Abstract
In a recent phase 3 trial, bortezomib-melphalan-prednisone-thalidomide followed by maintenance treatment with bortezomib-thalidomide demonstrated superior efficacy compared with bortezomib-melphalan-prednisone. To decrease neurologic toxicities, the protocol was amended and patients in both arms received once-weekly instead of the initial twice-weekly bortezomib infusions: 372 patients received once-weekly and 139 twice-weekly bortezomib. In this post-hoc analysis we assessed the impact of the schedule change on clinical outcomes and safety. Long-term outcomes appeared similar: 3-year progression-free survival rate was 50% in the once-weekly and 47% in the twice-weekly group (P > .999), and 3-year overall survival rate was 88% and 89%, respectively (P = .54). The complete response rate was 30% in the once-weekly and 35% in the twice-weekly group (P = .27). Nonhematologic grade 3/4 adverse events were reported in 35% of once-weekly patients and 51% of twice-weekly patients (P = .003). The incidence of grade 3/4 peripheral neuropathy was 8% in the once-weekly and 28% in the twice-weekly group (P < .001); 5% of patients in the once-weekly and 15% in the twice-weekly group discontinued therapy because of peripheral neuropathy (P < .001). This improvement in safety did not appear to affect efficacy. This study is registered at http://www.clinicaltrials.gov as NCT01063179.
Introduction
In recent years, novel agents, including bortezomib, have changed the management of multiple myeloma (MM).1 In the large randomized phase 3 Velcade as Initial Standard Therapy in Multiple Myeloma (VISTA) trial in transplantation-ineligible patients with newly diagnosed MM, bortezomib-melphalan-prednisone (VMP) was shown to be significantly superior to melphalan-prednisone, a previous standard of care, in all end points assessed, including complete response (CR) rate, time to progression, and overall survival.2 This superiority was further demonstrated in a recent updated analysis with more than 3 years of follow-up.3 On the basis of the results of VISTA, VMP is considered a new standard of care as initial treatment for newly diagnosed patients with MM who were not eligible for high-dose therapy and autologous stem cell transplantation.2-5
Nearly one-half (46%) of patients who received VMP in VISTA experienced serious adverse events, and approximately one-third discontinued VMP treatment or discontinued only bortezomib because of adverse events.2 Peripheral neuropathy (PN) is a key iatrogenic toxicity in patients with MM, often leading to dose modification and drug discontinuation.6 In newly diagnosed patients, bortezomib-induced PN incidences of 47%-64% and rates of dose modification or drug discontinuation attributable to PN of 14%-30% have been reported.2,7,8
Bortezomib-induced PN is considered a secondary effect of proteasome inhibition,9-11 producing a primarily small fiber and painful, axonal, sensory distal neuropathy; motor neuropathy is rare.12 The mechanism underlying bortezomib-induced PN is unknown. Metabolic changes attributable to the accumulation of bortezomib in the dorsal root ganglia cells, mitochondrial-mediated dysregulation of Ca2+ homeostasis, and dysregulation of neurotrophins may contribute to the pathogenesis of PN.13 Bortezomib-induced PN also seems to be dependent on dose and treatment exposure. In both newly diagnosed and relapsed/refractory patients, bortezomib-induced PN symptoms improve or stabilize after stopping or decreasing the dose of bortezomib within a median of approximately 3-6 months in approximately 70% of patients.2,7,8,14,15
In the randomized, phase 3 GIMEMA (Gruppo Italiano Malattie Ematologiche dell'Adulto) trial in which the authors compared bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide (VMPT-VT) versus VMP, VMPT-VT was superior for response rate (CR rate of 38% vs 24%) and progression-free survival (PFS; 3-year estimated PFS rate of 56% vs 41%).16 In both arms patients initially were treated by the use of a twice-weekly bortezomib schedule. In March 2007, the protocol was amended to evaluate whether the treatment regimens could be further optimized by decreasing toxicity while maintaining efficacy; in both arms, bortezomib schedules were reduced from twice-weekly to once-weekly infusions. In this posthoc analysis, we assessed the impact of these schedule changes on clinical outcomes and safety, especially on the incidence and reversibility of bortezomib-induced PN.
Methods
Patients
The eligibility criteria for the GIMEMA phase 3 trial of VMPT-VT versus VMP have been previously reported.16 In brief, patients with newly diagnosed MM who were not eligible for high-dose therapy followed by stem cell transplantation because of age (≥ 65 years) or coexisting comorbidities and who had measurable disease17 and Karnofsky performance status ≥ 60% were eligible. Patients with renal insufficiency (creatinine > 2.5 mg/dL), uncontrolled or severe cardiovascular disease, psychiatric disease, any grade ≥ 2 PN, or any other malignancy within the past 5 years were excluded. The study was approved by the institutional review board at each of the 61 participating centers. All patients provided written informed consent before entering the study, which was performed in accordance with the Declaration of Helsinki. The study is registered at http://www.clinicaltrials.gov as NCT01063179.
Treatment and study design
The study design and randomization procedure for the VMPT-VT versus VMP phase 3 trial has been previously reported.16 Patients were randomized (1:1) to receive VMPT-VT or VMP therapy and received 9 6-week cycles of intravenous bortezomib 1.3 mg/m2 on days 1, 4, 8, 11, 22, 25, 29, and 32 of cycles 1-4, and days 1, 8, 22, and 29 of cycles 5–9, oral melphalan 9 mg/m2 on days 1-4, and oral prednisone 60 mg/m2 on days 1-4, alone or in combination with thalidomide 50 mg/day. After the last course of VMPT, patients in the VMPT-VT arm received maintenance therapy with bortezomib 1.3 mg/m2 every 14 days and thalidomide 50 mg/day for 2 years or until disease progression or relapse. After the first 139 patients were enrolled, both VMPT-VT and VMP induction schedules were changed to 9 5-week cycles, and the bortezomib dose was modified to 1.3 mg/m2 on days 1, 8, 15, and 22 (cycles 1-9). Treatment was withheld on withdrawal of patient consent, disease progression, or the occurrence of any grade 4 hematologic or grade 3/4 nonhematologic adverse events.
Assessment
PFS was calculated from the time of diagnosis until the date of progression, relapse, death from any cause, or the date the patient was last known to be in remission. Time to next therapy was calculated from the time of diagnosis until the date of subsequent myeloma therapy caused by progression or relapse, the date of death caused by progressive disease, or the date the patient was last known to be in remission. Overall survival was calculated from the time of diagnosis until the date of death or the date the patient was last known to be alive. Response to treatment was defined by use of the International Uniform Response Criteria.17
Evaluation and management of adverse events
All adverse events were assessed at each visit and graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 3.0).18 In patients with grade ≥ 2 PN, improvement, resolution, and time to recovery were assessed. Cumulative incidences of overall and grade ≥ 3 PN were determined in patient subgroups according to age (≤ 75 vs > 75 years), sex, type of myeloma therapy (with vs without concomitant thalidomide), creatinine clearance (≤ 60 mL/min vs > 60 mL/min), bortezomib schedule (once weekly vs twice weekly), International Staging System (ISS), cytogenetic risk (presence vs absence of t(4;14), t(4;16), or del17p by fluorescence in situ hybridization), history of diabetes, and cardiac disease. PN was managed by the use of established bortezomib dose-modification guidelines.14,15 Pharmacologic interventions were not protocol-specified, and patients who developed PN could receive various interventions, including vitamins and/or nutritional supplements, gabapentin, and nortriptyline; use of these concomitant medications was recorded.
Statistical analysis
Data cutoff was February 1, 2010. For this posthoc nonspecified analysis, patients receiving VMPT-VT or VMP were pooled together and stratified according to the once-weekly or twice-weekly infusion modality; analyses also were conducted for patients receiving VMP only to eliminate the influence of thalidomide use and the addition of maintenance therapy on efficacy and safety. Patients were analyzed on an intention-to-treat basis for all time-to-event end points. Response rates and safety were analyzed in patients who received at least one dose of study drug. Response rates and the incidence of any adverse event were compared by use of the χ2 test or Fisher exact test as appropriate. Survival data were analyzed by the Kaplan-Meier method,19 and treatment groups compared with the log-rank test. The Cox proportional hazard model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the intention-to-treat population.20 Continuous variables and time-to-event data were expressed as median plus interquartile range (IQR). The cumulative incidence of PN and its associations with patient characteristics were analyzed accounting for competing events (death from any cause and early drug discontinuation not caused by PN) by use of the method of Gooley et al.21 Differences between groups were assessed by the use of the Gray test,22 and the HRs were estimated by use of the Fine and Gray proportional-hazards model.23
Results
Patients and treatment
A total of 511 patients were enrolled; 139 patients (73 VMPT-VT, 66 VMP) received the twice-weekly bortezomib schedule, and 372 patients (181 VMPT-VT, 191 VMP) received the once-weekly schedule. Baseline demographic and disease characteristics were well balanced in the 2 groups (Table 1): the median age was 71 years, and approximately one-quarter of patients were older than 75 years of age. Eight patients, 3 (0.8%) in the once-weekly group and 5 (3.6%) in the twice-weekly group, were not treated as assigned because of withdrawal of consent (2 patients in each group), progressive disease (2 patients in the twice-weekly group), and physician choice (1 patient in each group).
Baseline patient demographics and disease characteristics
| Variable . | Once-weekly bortezomib (n = 372) . | Twice-weekly bortezomib (n = 139) . |
|---|---|---|
| Age | ||
| Median, y | 71 | 71 |
| IQR, y | 68-75 | 68-74 |
| Subgroup, n (%) | ||
| < 65 y | 15 (4) | 3 (2) |
| 65-74 y | 254 (68) | 102 (73) |
| ≥ 75 y | 103 (28) | 34 (24) |
| Male sex, n (%) | 176 (47) | 76 (55) |
| Karnofsky performance status ≤ 70%, n (%) | 105 (28) | 40 (29) |
| Serum β2-microglobulin level | ||
| Median, mg/L | 3.8 | 3.9 |
| IQR, mg/L | 2.8-5.4 | 3.0-5.5 |
| Subgroup, n (%) | ||
| ≤ 3.5 mg/L | 127 (43) | 50 (41) |
| > 3.5 mg/L | 170 (57) | 73 (59) |
| Data missing, n (%) | 75 (20) | 16 (12) |
| Albumin level | ||
| Median, g/dL | 3.8 | 3.7 |
| IQR, g/dL | 3.3-4.1 | 3.4-4.1 |
| Data missing, n (%) | 52 (14) | 14 (10) |
| International Staging System stage, n (%) | ||
| I | 80 (21) | 35 (25) |
| II | 133 (36) | 55 (40) |
| III | 73 (20) | 31 (22) |
| Data missing | 86 (23) | 18 (13) |
| Creatinine clearance (calculated), n (%) | ||
| < 30 mL/min | 37 (10) | 8 (6) |
| 30-60 mL/min | 219 (59) | 88 (63) |
| > 60 mL/min | 116 (31) | 43 (31) |
| Chromosome abnormalities, n (%) | ||
| del13 | 141/281 (50) | 46/95 (48) |
| t(4;14) | 39/281 (14) | 20/95 (21) |
| t(11;14) | 32/281 (11) | 19/95 (20) |
| t(14;16) | 13/281 (5) | 2/95 (2) |
| del17 | 42/281 (15) | 13/95 (14) |
| Baseline diabetes mellitus, n (%) | 24 (6) | 9 (6) |
| Baseline hyperglycemia, n (%) | 124 (33) | 51 (37) |
| Baseline cardiopathy, n (%) | 93 (25) | 42 (30) |
| Myeloma induction therapy, n (%) | ||
| VMPT-VT | 181 (49) | 73 (53) |
| VMP | 191 (51) | 66 (47) |
| Variable . | Once-weekly bortezomib (n = 372) . | Twice-weekly bortezomib (n = 139) . |
|---|---|---|
| Age | ||
| Median, y | 71 | 71 |
| IQR, y | 68-75 | 68-74 |
| Subgroup, n (%) | ||
| < 65 y | 15 (4) | 3 (2) |
| 65-74 y | 254 (68) | 102 (73) |
| ≥ 75 y | 103 (28) | 34 (24) |
| Male sex, n (%) | 176 (47) | 76 (55) |
| Karnofsky performance status ≤ 70%, n (%) | 105 (28) | 40 (29) |
| Serum β2-microglobulin level | ||
| Median, mg/L | 3.8 | 3.9 |
| IQR, mg/L | 2.8-5.4 | 3.0-5.5 |
| Subgroup, n (%) | ||
| ≤ 3.5 mg/L | 127 (43) | 50 (41) |
| > 3.5 mg/L | 170 (57) | 73 (59) |
| Data missing, n (%) | 75 (20) | 16 (12) |
| Albumin level | ||
| Median, g/dL | 3.8 | 3.7 |
| IQR, g/dL | 3.3-4.1 | 3.4-4.1 |
| Data missing, n (%) | 52 (14) | 14 (10) |
| International Staging System stage, n (%) | ||
| I | 80 (21) | 35 (25) |
| II | 133 (36) | 55 (40) |
| III | 73 (20) | 31 (22) |
| Data missing | 86 (23) | 18 (13) |
| Creatinine clearance (calculated), n (%) | ||
| < 30 mL/min | 37 (10) | 8 (6) |
| 30-60 mL/min | 219 (59) | 88 (63) |
| > 60 mL/min | 116 (31) | 43 (31) |
| Chromosome abnormalities, n (%) | ||
| del13 | 141/281 (50) | 46/95 (48) |
| t(4;14) | 39/281 (14) | 20/95 (21) |
| t(11;14) | 32/281 (11) | 19/95 (20) |
| t(14;16) | 13/281 (5) | 2/95 (2) |
| del17 | 42/281 (15) | 13/95 (14) |
| Baseline diabetes mellitus, n (%) | 24 (6) | 9 (6) |
| Baseline hyperglycemia, n (%) | 124 (33) | 51 (37) |
| Baseline cardiopathy, n (%) | 93 (25) | 42 (30) |
| Myeloma induction therapy, n (%) | ||
| VMPT-VT | 181 (49) | 73 (53) |
| VMP | 191 (51) | 66 (47) |
IQR indicates interquartile range; VMP, bortezomib-melphalan-prednisone; and VMPT-VT, bortezomib-melphalan-prednisone-thalidomide followed by maintenance therapy with bortezomib and thalidomide.
Efficacy
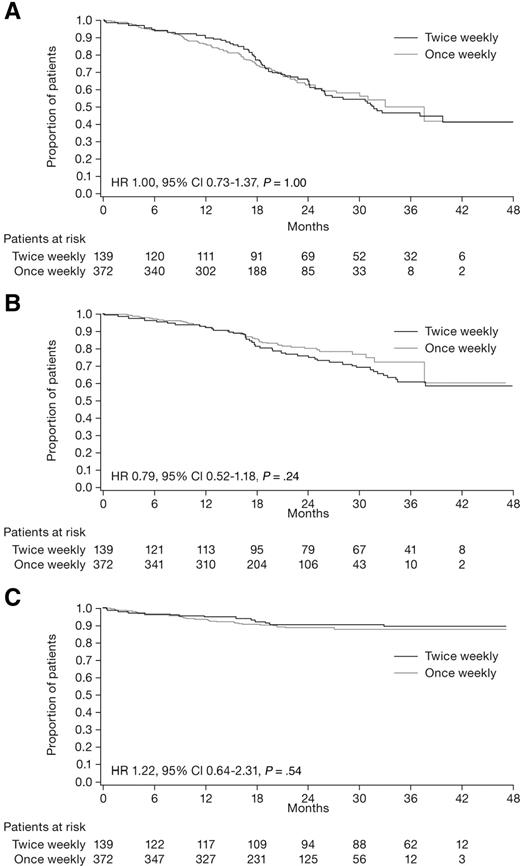
After a median follow-up of 23.2 months (IQR, 17.1-32.1) from time of diagnosis, median PFS was 33.1 months (IQR, 17.9 to not reached [NR]) in the once-weekly group and 31.7 months (IQR, 18.6 to NR) in the twice-weekly group. The 3-year PFS rate was 50% in patients receiving once-weekly bortezomib and 47% in those receiving twice-weekly bortezomib (HR = 1, 95% CI 0.73-1.37, P > .999; Figure 1A). The 3-year time-to-next-therapy rate was 72% in the once-weekly group and 61% in the twice-weekly group (HR = 0.79, 95% CI 0.52-1.18, P = .24; Figure 1B). At data cut-off, 51 patients had died; 38 (10%) in the once-weekly and 13 (9%) in the twice-weekly group. The 3-year overall survival rate was 88% in the once-weekly and 89% in the twice-weekly group (HR = 1.22, 95% CI 0.64-2.31, P = .54; Figure 1C).
Kaplan-Meier survival curves for patients who received once-weekly (gray line) or twice-weekly (black line) bortezomib (intention-to-treat population). (A) PFS. (B) Time to next therapy. (C) Overall survival.
Kaplan-Meier survival curves for patients who received once-weekly (gray line) or twice-weekly (black line) bortezomib (intention-to-treat population). (A) PFS. (B) Time to next therapy. (C) Overall survival.
In the analysis restricted to VMP patients, the median PFS was 27.3 months (IQR, 17.4 to NR) in the once-weekly group and 26.0 months (IQR, 17.7 to NR) in the twice-weekly group. The respective 3-year PFS rates were 46% and 39% (HR = 0.96, 95% CI 0.64-1.46, P = .86), and the respective 3-year OS rates were 87% and 89% (HR = 1.41, 95% CI 0.56-3.56, P = .47).
There were no significant differences in the rates of CR, very good partial response (VGPR), or partial response (PR) to induction therapy, or in time to response and response duration between the 2 treatment groups (Table 2). The overall response rate (≥ PR) was 85% in the once-weekly group and 86% in the twice-weekly group (P = .78), which included CR rates of 30% and 35% (P = .27), and ≥ VGPR rates of 55% and 54% (P = .84), respectively. In the analysis restricted to VMP patients, the overall response rate (≥ PR) was 79% in the once-weekly group and 86% in the twice-weekly group (P = .27), which included CR rates of 23% and 27% (P = .54), and ≥ VGPR rates of 49% and 52% (P = .64), respectively.
Best response to treatment and time-to-event data
| . | Once-weekly bortezomib (n = 369) . | Twice-weekly bortezomib (n = 134) . | P . |
|---|---|---|---|
| Best response by International Uniform Response Criteria, n (%) | |||
| Overall response rate | 312 (85) | 115 (86) | .78 |
| Complete response | 109 (30) | 47 (35) | .27 |
| Very good partial response | 93 (25) | 25 (19) | .15 |
| Partial response | 110 (30) | 43 (32) | .66 |
| Stable disease | 47 (13) | 12 (9) | .27 |
| Progressive disease | 4 (1) | 1 (1) | .61 |
| Time-to-event data | |||
| Median time to response, mo (IQR) | |||
| Partial response | 1.1 (1.1-2.3) | 1.4 (1.4-2.8) | .57 |
| Complete response | 5.7 (3.4-8.0) | 5.5 (2.8-6.9) | .40 |
| Duration of response | |||
| Complete or partial response | |||
| Median, months (IQR) | NR (18.1-NR) | 28.4 (17.0-NR) | .50 |
| Patients in remission at 2 y, n (%) | 52 (65) | 50 (57) | .50 |
| Complete response | |||
| Median, mo (IQR) | NR (27.5-NR) | NR (22.2-NR) | .79 |
| Patients in remission at 2 y, n (%) | 26 (84) | 28 (73) | .79 |
| . | Once-weekly bortezomib (n = 369) . | Twice-weekly bortezomib (n = 134) . | P . |
|---|---|---|---|
| Best response by International Uniform Response Criteria, n (%) | |||
| Overall response rate | 312 (85) | 115 (86) | .78 |
| Complete response | 109 (30) | 47 (35) | .27 |
| Very good partial response | 93 (25) | 25 (19) | .15 |
| Partial response | 110 (30) | 43 (32) | .66 |
| Stable disease | 47 (13) | 12 (9) | .27 |
| Progressive disease | 4 (1) | 1 (1) | .61 |
| Time-to-event data | |||
| Median time to response, mo (IQR) | |||
| Partial response | 1.1 (1.1-2.3) | 1.4 (1.4-2.8) | .57 |
| Complete response | 5.7 (3.4-8.0) | 5.5 (2.8-6.9) | .40 |
| Duration of response | |||
| Complete or partial response | |||
| Median, months (IQR) | NR (18.1-NR) | 28.4 (17.0-NR) | .50 |
| Patients in remission at 2 y, n (%) | 52 (65) | 50 (57) | .50 |
| Complete response | |||
| Median, mo (IQR) | NR (27.5-NR) | NR (22.2-NR) | .79 |
| Patients in remission at 2 y, n (%) | 26 (84) | 28 (73) | .79 |
IQR indicates interquartile range; and NR, not reached.
Treatment exposure and adverse events
In both groups, patients received a median of 9 treatment cycles. Patients received a median cumulative bortezomib dose of 39.4 mg/m2 in the once-weekly group and 40.1 mg/m2 in the twice-weekly group, corresponding to 84% and 59%, respectively, of the planned total dose of bortezomib. One hundred forty-four patients (39%) in the once-weekly group and 17 patients (13%) in the twice-weekly group received more than 90% of the planned bortezomib dose (P < .001; Table 3).
Bortezomib treatment exposure and grade 3/4 adverse events reported during treatment
| . | Once-weekly bortezomib (n = 369) . | Twice-weekly bortezomib (n = 134) . | P . |
|---|---|---|---|
| Bortezomib exposure | |||
| Cumulative planned dose, mg/m2 | 46.8 | 67.6 | |
| Median cumulative dose delivered, mg/m2 (IQR) | 39.4 (22.2-45.5) | 40.1 (26.3-52.0) | .65 |
| Percentage of planned dose delivered, % | 84 | 59 | |
| Patients who received ≥ 90% of planned dose, n (%) | 144 (39) | 17 (13) | < .001 |
| Adverse events, n (%) | |||
| Hematologic events | 161 (44) | 60 (45) | .83 |
| Neutropenia | 120 (33) | 46 (34) | .74 |
| Thrombocytopenia | 69 (19) | 35 (26) | .08 |
| Anemia | 38 (10) | 12 (9) | .74 |
| Nonhematologic events | 131 (35) | 68 (51) | .003 |
| Infections | 41 (11) | 14 (10) | > .999 |
| Cardiac events | 31 (8) | 9 (7) | .70 |
| Neuropathy | 30 (8) | 38 (28) | < .001 |
| Sensory | 11 (3) | 22 (16) | < .001 |
| Neuralgia | 10 (3) | 6 (5) | .25 |
| Sensory and neuralgia | 9 (3) | 10 (8) | .01 |
| Gastrointestinal events | 22 (6) | 15 (11) | .08 |
| Systemic events | 14 (4) | 10 (7) | .09 |
| Vascular events | 13 (4) | 5 (4) | > .999 |
| Deep-vein thrombosis/pulmonary embolism | 12 (3) | 5 (4) | .78 |
| Dermatologic events | 6 (2) | 9 (7) | .006 |
| Bleeding | 1 (< 1) | 1 (< 1) | .46 |
| Other conditions | 22 (6) | 5 (4) | .66 |
| . | Once-weekly bortezomib (n = 369) . | Twice-weekly bortezomib (n = 134) . | P . |
|---|---|---|---|
| Bortezomib exposure | |||
| Cumulative planned dose, mg/m2 | 46.8 | 67.6 | |
| Median cumulative dose delivered, mg/m2 (IQR) | 39.4 (22.2-45.5) | 40.1 (26.3-52.0) | .65 |
| Percentage of planned dose delivered, % | 84 | 59 | |
| Patients who received ≥ 90% of planned dose, n (%) | 144 (39) | 17 (13) | < .001 |
| Adverse events, n (%) | |||
| Hematologic events | 161 (44) | 60 (45) | .83 |
| Neutropenia | 120 (33) | 46 (34) | .74 |
| Thrombocytopenia | 69 (19) | 35 (26) | .08 |
| Anemia | 38 (10) | 12 (9) | .74 |
| Nonhematologic events | 131 (35) | 68 (51) | .003 |
| Infections | 41 (11) | 14 (10) | > .999 |
| Cardiac events | 31 (8) | 9 (7) | .70 |
| Neuropathy | 30 (8) | 38 (28) | < .001 |
| Sensory | 11 (3) | 22 (16) | < .001 |
| Neuralgia | 10 (3) | 6 (5) | .25 |
| Sensory and neuralgia | 9 (3) | 10 (8) | .01 |
| Gastrointestinal events | 22 (6) | 15 (11) | .08 |
| Systemic events | 14 (4) | 10 (7) | .09 |
| Vascular events | 13 (4) | 5 (4) | > .999 |
| Deep-vein thrombosis/pulmonary embolism | 12 (3) | 5 (4) | .78 |
| Dermatologic events | 6 (2) | 9 (7) | .006 |
| Bleeding | 1 (< 1) | 1 (< 1) | .46 |
| Other conditions | 22 (6) | 5 (4) | .66 |
IQR indicates interquartile range.
There was no difference in the rate of treatment-related deaths between the 2 groups; 14 patients (4%) in the once-weekly group and 2 (1%) in the twice-weekly group (P = .26) had died by data cut-off. The most common causes of death were cardiac, pulmonary, and infection events.
The incidence of any grade 3/4 hematologic toxicity (Table 3) was similar in the 2 groups (44% vs 45% in once- and twice-weekly patients, respectively; P = .83), but severe thrombocytopenia was slightly less common in the once-weekly patients (19% vs 26%, P = .08). The incidence of nonhematologic grade 3/4 adverse events was significantly reduced in the once-weekly versus twice-weekly group; 131 patients (35%) in the once-weekly group versus 68 (51%) in the twice-weekly group (P = .003). Grade 3/4 gastrointestinal events, primarily diarrhea, constipation, nausea, and vomiting, were less frequent in the once-weekly versus twice-weekly group (6% vs 11%, P = .08). Severe systemic events, which consisted of fatigue and fever, were also slightly less frequent in the once-weekly versus twice-weekly group (4% vs 7%, P = .09). Grade 3/4 dermatologic events were rare and less frequent in patients receiving once-weekly versus twice-weekly bortezomib (2% vs 7%, P = .006).
There was a significantly reduced overall incidence of grade 3/4 PN (8% vs 28%, P < .001) in the once-weekly versus twice-weekly group. PN was predominantly sensory. Among 82 VMPT-VT patients who received at least 6 months of maintenance therapy with bortezomib twice-monthly and thalidomide, only 3 developed de novo grade 3 PN, and no grade 4 PN was reported. The incidence of PN thus did not substantially increase during maintenance therapy. Patients who received maintenance therapy were distributed approximately equally between the once-weekly and twice-weekly groups, per protocol; thus, there was no imbalance affecting the comparison of PN incidence between groups. Illustrating this, similar differences between groups were observed in the analysis restricted to VMP patients only, which also eliminated the contribution of thalidomide treatment to the incidence of PN. The incidence of grade 3/4 PN (7% vs 29%, P < .001) was lower in the once-weekly versus twice-weekly VMP group.
In total, 5% of the once-weekly patients and 15% of the twice-weekly patients did not complete the assigned treatment schedule because of PN (P < .001, Table 4). The proportion of patients who required dose reductions because of PN was also lower in the once-weekly (17%) than the twice-weekly group (41%; P < .001). In the analysis restricted to VMP patients only, 4% of the once-weekly patients and 16% of the twice-weekly patients discontinued due to PN (P = .002).
Features of peripheral neuropathy
| . | Once-weekly bortezomib (n = 369) . | Twice-weekly bortezomib (n = 134) . | P . |
|---|---|---|---|
| Cumulative proportion of patients with PN at 18 mo (95% CI) | |||
| Any grade | 0.40 (0.35-0.45) | 0.72 (0.64-0.79) | < .001 |
| Sensory neuropathy | 0.27 (0.22-0.32) | 0.46 (0.37-0.54) | < .001 |
| Neuralgia | 0.08 (0.05-0.10) | 0.13 (0.07-0.19) | .058 |
| Sensory and neuralgia | 0.06 (0.03-0.08) | 0.13 (0.07-0.19) | .008 |
| Grade 3/4 | 0.09 (0.06-0.12) | 0.36 (0.26-0.45) | < .001 |
| Sensory neuropathy | 0.04 (0.01-0.06) | 0.21 (0.13-0.29) | < .001 |
| Neuralgia | 0.03 (0.01-0.05) | 0.05 (0.01-0.10) | .234 |
| Sensory and neuralgia | 0.03 (0.01-0.04) | 0.09 (0.04-0.15) | .006 |
| Bortezomib dose modification caused by PN | |||
| Dose reduction, n (%) | 63 (17) | 55 (41) | < .001 |
| Drug discontinuation, n (%) | 20 (5) | 20 (15) | < .001 |
| Median time to dose reduction, mo (IQR) | 3.8 (1.8-5.9) | 2.8 (1.6-4.8) | .08 |
| Median time to discontinuation, mo (IQR) | 7.6 (5.7-9.6) | 7.0 (4.7-11.1) | .91 |
| Outcome of grade 2-4 PN | n = 77 | n = 73 | |
| Resolution, n (%) | 26 (34) | 29 (40) | .74 |
| Improvement, n (%) | 23 (30) | 19 (26) | |
| Persistence, n (%) | 28 (36) | 25 (34) | |
| Median time to recovery, mo (IQR) | 2.3 (1.1-4.0) | 3.2 (2.1-4.8) | .005 |
| . | Once-weekly bortezomib (n = 369) . | Twice-weekly bortezomib (n = 134) . | P . |
|---|---|---|---|
| Cumulative proportion of patients with PN at 18 mo (95% CI) | |||
| Any grade | 0.40 (0.35-0.45) | 0.72 (0.64-0.79) | < .001 |
| Sensory neuropathy | 0.27 (0.22-0.32) | 0.46 (0.37-0.54) | < .001 |
| Neuralgia | 0.08 (0.05-0.10) | 0.13 (0.07-0.19) | .058 |
| Sensory and neuralgia | 0.06 (0.03-0.08) | 0.13 (0.07-0.19) | .008 |
| Grade 3/4 | 0.09 (0.06-0.12) | 0.36 (0.26-0.45) | < .001 |
| Sensory neuropathy | 0.04 (0.01-0.06) | 0.21 (0.13-0.29) | < .001 |
| Neuralgia | 0.03 (0.01-0.05) | 0.05 (0.01-0.10) | .234 |
| Sensory and neuralgia | 0.03 (0.01-0.04) | 0.09 (0.04-0.15) | .006 |
| Bortezomib dose modification caused by PN | |||
| Dose reduction, n (%) | 63 (17) | 55 (41) | < .001 |
| Drug discontinuation, n (%) | 20 (5) | 20 (15) | < .001 |
| Median time to dose reduction, mo (IQR) | 3.8 (1.8-5.9) | 2.8 (1.6-4.8) | .08 |
| Median time to discontinuation, mo (IQR) | 7.6 (5.7-9.6) | 7.0 (4.7-11.1) | .91 |
| Outcome of grade 2-4 PN | n = 77 | n = 73 | |
| Resolution, n (%) | 26 (34) | 29 (40) | .74 |
| Improvement, n (%) | 23 (30) | 19 (26) | |
| Persistence, n (%) | 28 (36) | 25 (34) | |
| Median time to recovery, mo (IQR) | 2.3 (1.1-4.0) | 3.2 (2.1-4.8) | .005 |
CI indicates confidence interval; IQR, interquartile range; and PN, peripheral neuropathy.
Onset and reversibility of PN
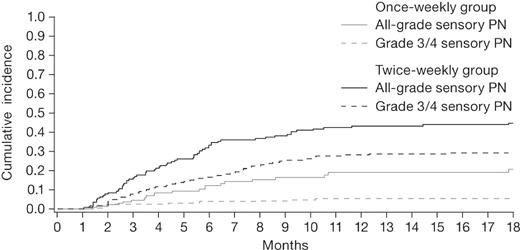
The median time to onset of sensory PN was 4.4 months (IQR, 2.6-6.7) in the once-weekly group and 3.5 months (IQR, 2.4-6.9) in the twice-weekly group (P = .61). The median time to onset of grade 3/4 sensory PN was 4.3 months (IQR, 3.4-7.4) in the once-weekly group and 3.2 months (IQR, 1.9-5.1) in the twice-weekly group (P = .10). The cumulative incidence of sensory PN appeared to plateau after 12 months of therapy in both groups (Figure 2). At 18 months, the cumulative incidence of any-grade sensory PN was 27% in the once-weekly group and 46% in the twice-weekly group (P < .001), including 4% and 21% grade 3/4 sensory PN, respectively (Table 4).
Cumulative incidence of sensory PN, accounting for competing events (death and any other PN type) in patients who received once-weekly or twice-weekly bortezomib. The cumulative incidence of PN rate increased over time, reaching a plateau after 12 months of therapy.
Cumulative incidence of sensory PN, accounting for competing events (death and any other PN type) in patients who received once-weekly or twice-weekly bortezomib. The cumulative incidence of PN rate increased over time, reaching a plateau after 12 months of therapy.
In the once-weekly group, among 77 patients with grade ≥ 2 PN, 49 (64%) experienced improvement or resolution by data cut-off. Similarly, among 73 patients with grade ≥ 2 PN in the twice-weekly group, 48 (66%) experienced improvement or resolution by data cut-off. Median time to improvement or resolution was 2.3 months (IQR, 1.1-4.0) in the once-weekly group and 3.2 months (IQR, 2.1-4.8) in the twice-weekly group (P = .005; Table 4).
Factors associated with PN incidence and grade
PN incidence and grade were not associated with age (≤ 75 years vs > 75 years), sex, creatinine clearance (≤ 60 mL/min vs > 60 mL/min), ISS stage, high-risk cytogenetics, that is, t(4;14) or t(14;16) or del17p, history of diabetes, or cardiac disease (Table 5). Concomitant thalidomide correlated with an increased incidence of any-grade PN (HR 1.32, 95% CI 1.03-1.70) but not with grade 3/4 PN (HR 1.24, 95% CI 0.74-2.06, P = .41). Once-weekly bortezomib was the only factor associated with a reduced incidence of any-grade PN (HR 0.42, 95% CI 0.32-0.54, P < .001) and grade 3/4 PN (HR 0.24, (95% CI 0.15-0.39, P < .001). Patients received various interventions for PN, including gabapentin, vitamins, nutritional supplements, or nortriptyline, but insufficient data were available for analysis of the impact of these interventions on the incidence or severity of PN.
Association between patient characteristics and cumulative incidence of peripheral neuropathy
| Variable . | Any grade PN . | Grade 3/4 PN . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Thalidomide | 1.32 | 1.03-1.7 | .029 | 1.24 | 0.74-2.06 | .41 |
| Once-weekly bortezomib | 0.42 | 0.32-0.54 | < .001 | 0.24 | 0.15-0.39 | < .001 |
| Age > 75 y | 0.98 | 0.96-1.01 | .162 | 1.01 | 0.96-1.06 | .744 |
| Male | 0.95 | 0.73-1.24 | .721 | 1.21 | 0.72-2.04 | .469 |
| Creatinine clearance ≤ 60 mL/min | 0.94 | 0.7-1.26 | .658 | 0.89 | 0.51-1.56 | .688 |
| ISS stage | ||||||
| I | 1 | 1 | ||||
| II | 0.75 | 0.55-1.01 | .061 | 0.66 | 0.37-1.18 | .164 |
| III | 0.85 | 0.57-1.27 | .429 | 0.77 | 0.37-1.6 | .483 |
| Cytogenetic risk | ||||||
| Standard | 1 | 1 | ||||
| High* | 1.05 | 0.74-1.48 | .793 | 1.45 | 0.79-2.65 | .228 |
| Diabetes | 0.86 | 0.52-1.41 | .545 | 0.46 | 0.11-1.97 | .293 |
| Cardiopathy | 0.93 | 0.7-1.23 | .601 | 0.75 | 0.41-1.37 | .351 |
| Variable . | Any grade PN . | Grade 3/4 PN . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Thalidomide | 1.32 | 1.03-1.7 | .029 | 1.24 | 0.74-2.06 | .41 |
| Once-weekly bortezomib | 0.42 | 0.32-0.54 | < .001 | 0.24 | 0.15-0.39 | < .001 |
| Age > 75 y | 0.98 | 0.96-1.01 | .162 | 1.01 | 0.96-1.06 | .744 |
| Male | 0.95 | 0.73-1.24 | .721 | 1.21 | 0.72-2.04 | .469 |
| Creatinine clearance ≤ 60 mL/min | 0.94 | 0.7-1.26 | .658 | 0.89 | 0.51-1.56 | .688 |
| ISS stage | ||||||
| I | 1 | 1 | ||||
| II | 0.75 | 0.55-1.01 | .061 | 0.66 | 0.37-1.18 | .164 |
| III | 0.85 | 0.57-1.27 | .429 | 0.77 | 0.37-1.6 | .483 |
| Cytogenetic risk | ||||||
| Standard | 1 | 1 | ||||
| High* | 1.05 | 0.74-1.48 | .793 | 1.45 | 0.79-2.65 | .228 |
| Diabetes | 0.86 | 0.52-1.41 | .545 | 0.46 | 0.11-1.97 | .293 |
| Cardiopathy | 0.93 | 0.7-1.23 | .601 | 0.75 | 0.41-1.37 | .351 |
Analyses performed accounting for competing events (Fine and Gray model).
CI indicates confidence interval; HR, hazard ratio; ISS, International Staging System.
High-risk cytogenetic profile was defined as the presence of a t(4;14) or t(14;16) translocation or a 17p deletion on the basis of fluorescence in situ hybridization performed at a centralized laboratory.
Discussion
These results demonstrate that both once-weekly and twice-weekly schedules of bortezomib in combination with melphalan-prednisone ± thalidomide are highly active regimens in newly diagnosed patients with MM who are ineligible for autologous stem cell transplantation. Within the limitations of this nonrandomized post-hoc analysis, once-weekly bortezomib seems to be equally effective and better tolerated than the standard twice-weekly schedule. The outcomes and response rate did not appear to be affected by the bortezomib dosing schedule. Durability of response was similar between groups: median PFS was 33.1 months in the once-weekly group and 31.7 months in the twice-weekly group. Overall response rates were 85% and 86%, including CR rates of 30% and 35%, in the once-weekly and twice-weekly groups, respectively. Similarly, the rapidity of response, which is important to rapidly reduce symptoms, and in particular pain, was comparable between the 2 groups. These results do not appear to be the result of differences in prognostic profile between the 2 groups; the proportions of patients older than 75 years or who had high cytogenetic risk or high ISS stage were similar in the once- and twice-weekly groups.
Similar outcomes and response rates were seen with the once-weekly and twice-weekly bortezomib schedules in our analyses restricted to patients receiving VMP induction, including 3-year PFS rates of 46% and 39%, respectively, and CR rates of 23% and 27%, respectively. Although comparisons between studies should always be interpreted with caution, our efficacy data appear comparable with those from other studies of VMP conducted in similar populations of previously untreated, elderly, transplant-ineligible MM patients (Table 6).2,3,7,24 Time-to-event data with VMP therapy appeared similar between studies, with median PFS or time to next therapy of approximately 2.2-3 years; these findings suggest that our use of once-weekly bortezomib within a VMP regimen did not adversely affect the established long-term outcome benefit of this regimen.3,24 In addition, overall response rates and CR rates appeared generally similar between studies2,24 ; notably, CR rates substantially increased with bortezomib plus thalidomide or prednisone maintenance in the GEM05 > 65 study reported by Mateos et al,24 perhaps reflecting the grater CR rate found with VMPT-VT in the primary analysis of our study.16
Comparison between efficacy and safety data reported for different VMP regimens
| Parameter . | This study . | GEM05>6524 . | VISTA2,3,7 . | |
|---|---|---|---|---|
| VMP (9 once-weekly cycles)* . | VMP (4/5 twice-/once-weekly cycles)† . | VMP (1/5 twice-/once-weekly cycles)‡ . | VMP (4/5 twice-/once-weekly cycles)† . | |
| n | 191 | 66 | 130 | 344 |
| Median age, y (range) | 71 (56-86) | 72 (65-85) | 73 (65-83) | 71 (57-90) |
| Overall response rate (≥PR), % | 79# | 86# | 80 (98 on maintenance) | 71§/74# |
| CR rate, % | 23 | 27 | 20 (44 on maintenance) | 30/33 |
| CR/nCR rate, % | NA | NA | 32 (59 on maintenance) | NR |
| Median follow-up, mo (range) | 20.9 (0.3-48.6) | 35.3 (0.1-48.0) | 24 (12-43) | 36.7 (NR) |
| Median PFS, mo | 27 | 26 | 34 | 22 |
| 3-y PFS rate, % | 46 | 39 | NR | NR |
| 3-y OS rate, % | 87 | 89 | 80 | 68.5 |
| Median no. cycles, n | 9 | 9 | NR | 9 |
| Sensory PN (any grade), % | 22 | 44 | NR | 44 |
| Grade 3/4, % | 2 | 14 | 5 (2/5 on VP/VT maintenance) | 13 |
| Discontinuation caused by PN, % | 4 | 16 | NR | 15 (3.2 VMP, 11.8 V only) |
| Bortezomib dose reductions caused by PN, % | 14 | 40 | NR | 22 |
| Bortezomib planned dose intensity, mg/m2/wk | 1.04 | 1.73 (cycles 1-4) | 1.73 (cycle 1) | 1.73 (cycles 1-4) |
| 0.87 (cycles 5-9) | 1.04 (cycles 2-6) | 0.87 (cycles 5-9) | ||
| Parameter . | This study . | GEM05>6524 . | VISTA2,3,7 . | |
|---|---|---|---|---|
| VMP (9 once-weekly cycles)* . | VMP (4/5 twice-/once-weekly cycles)† . | VMP (1/5 twice-/once-weekly cycles)‡ . | VMP (4/5 twice-/once-weekly cycles)† . | |
| n | 191 | 66 | 130 | 344 |
| Median age, y (range) | 71 (56-86) | 72 (65-85) | 73 (65-83) | 71 (57-90) |
| Overall response rate (≥PR), % | 79# | 86# | 80 (98 on maintenance) | 71§/74# |
| CR rate, % | 23 | 27 | 20 (44 on maintenance) | 30/33 |
| CR/nCR rate, % | NA | NA | 32 (59 on maintenance) | NR |
| Median follow-up, mo (range) | 20.9 (0.3-48.6) | 35.3 (0.1-48.0) | 24 (12-43) | 36.7 (NR) |
| Median PFS, mo | 27 | 26 | 34 | 22 |
| 3-y PFS rate, % | 46 | 39 | NR | NR |
| 3-y OS rate, % | 87 | 89 | 80 | 68.5 |
| Median no. cycles, n | 9 | 9 | NR | 9 |
| Sensory PN (any grade), % | 22 | 44 | NR | 44 |
| Grade 3/4, % | 2 | 14 | 5 (2/5 on VP/VT maintenance) | 13 |
| Discontinuation caused by PN, % | 4 | 16 | NR | 15 (3.2 VMP, 11.8 V only) |
| Bortezomib dose reductions caused by PN, % | 14 | 40 | NR | 22 |
| Bortezomib planned dose intensity, mg/m2/wk | 1.04 | 1.73 (cycles 1-4) | 1.73 (cycle 1) | 1.73 (cycles 1-4) |
| 0.87 (cycles 5-9) | 1.04 (cycles 2-6) | 0.87 (cycles 5-9) | ||
CR indicates complete response; NA, not applicable; nCR, near-complete response; NR, not reported; OS, overall survival; PFS, progression-free survival; PN, peripheral neuropathy; PR, partial response; TNT, time to next therapy; VMP, bortezomib, melphalan, prednisone; VP, bortezomib plus prednisone; and VT, bortezomib plus thalidomide.
Bortezomib 1.3 mg/m2 on days 1, 8, 15, and 22 for 9 5-week cycles.
Bortezomib 1.3 mg/m2 on days 1, 4, 8, 11, 22, 25, 29, and 32 for 4 6-week cycles, and on days 1, 8, 22, and 29 for 56-week cycles.
Bortezomib 1.3 mg/m2 on days 1, 4, 8, 11, 22, 25, 29, and 32 for 1 6-week cycle, and on days 1, 8, 15, and 22 for 55-week cycles; followed by maintenance including bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11 every 3 months, for up to 3 years.
International Myeloma Working Group uniform criteria.17
European Group for Blood and Marrow Transplantation criteria.
Although bortezomib- and/or thalidomide-based combinations have shown good efficacy in newly diagnosed and relapsed patients with MM in several trials,2,25-30 high rates of adverse events have been reported, and modification of the dosing schedule could reduce the incidence of severe adverse events, especially in elderly patients. Our analysis showed that the use of once-weekly versus twice-weekly bortezomib in combination with melphalan-prednisone ± thalidomide was associated with a significantly improved safety profile. Although the overall rate of grade 3/4 hematologic adverse events was similar between the once-weekly and twice-weekly groups, thrombocytopenia, a major side effect of bortezomib, was slightly less frequent in the once-weekly patients. Similarly, the rate of grade 3/4 nonhematologic adverse events was lower in the once-weekly versus twice-weekly group, with gastrointestinal, systemic, and dermatologic events all less frequent with once-weekly bortezomib. As expected, the main dose-limiting toxicity was PN, which was predominantly sensory. The incidence was significantly reduced in the once-weekly group; cumulative incidence of any-grade sensory PN at 18 months was 27% in the once-weekly group and 46% in the twice-weekly group, including 4% and 21% grade 3/4 sensory PN, respectively. Rates of discontinuations (5% vs 15%) and dose reductions (17% vs 41%) as the result of PN were also significantly lower in the once-weekly group.
These differences in the incidence of PN and dose modifications caused by PN were reflected in our analysis restricted to VMP patients, in which rates of sensory PN were 22% versus 44%, including 2% versus 14% grade 3/4 events, with once-weekly versus twice-weekly bortezomib. Our findings in the twice-weekly group were consistent with the incidence of PN and discontinuation caused by PN reported in the VISTA study (Table 6).2,7 In comparison, the rate of grade 3/4 PN with VMP in the GEM05 > 65 study, in which one cycle of twice-weekly bortezomib was followed by once-weekly dosing, was 5%24 ; the differences between the rates of grade 3/4 PN between these studies of VMP in similar populations of elderly MM patients may therefore be associated with the different numbers of treatment cycles of twice-weekly bortezomib and the consequent differences in bortezomib dose intensity.
As noted, rates of discontinuations and bortezomib dose reductions were significantly lower in the once-weekly versus twice-weekly group, which explains why the median percentage of the planned dose that was actually delivered was greater (84% vs 59%) in the once-weekly group. These data also explain why, despite the cumulative planned dose being lower in the once-weekly group (46.8 mg/m2 vs 67.6 mg/m2), the delivered cumulative dose of bortezomib was similar in the 2 groups (39.4 mg/m2 in the once-weekly and 40.1 mg/m2 in the twice-weekly group) and thus the efficacy was also similar between groups. However, because the same cumulative bortezomib dose was delivered over the course of a longer period in the once-weekly group, the dose intensity was lower and the safety profile was improved, notably regarding PN. Our analysis showed that in both the once-weekly and twice-weekly groups, the onset of PN increased over time, reaching a plateau after approximately 12 months of therapy, beyond which the risk of PN did not increase. This finding is in agreement with the results of previous bortezomib studies7,14,15 in which the incidence of bortezomib-induced PN reached a plateau at a cumulative dose of 42-45 mg/m2 in relapsed and newly diagnosed MM patients. In our study, this cumulative dose plateau for PN risk was achieved via a longer, less intensive treatment schedule, thereby lowering the overall risk of PN onset (Figure 2). Importantly, when this approach is used, our findings suggest that bortezomib can subsequently be used for maintenance therapy without any further substantial increase in the risk of PN, which appears in contrast to thalidomide-associated PN for which the risk may continue to increase over time on treatment.31
In this study, age (> 75 years), sex, renal function (creatinine clearance ≤ 60 mL/min), poor prognostic disease characteristics (ISS stage and high-risk cytogenetics), a history of diabetes, or cardiac disease did not significantly affect the incidence and severity of PN. This finding is consistent with previous observations demonstrating no association of bortezomib-induced PN with age or other baseline characteristics.7,14,15 Concomitant therapy with the neurotoxic agent thalidomide increased the risk of any-grade PN, but not the risk of grade 3/4 PN, in our analysis; of note, in the GEM05 > 65 study, Mateos et al24 reported rates of grade 3/4 PN of 5% and 9% in the VMP and VTP arms, respectively, although this difference was not significant. In this regard, the use of lenalidomide, which is associated with a lower risk of PN, in place of thalidomide may represent an interesting alternative; in phase 3 studies of thalidomide plus melphalan–prednisone, the rate of grade 3/4 PN was 2%-8%,25-27 whereas no grade 3/4 PN has been seen in studies of lenalidomide plus melphalan-prednisone.32,33 Lenalidomide in combination with VMP may result in an elevated hematologic toxicity profile, whereas in combination with bortezomib without chemotherapeutic agents it has a good safety profile with a severe PN incidence of 2%.34
The twice-weekly infusion of bortezomib was the only strong risk factor for any-grade PN and grade 3/4 PN. These findings support the hypothesis that bortezomib-induced PN is mechanistically distinct from thalidomide-associated and other forms of PN. Prior or concomitant exposure to other neurotoxic MM agents or history of diabetes should not exclude patients from receiving bortezomib therapy, provided baseline symptoms of sensory PN do not interfere with function or daily activities (grade 1 PN). Patients with baseline grade ≥ 2 PN were excluded from our study, and in the clinic patients with symptoms interfering with function or daily activities, with pain or motor PN, should be excluded from bortezomib therapy.
The management of bortezomib-induced PN with adequate dose reductions results in a high proportion of improvement or resolution of symptoms.2,7,8,15,35 In our analysis, grade ≥ 2 PN resolved or improved in approximately two-thirds of patients in both groups after a median of 2-3 months, as seen in the VISTA trial.7 In the management of bortezomib-induced PN, prompt action and providing patients with information is mandatory; informed patients can immediately signal the worsening of symptoms, whereas uninformed patients may only report them much later. Typically, sensory PN in the extremities, such as hypoesthesia (numbness), paresthesias (tingling, pinprick sensation), and hyperesthesia in the toes and fingers are the most common presenting symptoms8,12-14 and require no further action. The occurrence of symptoms interfering with functions or daily activity, or pain, or motor PN such as muscle cramps, tremor, or loss of strength in distal muscles, require immediate withholding of treatment. Once toxicities resolve to sensory PN without loss of function, therapy should be reinitiated, but a 50% bortezomib dose reduction is recommended.
In conclusion, our findings suggest that the once-weekly infusion of bortezomib in combination with melphalan-prednisone ± thalidomide is a valuable treatment schedule for newly diagnosed patients at least 65 years of age. Initial twice weekly bortezomib followed by a rapid reduction to a once-weekly schedule may be suggested in selected patients with clinically aggressive disease, such as those with incipient renal failure or extensive pain. The once-weekly schedule significantly reduced the incidence of PN and decreased the rate of discontinuation compared with the twice-weekly schedule, resulting in similar cumulative bortezomib doses in the 2 groups. The improvement in the safety profile was not associated with any reduction in the efficacy of the regimen.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients who agreed to participate in this study, the nurses, in particular Elena Ponticelli and Maria Marcella Lionetti, and the data managers Tiziana Marangon and Giulia Lupparelli.
This study was sponsored and funded by Fondazione Neoplasie Sangue Onlus. Celgene and Pharmion supplied free thalidomide, and Janssen-Cilag supplied free bortezomib for this study. They had no role in the study design, data analysis, data interpretation, or writing of the report. The authors would also like to acknowledge the editorial assistance of Jane Saunders of FireKite during the development of this publication, which was funded by Millennium Pharmaceuticals.
Authorship
Contribution: M.B. and A.P. designed the research; S.B. and A.L. collected, analyzed, and interpreted data; A.E. performed statistical analysis; S.B., M.B., and A.P. wrote the manuscript; D.R., M. Cavalli, M. Genuardi, R.R., S.G., F.P., C.N., T.G., G.B., V.C., V.R., C.C., P.M., L.D.R., A.M.L., M. Grasso, A.P.F., A.E., M. Cavo, and G.G. enrolled patients; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: S.B. has received honoraria from Celgene, Janssen-Cilag, and Novartis and served on the advisory committee of Merck Sharp & Dohme; F.P. has served on the advisory board of Celgene, Janssen-Cilag, and Schering-Plough; M.C. has received honoraria from Janssen-Cilag, Celgene, and Novartis and served on the Speakers' Bureau of Janssen-Cilag and Millennium Pharmaceuticals; M.B. has received research support, consultancy, and served on the scientific advisory board from Celgene and Janssen Cilag; A.P. has received honoraria from and served on the advisory committee of Celgene and Janssen-Cilag. The remaining authors declare no competing financial interests.
For a complete list of Italian Multiple Myeloma Network GIMEMA participants, please see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Antonio Palumbo, MD, Myeloma Unit, University of Torino, Azienda Ospedaliero-Universitaria (A.O.U.) S. Giovanni Battista, Via Genova 3, 10126 Torino, Italy; e-mail: appalumbo@yahoo.com.