated with specificity for adenovirus, cytomegalovirus, and EBV10 by adapting an approach that has shown beneficial clinical activity upon infusing trivirus-specific T cells obtained from peripheral blood mononuclear cells (PBMC).11 Indeed, trivirus-specific PBMC-derived T cells are being evaluated in a multi-institution clinical trial as anoff-the-shelf therapy (ClinicalTrials.gov Identifier: NCT00711035). To broaden the therapeutic potential of UCB-derived T-cell therapy investigators are using gene-transfer approaches to redirect the specificity of T cells to introduce chimeric antigen receptor (CAR) for a tumor-associated antigen and expressing CAR on trivirus-specific T cells.12,13
The field of T-cell therapy is changing so that a given T-cell product can be infused into multiple recipients. Preclinical data are being generated to avoid immune recognition by down-regulating the expression of disparate MHC14 and using T-cell precursors from MHC-mismatched donors that can be infused across transplantation barriers.15 Regarding the clinical application of off-the-shelf T cells, questions remain for infrastructure, governance, and clinical conduct. Who will prepare these banks? What procedures will govern the distribution of cryopreserved T cells within state and across state lines? What are the avenues for reimbursement? Will these cells be dispensed using blood-banking practices? What informatics is needed to match candidate recipients with banked T cells?
There are also compelling questions that remain unanswered for how best to deploy off-the-shelf T cells. For example, should third-party effectors be infused after a lymphodepleting regimen to enable lymphopenia-induced proliferation of the adoptively transferred T cells as well as reduce the potential for immune-mediated rejection? Should the dose of T cells be increased per infusion rather than administering multiple infusions on the basis of reducing the chance of immunizing the recipient against disparate MHC?
The current approach of pairing a patient with cryopreserved T cells is based upon matching the recipient with the most closely matched MHC in the bank, with preference given to the cryopreserved product having the greatest number of shared MHC loci. In addition to typing, a bank's in vitro data should be queried so that priority is given to T-cell lines that indeed recognize the desired antigen through a restricting MHC molecule that is shared by the recipient.
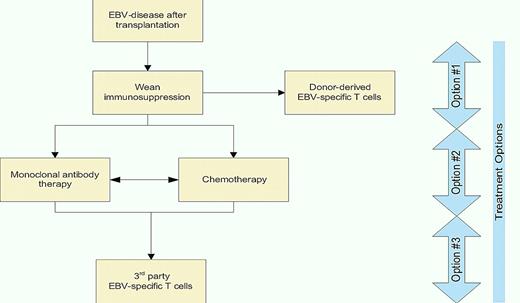
Off-the-shelf T-cell therapy is currently being used when other therapeutic options are exhausted. A decision tree (see figure) is offered that integrates immunotherapy with chemotherapy for the treatment of EBV-associated disease after solid organ and HSCT. Trials will be needed to compare third-party T cells with other treatment options. In this context, it is revealing to consider that the cost to generate and release clinical-grade T cells in facilities that operate in compliance with current good manufacturing practice continues to fall due to improved processing and economies of scale. Indeed, manufacturing and releasing a T-cell line with specificity for EBV costs approximately $6000 which may be used for multiple infusions.16 When one considers the cost of treating EBV-disease with rituximab (cumulatively $9000 per dose) or chemotherapy, there is considerable financial merit, in addition to scientific rationale, for infusing off-the-shelf EBV-specific T cells.
An algorithm for treating EBV-associated clinical disease after transplantation. Frontline treatment (Option #1) and second-line therapies (Option #2) are widely practiced. Option #3 is to be considered experimental, but encouraging, at this time.
An algorithm for treating EBV-associated clinical disease after transplantation. Frontline treatment (Option #1) and second-line therapies (Option #2) are widely practiced. Option #3 is to be considered experimental, but encouraging, at this time.
In summary, medical centers around the world are now stacking the shelves of their freezers with viral-specific T cells ready to be infused as third-party effectors. These off-the-self therapies are the first steps to transforming T cells as drugs that can be prepared a priori and infused on demand.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal