Abstract
Cellular therapy of Epstein-Barr virus (EBV)+ posttransplantation lymphoproliferative diseases (PTLD) in cord blood transplant (CBT) recipients is limited by lack of donor access and the donor's naive neonatal immune system. We therefore used partially human leukocyte antigen–matched third-party in vitro expanded EBV-specific cytotoxic T lymphocytes (CTLs) to treat 2 CBT recipients with life-threatening, donor-derived monoclonal EBV+ diffuse large B-cell lymphomas with extranodal involvement developing in the context of graft-versus-host disease. Both patients had failed immunosuppression taper and Rituximab. After 5 and 9 infusions of 106 EBV-CTL/kg, respectively, each patient achieved a sustained complete remission without toxicity or graft-versus-host disease. Each is alive without recurrence at 20 and 15 months, respectively, post–EBV-PTLD diagnosis. This approach demonstrates the efficacy of using “off-the-shelf,” virus-specific third-party CTLs restricted by human leukocyte antigens expressed by the tumor to treat otherwise lethal EBV-PTLD. Such therapy may also be applicable to the treatment of other infections and residual or recurrent malignancy after CBT.
Introduction
Cord blood transplantation (CBT) has been associated with a 2% Epstein-Barr virus–posttransplantation lymphoproliferative diseases (EBV-PTLD) risk,1 although an incidence of 21% has been documented when antithymocyte globulin (ATG) is added to nonmyeloablative conditioning.2 While EBV-PTLD risk can be reduced by avoidance of ATG or monitoring and preemptive Rituximab,3,4 once EBV-PTLD has developed, therapy after CBT is limited to rituximab ± chemotherapy. Herein, we describe 2 cases of successful treatment of CB-derived EBV-PTLD with partially human leukocyte antigen (HLA)–matched third-party EBV-specific cytotoxic T lymphocytes (EBV-CTLs).
Methods
Patients and disease characteristics
Patient #1 (Table 1) achieved 100% chimerism with 1 of 2 CB units by day 21. He developed grade 2 acute graft-versus-host disease (GVHD). This resolved with 2 mg/kg of intravenous methylprednisolone, although corticosteroid taper was slowed due to intermittent flares. After routine surveillance for EBV-DNA by quantitative polymerase chain reaction (qPCR) was negative for 6 months, it was discontinued. However, exacerbation of gut symptoms at 8 months prompted endoscopy. This revealed extensive intestinal ulceration with a donor-derived EBV+ diffuse large B-cell lymphoma. Simultaneously, blood EBV qPCR was > 8300 copies/mL 18-flourodeoxyglucose positron emission tomography (FDG-PET) demonstrated lymphoma in cervical and mesenteric lymph nodes and throughout the intestine (Figure 1A). Immunosuppression was stopped and rituximab (375 mg/m2/dose × 10) was administered without response. Disease progression with new lung lesions and extensive tonsillar and oropharyngeal ulceration prompted emergent treatment with third-party EBV-CTLs.
Patient, graft, and EBV-PTLD characteristics
| . | Patient #1 . | Patient #2 . |
|---|---|---|
| Age/sex | 32 y/male | 10 y/female |
| Diagnosis | Primary refractory peripheral T-cell NHL | AML |
| Remission status | 1st partial remission | 2nd complete remission |
| Conditioning | Cyclo 120 mg/kg | Busulfan 0.8 mg/kg × 16 |
| Flu 75 mg/m2 | Cyclo 200 mg/kg | |
| TBI 1375 cGy | Equine ATG 90 mg/kg | |
| Immune suppression | Cyclosporine-A Mycophenolate mofetil | Tacrolimus Prednisone (1 mg/kg) |
| Infused TNC dose | Double unit graft: | Single unit graft: 4.9 × 107/kg |
| Engrafting unit*: 2.4 × 107/kg | ||
| Non-engrafting unit: 2.5 × 107/kg | ||
| HLA-A,-B antigen, DRB1 allele | Engrafting unit*: 5/6 HLA Allele match; make donor | 4/6 HLA Allele match; male donor |
| Match | Non-engrafting unit: 5/6 HLA Allele match; donor | |
| GVHD/ GVHD therapy | Grade II skin and gut/corticosteroids | Grade II gut/corticosteroids |
| PTLD onset | +253 days | +244 days |
| Organ involvement | Tonsil, upper and lower GI tract, lungs | Brain, lung, upper and lower GI tract |
| Pathology | Monomorphic DLBCL; EBV (EBER-ISH)+, CD20−, CD79a+; Donor origin by qPCR for informative STR of tumor specimen | Monomorphic DLBCL; EBV (EBER-ISH)+, CD20+; Donor origin by FISH for the Y chromosome |
| . | Patient #1 . | Patient #2 . |
|---|---|---|
| Age/sex | 32 y/male | 10 y/female |
| Diagnosis | Primary refractory peripheral T-cell NHL | AML |
| Remission status | 1st partial remission | 2nd complete remission |
| Conditioning | Cyclo 120 mg/kg | Busulfan 0.8 mg/kg × 16 |
| Flu 75 mg/m2 | Cyclo 200 mg/kg | |
| TBI 1375 cGy | Equine ATG 90 mg/kg | |
| Immune suppression | Cyclosporine-A Mycophenolate mofetil | Tacrolimus Prednisone (1 mg/kg) |
| Infused TNC dose | Double unit graft: | Single unit graft: 4.9 × 107/kg |
| Engrafting unit*: 2.4 × 107/kg | ||
| Non-engrafting unit: 2.5 × 107/kg | ||
| HLA-A,-B antigen, DRB1 allele | Engrafting unit*: 5/6 HLA Allele match; make donor | 4/6 HLA Allele match; male donor |
| Match | Non-engrafting unit: 5/6 HLA Allele match; donor | |
| GVHD/ GVHD therapy | Grade II skin and gut/corticosteroids | Grade II gut/corticosteroids |
| PTLD onset | +253 days | +244 days |
| Organ involvement | Tonsil, upper and lower GI tract, lungs | Brain, lung, upper and lower GI tract |
| Pathology | Monomorphic DLBCL; EBV (EBER-ISH)+, CD20−, CD79a+; Donor origin by qPCR for informative STR of tumor specimen | Monomorphic DLBCL; EBV (EBER-ISH)+, CD20+; Donor origin by FISH for the Y chromosome |
NHL indicates non-Hodgkin lymphoma; AML, acute myelogenous leukemia; cyclo, cyclophosphamide; flu, fludarabine; TBI, total-body irradiation; ATG, anti-thymocyte globulin; TNC, total nucleated cell dose; HLA, human leukocyte antigen; GVHD, graft-versus-host disease; PTLD, posttransplantation lymphoproliferative disease; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus, EBER-ISH; EBV encoded RNA in-situ hybridization; qPCR, quantitative polymerase chain reaction; STR, short tandem repeats; and FISH, fluorescent in situ hybridization.
Patient was 100% donor in bone marrow and blood with the engrafting unit and at no time was the nonengrafting unit detected.
Patient #2 similarly developed grade 2 gut GVHD that responded to tacrolimus and prednisone. However, 8 months after transplantation, diarrhea and abdominal pain prompted colonic biopsy that revealed extensive infiltration with donor-derived EBV+ diffuse large B-cell lymphoma. FDG-PET revealed widely disseminated disease. Despite Rituxan (375 mg/m2/dose ×4), hydroxyurea, prednisone taper to physiologic levels, and cessation of tacrolimus, EBV-PTLD progressed with left hemiparesis and subsequent coma. Magnetic resonance imaging revealed new space-occupying lesions in the basal ganglia and cerebellum, and EBV-DNA was detected in spinal fluid. She was treated with whole-brain radiotherapy (2450 centi-Gray), dexamethasone, and 4 additional rituximab doses. All neurologic symptoms resolved except left-arm paresis, viremia cleared, and abdominal disease improved on FDG-PET. However, within 2 months FDG-PET scan showed progression in the colon. Hence, the patient was referred for third-party EBV-specific CTL therapy.
Generation and selection of third-party EBV-specific T cells
We have generated more than 330 fully characterized EBV-CTLs from consenting healthy donors under good manufacturing practice conditions for adoptive therapy of high-risk allograft recipients. EBV-CTLs were generated as previously described.5,6 Briefly, monocyte/natural killer cell–depleted mononuclear cells were stimulated in vitro at a 20:1 ratio with irradiated autologous EBV-transformed B-cell lines (EBV-BLCLs). After 11 days, T cells were restimulated weekly with irradiated EBV-BLCLs at a 4:1 ratio with interleukin-2. After at least 28 days, T cells were tested forEBV-specificity and their HLA-restriction established by examining their cytotoxic activity in 5Cr release assays against a panel of EBV+ and EBV− targets, each expressing one of the set of HLA A, B, C, DR and DQ HLA-alleles shared by the T-cell donor as previously described.7 T-cell lines lacked alloreactivity and were sterile and endotoxin-free. CTLs with the closest HLA-match to the CB donor (and hence the lymphoma) that were restricted by 1 or more of that donor's HLA-alleles were selected for administration. Memorial Sloan-Kettering Cancer Center Institutional Review Board–approved informed consent was obtained before EBV-CTL administration in accordance with the Declaration of Helsinki. The donors from whom the lines were generated also consented to the use of their cells in a patient other than for who they had donated.
Results and discussion
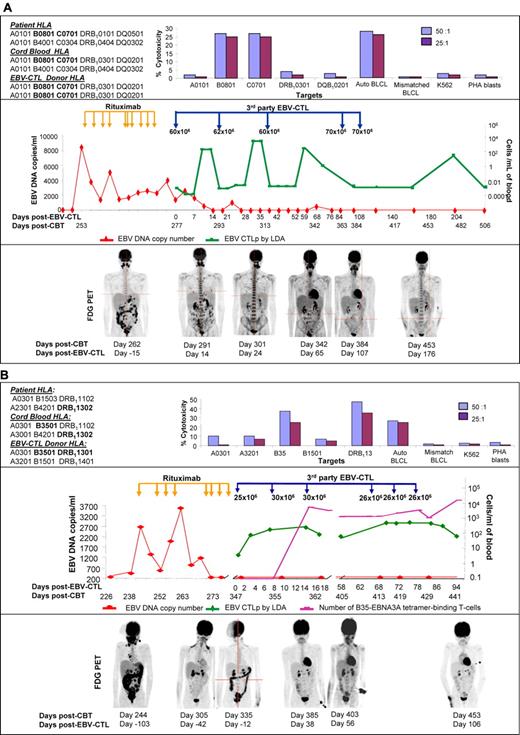
Figure 1A-B shows the EBV-CTL administration time-line, EBV-specific CTL precursor (CTLp) blood levels, and the EBV qPCR and FDG-PET disease course for each patient. Patient #1 received EBV-CTLs (1 × 106T cells/kg/dose ×5) every 21 days ×3, and then at 86 and 108 days. They were homozygous for class I and II HLA-alleles and restricted by HLA-B*0801 and HLA-C*0701 shared by the lymphoma. By day 10 after each infusion, a 2-3 log10 increase in EBV-CTLp frequency was detected that persisted for 7-10 days. By 48 days after the third infusion, and 7 days after infusion 4, EBV-CTLs could not be detected in the blood by PCR analysis. Clinical improvement was documented by resolution of fever, abdominal pain, and melena over the first 18 days after the first infusion. Reduction in PET-avid lesions was documented 14 days after the initial infusion, with marked reductions of all lesions by day 24. Imaging 107 days after CTL initiation revealed near resolution, but colonic lesions remained. Colonic biopsy demonstrated persistent EBV-PTLD. Repeat imaging 176 days after EBV-CTL demonstrated complete remission that is maintained 20 months after EBV-PTLD diagnosis.
EBV-CTL administration timeline, EBV-specific CTL precursor (CTLp) blood levels, and the EBV-qPCR and FDG-PET disease course. (A) Patient #1. Top panel: T cells generated from the third-party donor were restricted by the B*0801 and C*0701 HLA-alleles presented on the EBV+ tumor of the patient and were EBV-specific. The HLA-typing of the engrafting unit is shown. The HLA-typing of the other unit was A*0101, A*0101; B*0801, B*2705; C*0701, C*0102; DRB1*0101, DRB1*0404; DQ*0501, DQ*0302. However, this unit did not engraft. Middle panel: EBV DNA increased on day 253 after CBT (red line) and partially responded to 10 doses of Rituximab. EBV viremia resolved completely after EBV-specific T-cell administration with partial clinical remission after 3 infusions and complete remission after 5 infusions. Peripheral blood EBV-specific CTLp measured by limiting dilution analysis (LDA) rose after each administration indicating transient in vivo expansion (green line) with subsequent decrease in frequencies after 10 days. Bottom panel: PET images demonstrating extensive abdominal and cervical lesions, persistence of abdominal and lesions as well as pulmonary lesions, marked resolution in abdominal lesions at 24 days, near resolution of abdominal and pulmonary lesions at 107 days, and complete remission 176 days after CTL initiation. (B) Patient #2. Top panel: The cytotoxic EBV-specific CTL activity was restricted by the HLA-B*3501 and DRB1*13 HLA-antigens shared with the CB donor. Middle panel: Increased EBV DNA was first detected in the blood 244 days after CBT (red line). This resolved after 7 doses of Rituximab. However, because of relapsed lymphoma in the intestine the patient received EBV-CTLs on day 347. LDA demonstrated 4-fold increase in EBV-CTLp frequency (green line) by day 7 that remained high until 18 days after the first CTL dose. The second course of 3 infusions resulted in a 10-fold increase of EBV-CTLp for another 30 days. T cells binding an HLA-B35-EBNA3A tetramer (purple line), which were the major class I restricted T cells in the T-cell adoptive therapy, were not detectable before CTL infusion, but increased 4-5 logs by 16 days after CTL initiation. Bottom panel: Extensive abdominal and cervical lymphoma 244 days after CBT largely resolved after the 8 doses of Rituximab (day +305). However, abdominal disease recurred that was successfully treated with EBV-CTLs that induced partial remission by 56 days and complete remission by 106 days after CTL initiation.
EBV-CTL administration timeline, EBV-specific CTL precursor (CTLp) blood levels, and the EBV-qPCR and FDG-PET disease course. (A) Patient #1. Top panel: T cells generated from the third-party donor were restricted by the B*0801 and C*0701 HLA-alleles presented on the EBV+ tumor of the patient and were EBV-specific. The HLA-typing of the engrafting unit is shown. The HLA-typing of the other unit was A*0101, A*0101; B*0801, B*2705; C*0701, C*0102; DRB1*0101, DRB1*0404; DQ*0501, DQ*0302. However, this unit did not engraft. Middle panel: EBV DNA increased on day 253 after CBT (red line) and partially responded to 10 doses of Rituximab. EBV viremia resolved completely after EBV-specific T-cell administration with partial clinical remission after 3 infusions and complete remission after 5 infusions. Peripheral blood EBV-specific CTLp measured by limiting dilution analysis (LDA) rose after each administration indicating transient in vivo expansion (green line) with subsequent decrease in frequencies after 10 days. Bottom panel: PET images demonstrating extensive abdominal and cervical lesions, persistence of abdominal and lesions as well as pulmonary lesions, marked resolution in abdominal lesions at 24 days, near resolution of abdominal and pulmonary lesions at 107 days, and complete remission 176 days after CTL initiation. (B) Patient #2. Top panel: The cytotoxic EBV-specific CTL activity was restricted by the HLA-B*3501 and DRB1*13 HLA-antigens shared with the CB donor. Middle panel: Increased EBV DNA was first detected in the blood 244 days after CBT (red line). This resolved after 7 doses of Rituximab. However, because of relapsed lymphoma in the intestine the patient received EBV-CTLs on day 347. LDA demonstrated 4-fold increase in EBV-CTLp frequency (green line) by day 7 that remained high until 18 days after the first CTL dose. The second course of 3 infusions resulted in a 10-fold increase of EBV-CTLp for another 30 days. T cells binding an HLA-B35-EBNA3A tetramer (purple line), which were the major class I restricted T cells in the T-cell adoptive therapy, were not detectable before CTL infusion, but increased 4-5 logs by 16 days after CTL initiation. Bottom panel: Extensive abdominal and cervical lymphoma 244 days after CBT largely resolved after the 8 doses of Rituximab (day +305). However, abdominal disease recurred that was successfully treated with EBV-CTLs that induced partial remission by 56 days and complete remission by 106 days after CTL initiation.
Patient #2 initially received 2 courses of 3 weekly infusions of EBV-CTLs (1 × 106 T cells/kg/dose ×6). These were matched to the lymphoma at HLA-A*0301 and B*3501 but differed for alleles of HLA-DRB1*13 (CB donor/lymphoma DRB1*1302 and EBV-CTL DRB1*1301). The EBV-CTLs included cytotoxic CD8+ T cells restricted by HLA-B*3501 and CD4+ T cells restricted by HLA-DRB1*1301. This resulted in partial remission after course 1 (day +38), and complete remission after course 2 (day +106). However, because of persistent left-arm paresis, 3 additional infusions were given at the same dose. Disease response was correlated with a 4-fold EBV-CTLp increase after the first, and a 10-fold increase after the second EBV-CTL course. The persistence of the CTLp frequencies likely reflects the shorter intervals between infusions. We also analyzed the number of tetramer-binding HLA-B*3501–restricted EBV-specific T cells before and after CTL administration. CD8+ EBV-CTLs restricted by HLA-B3501 were a dominant population in the CTLs. They were not detected before treatment, but dramatically increased by 16 days after the first CTL infusion. The contribution of HLA-DRB1*13–restricted T cells is unclear. We did not have tetramers to measure HLA-DRB1*1301–restricted T cells after infusion. Furthermore, their activity against targets expressing the HLA-DRB1*1302 of the lymphoma could not be ascertained. Subsequently, the patient improved with complete remission maintained at 15 months after EBV-PTLD diagnosis. In both patients infusions were without toxicity; neither patient had GVHD flare despite cessation of GVHD therapy and CTL administration.
Rituximab monotherapy in EBV-PTLD has yielded a response rate of only 44%,8 and can be limited by lack of CD20 expression.9 However, chemotherapy (± rituximab) after allograft can be associated with significant toxicity and mortality.8 An alternative therapy is the administration of donor-derived EBV-CTLs.9 However, the requirement for a seropositive donor and the time needed for CTL production has limited application of this approach. Moreover, until recently generation of donor-derived virus-specific CTLs from naive CB T cells was not thought possible. Hanley et al10 have now confirmed 2 earlier studies11,12 indicating that cytomegalovirus and EBV-specific T cells can be generated from CB. However, the EBV-specific T cells generated from naive CB-derived T cells are specific for epitopes not targeted by adult memory T cells. While these T cells can lyse autologous EBV-BLCL in vitro, it remains to be determined whether they have the requisite avidity and resistance to apoptosis to be effective in vivo. Furthermore, production time remains lengthy (30 days for EBV-BLCL sensitization plus 21 days for CTL production).
In contrast to transplant donor–derived T cells, cryopreserved third-party EBV-specific T cells are immediately available and can be selected on the basis of their partial HLA-restriction and their level of HLA-match. Haque et al were the first to demonstrate that partially HLA-matched, third-party, EBV-specific T cells can induce remissions of EBV-PTLD in organ allograft recipients and 2 marrow allograft recipients.13,14 However, they did not establish the clonality of the EBV-PTLD. In our patients, the lymphomas were monoclonal and had failed immunosuppression taper and Rituximab. Historically, such lymphomas are usually lethal. In our patients, sequential third-party T-cell infusions induced transient increments in circulating EBV-specific T cells, clearance of EBV-DNA, and clinical and radiologic remissions. In contrast to the extended survival that has been reported for donor-derived EBV-specific T cells,15 the third-party T cells did not durably engraft. Thus, multiple infusions were required. Nevertheless, the disease regressions achieved have been sustained, likely reflecting subsequent establishment of effective transplant donor-derived EBV-specific T cells. Our results provide evidence that “off-the-shelf” third-party CTLs selected on the basis of partial HLA-matching and HLA-restriction can be effective treatment of monoclonal EBV-PTLD after CBT. While transplant donor–derived T cells may constitute the only option for adoptive cell therapy for a proportion of CBT recipients who inherit very rare HLA genotypes, a review of the HLA typing of marrow and CB allograft recipients transplanted at our center also suggests that our diverse bank of fully characterized EBV-CTLs derived from consenting donors and manufactured under good manufacturing practice conditions could provide effective treatment for a large proportion of EBV-PTLD patients. This treatment approach could also be applied to other infections, and, potentially, to the eradication of residual or recurrent malignancy.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the P01 CA23766 and CA59350 from the National Institutes of Health, The Tow Foundation, The Aubrey Fund, The Smead Foundation, and The Laura Rosenberg Foundation.
National Institutes of Health
Authorship
Contribution: J.N.B., G.K., and R.J.O. performed the research, analyzed and interpreted the data, and wrote the manuscript; E.D. generated and characterized the EBV-CTLs and the immunologic responses and assisted in writing the manuscript; C.S. assisted in writing the manuscript; J.J.J., M.A.P., and S.E.P. assisted in clinical management; and M.D. assisted in the analysis of imaging studies and in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juliet N. Barker, MBBS (Hons), Department of Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: barkerj@mskcc.org.
References
Author notes
J.N.B. and E.D. are equal first authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal