Abstract
Warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome is a genetic disease that is caused by heterozygous mutations of the CXCR4 gene. These mutations confer an increased leukocyte response to the CXCR4-ligand CXCL12, resulting in abnormal homeostasis of many leukocyte types, including neutrophils and lymphocytes. Analysis of the myeloid and plasmacytoid dendritic cell blood counts in WHIM patients revealed a striking defect in the number of plasmacytoid dendritic cells as well as a partial reduction of the number of myeloid dendritic cells, compared with healthy subjects. Moreover, the production of interferon-α by mononuclear cells in response to herpes simplex infection, or after stimulation with the Toll-like receptor 9 ligand CpG, was undetectable in WHIM patients. Because plasmacytoid dendritic cells play a key role in the defense against viruses and their generation and motility are in part dependent on CXCR4, we hypothesized that the susceptibility of WHIM patients to warts is related to the abnormal homeostasis of plasmacytoid dendritic cells.
Introduction
Warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome is an autosomal dominant genetic disease that is characterized by severe/moderate neutropenia and leukopenia (despite the retention of mature neutrophils in the bone marrow, eg, myelokathexis), hypogammaglobulinemia, recurrent respiratory infections, and severe verrucosis, which are caused by common human papillomavirus (HPV) strains. WHIM is caused by heterozygous mutations at the C-terminus of the chemokine receptor protein CXCR4, and these mutations affect the intracellular signaling of the receptor in response to the ligand CXCL12.1-7 Because leukocytes that express CXCR4 have an increased chemotactic response to the CXCL12 ligand, these cells are retained in the bone marrow and lymphoid compartment, thereby reducing their blood counts.3-6 Although several studies have provided insight into the pathogenesis of WHIM syndrome, some clinical features of this genetic disorder remain unexplained. In particular, it is still unclear why WHIM patients develop verrucosis that affects both the hands and genitalia, which are extremely refractory to treatment.7,8
Recent studies suggest that plasmacytoid dendritic cells (pDCs) might have a role in protecting against HPV infection, as pDCs were present in the epidermis of patients diagnosed with chronic genital HPV infections. pDCs secrete the antiviral cytokine interferon-α (IFN-α) in response to stimulation with HPV virus-like particles9,10 and abnormalities in DC homeostasis have been implicated in various human diseases, including cancer, autoimmune disease, allergy, and infection.11-14 Because pDC generation and trafficking among tissues is regulated by CXCR4,15-17 we investigated the number of circulating pDCs and their ability to secrete IFN-α in WHIM patients.
Methods
Informed consent was obtained from all WHIM patients in accordance with the Declaration of Helsinki according to a protocol approved by the Hospital Ethical Committee (Spedali Civili, Brescia, Italy). Ethylenediaminetetraacetic acid-treated blood samples were collected from 5 WHIM patients (patients 1-5)8 and healthy subjects, who were age-matched with the patients. Immunophenotyping was performed by flow cytometry (FACScan, BD Biosciences) using the following antihuman antibodies (BD Biosciences): anti-CD14/anti-CD15/anti-CD19/anti-CD20–fluorescein isothiocyanate (lineage cocktail), anti-CD1c-phycoerythrin, anti-CD4-peridinin chlorophyll protein, and anti–BDCA-2-allophycocyanin. The DC subsets were then identified and gated based on CD1c and BDCA-2 surface marker expression. Myeloid DCs were defined as CD4+CD1c+BDCA-2− cells, and plasmacytoid DCs were defined as CD4+CD1c−BDCA-2+ cells.18
Heparin-treated blood samples were collected from patient 1, patient 2, patient 3, and the controls, and peripheral blood mononuclear cells (PBMCs) were isolated using Lympholyte H density gradient centrifugation.
Formalin-fixed, paraffin-embedded skin biopsies were obtained from a WHIM patient and 10 control cases. For immunohistochemical staining, primary antibodies to the following antigens were used: CD1a (mouse IgG1, clone 010, Dako Denmark), CD207/Langherin (mouse IgG2b, clone 12D6, Vector Laboratories), MxA (mouse IgG2a, kindly provided by Dr O. Haller), BDCA2 (mouse IgG1, clone 124B3.13, Dendritics), and anti-HPV clone K1H8 (Dako Denmark), which recognizes a 57-kDa capsid protein of HPV-1.19 All sections were counterstained with hematoxylin.
A 2-tailed Mann-Whitney U test (nonparametric analysis) was used for statistical comparison of the patients to the healthy controls. A P value less than .05 was considered significant.
Results and discussion
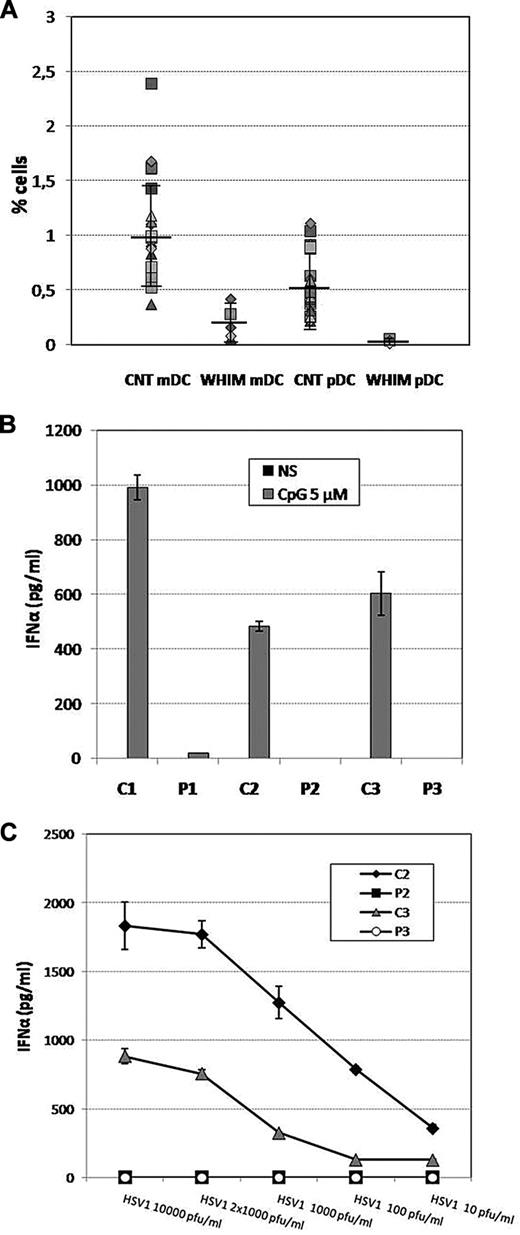
To assess the effect of CXCR4 mutations on pDC homeostasis in WHIM patients, we performed a quantitative evaluation of the DC subpopulations (myeloid DCs [mDCs] and pDCs)20 in the peripheral blood of 24 healthy subjects and 5 WHIM patients bearing p.Arg334X or p.Gly336X mutations. As shown in Figure 1A, we observed a significant reduction in both DC subsets in the blood of WHIM patients compared with healthy subjects. In particular, the percentages of pDCs were markedly decreased in the patients (mean ± SE, 0.032% ± 0.016%; range, 0.01%-0.05%) compared with the healthy subjects (mean ± SE, 0.53% ± 0.278%; range, 0.22%-1.11%, P < .05). In addition, the numbers of mDCs were reduced in WHIM patients (mean ± SE, 0.2% ± 0.15%; range, 0.06%-0.42%), although to a lesser extent, compared with healthy subject levels (mean ± SE, 0.99% ± 0.466%; range, 0.37%-2.39%, P < .05; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Evaluation of the pDCs and mDCs in WHIM patients. (A) Percentages of mDCs and pDCs (calculated as the percentage of the lymphocyte population) in 5 WHIM patients compared with 24 healthy subjects. To identify pDCs and mDCs via flow cytometry, we used the following gating strategy. Side scatter and staining with anti-CD4-peridinin chlorophyll protein and anti-CD14/anti-CD15/anti-CD19/anti-CD20-fluorescein isothiocyanate were used to exclude B cells, neutrophils, and monocytes. (B) IFN-α production was evaluated after HSV1 infection (dose ranging from 10 to 10 000 pfu/mL, for 24 hours; B) or CpG (Toll-like receptor 9 ligand, 5μM for 24 hours) stimulation (C) in PBMCs of 3 WHIM patients compared with healthy subjects. PBMCs (2.5 × 106/mL) were plated in U-bottomed 96-well plates and cultured with 5μM CpG (ODN2216, InvivoGen) or infected with HSV1 (strain MacIntyre; from 10 up to 10 000 pfu/mL) for 24 hours. IFN-α levels in culture supernatants were determined using a VeriKine human IFN-α ELISA Kit (PBL InterferonSource) according to the manufacturer's instructions. (B-C) Results are presented as average of duplicate data. Error bars represent SE. Comparison of cytokine production by nonparametric analysis shows significant differences between WHIM patients and control subjects.
Evaluation of the pDCs and mDCs in WHIM patients. (A) Percentages of mDCs and pDCs (calculated as the percentage of the lymphocyte population) in 5 WHIM patients compared with 24 healthy subjects. To identify pDCs and mDCs via flow cytometry, we used the following gating strategy. Side scatter and staining with anti-CD4-peridinin chlorophyll protein and anti-CD14/anti-CD15/anti-CD19/anti-CD20-fluorescein isothiocyanate were used to exclude B cells, neutrophils, and monocytes. (B) IFN-α production was evaluated after HSV1 infection (dose ranging from 10 to 10 000 pfu/mL, for 24 hours; B) or CpG (Toll-like receptor 9 ligand, 5μM for 24 hours) stimulation (C) in PBMCs of 3 WHIM patients compared with healthy subjects. PBMCs (2.5 × 106/mL) were plated in U-bottomed 96-well plates and cultured with 5μM CpG (ODN2216, InvivoGen) or infected with HSV1 (strain MacIntyre; from 10 up to 10 000 pfu/mL) for 24 hours. IFN-α levels in culture supernatants were determined using a VeriKine human IFN-α ELISA Kit (PBL InterferonSource) according to the manufacturer's instructions. (B-C) Results are presented as average of duplicate data. Error bars represent SE. Comparison of cytokine production by nonparametric analysis shows significant differences between WHIM patients and control subjects.
Because of the paucity of circulating mDCs, we have evaluated cytokine production in DCs derived in vitro from the monocytes isolated from blood of a WHIM patient and from a control subject. We specifically analyzed the production of interleukin-12, interleukin-6, and tumor necrosis factor-α, which are secreted by mature monocyte-derived DCs after 24 hours of stimulation with lipopolysaccharide and IFN-γ.21 Our results showed normal production of interleukin-12 (p70), interleukin-6, and tumor necrosis factor-α by mature DCs from WHIM patients compared with the cells derived from healthy donors (supplemental Figure 1).
Because pDCs are the most potent secretors of IFN-α in response to viral stimuli, they are essential for the antiviral response.22,23 To study the effect of the reduced pDC number in WHIM patients, we analyzed PBMC IFN-α expression. We infected PBMCs derived from 2 WHIM patients and 2 control subjects with an increasing number of type 1 herpes simplex virus particles (HSV1, from 10 up to 10 000 pfu/mL). Analysis of the IFN-α supernatant concentration after 24 hours of culture showed that, with the lowest number of HSV1 copies, cytokine release by healthy PBMCs was already detectable. In contrast, WHIM-PBMC cytokine production remained undetectable, even after infection with 10 000 pfu/mL of HSV1 (Figure 1B). We subsequently analyzed the WHIM-PBMC IFN-α production levels in response to CpG (5μM), which stimulates pDCs by activating Toll-like receptor 9. Even under these experimental conditions, we observed a severe defect in IFN-α production by WHIM-PBMCs compared with the healthy, control PBMCs (Figure 1C). These data suggest that WHIM patients have a severely impaired capacity to produce IFN-α in response to Toll-like receptor 9 stimulation, which is probably the result of the reduction of the pDC count.
Next, we analyzed the dermal mononuclear infiltrate of HPV-associated Verruca vulgaris from one WHIM patient (Figure 2A-B) and from control subjects (supplemental Figure 2A). Dermal BDCA2+ pDCs were not detected in multistep sections of skin biopsies of the WHIM patient but were present at variable extent in warts from control subjects (Figure 2E-F; supplemental Figure 2B). Because IFN-α secretion by pDC results in the expression of the antiviral protein MxA, skin biopsies were stained with anti-MXA monoclonal antibody. Remarkably, all control cases showed variable intraepithelial reactivity for MxA, whereas Verruca vulgaris from the WHIM patient was completely negative (Figure 2G-H; supplemental Figure 2C-D). In contrast, intraepidermal CD1a+CD207+ Langerhans cells and dermal CD1a+ cells were detected in the biopsies from the WHIM patient as well as in control biopsies (Figure 2C-D).
DCs in Verruca vulgaris. Sections of Verruca vulgaris were obtained from a WHIM patient (A-D,F,H) and a control case (E,G) and stained for hematoxylin and eosin (A), HPV (brown, B), CD1a (blue in C; and brown D), CD207 (brown, C), BDCA2 (brown, E-F), and MxA (brown, G-H). Verruca vulgaris from WHIM patient 4 showed a moderate dermal mononuclear infiltrate (A) and contained HPV-infected keratinocytes (B). By immunohistochemistry, intraepidermal CD1a+CD207+ Langerhans cells (C) and dermal CD1a+ cells (D) were detected in WHIM patient biopsy. *Position of dermal papillae. Dermal BDCA2+ cells were detectable in control cases (E) but not seen in the patient biopsy (F). Strong reactivity for MxA was observed in keratinocytes from a control biopsy (G and inset), whereas it was completely negative in WHIM patient biopsy (H). Original magnifications are as follows: A,G-H, ×40 (scale bar represents 500 μm); B, ×600 (scale bar represents 20 μm); and C-F, inset in G, ×400 (scale bar represents 50 μm).
DCs in Verruca vulgaris. Sections of Verruca vulgaris were obtained from a WHIM patient (A-D,F,H) and a control case (E,G) and stained for hematoxylin and eosin (A), HPV (brown, B), CD1a (blue in C; and brown D), CD207 (brown, C), BDCA2 (brown, E-F), and MxA (brown, G-H). Verruca vulgaris from WHIM patient 4 showed a moderate dermal mononuclear infiltrate (A) and contained HPV-infected keratinocytes (B). By immunohistochemistry, intraepidermal CD1a+CD207+ Langerhans cells (C) and dermal CD1a+ cells (D) were detected in WHIM patient biopsy. *Position of dermal papillae. Dermal BDCA2+ cells were detectable in control cases (E) but not seen in the patient biopsy (F). Strong reactivity for MxA was observed in keratinocytes from a control biopsy (G and inset), whereas it was completely negative in WHIM patient biopsy (H). Original magnifications are as follows: A,G-H, ×40 (scale bar represents 500 μm); B, ×600 (scale bar represents 20 μm); and C-F, inset in G, ×400 (scale bar represents 50 μm).
Taken together, our results demonstrate that the number of circulating pDCs and mDCs is markedly reduced in WHIM patients. In particular, we show that the decline in pDC numbers is associated with a decreased production of IFN-α in response to viral infection or CpG stimulation. We speculate that the high levels of CXCR4 expression by pDCs12,15 (data not shown), along with the increased responsiveness of WHIM patient leukocytes to CXCL12, may account for the low percentage of circulating pDCs in WHIM patients.3,5,6,24,25 Because of the disturbed homeostasis of pDCs in WHIM patients, these cells may not be able to migrate to the skin, where they supply antiviral activity against HPV infection. This migration defect may lead to the development of severe, untreatable warts, which are typically observed in WHIM patients. However, the mechanisms behind the immune response to HPV are not dependent on the production of interferon by pDCs and might involve other cell types, including mDCs and T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Telethon (grant GGP07134), Fondazione Cariplo (NOBEL Grant), EU Grant FP7 HLH-cure (project 201461), and PRIN 2007 (2007ACZMMZ-005; R.B.).
Authorship
Contribution: L.T. performed the studies with the dendritic cells and wrote the manuscript; D.M. performed the flow cytometric analysis; W.V. and F.F. performed immunohistopathology studies; M.D.F. studied the response of cells to herpes infection; L.D.N., F.P., V.L., and A.P. were in charge of the patients' follow-up; and R.B. supervised the project and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raffaele Badolato, Istituto di Medicina Molecolare Angelo Nocivelli, Università di Brescia, c/o Spedali Civili, 25123 Brescia, Italy; e-mail: badolato@med.unibs.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal