To the editor:
The so-called controversy about the presence of tissue factor (TF) in platelets is a reality that our research group has been dealing with since 2003, when we published our first data on this topic.
We have read with interest the letter by Bouchard et al,1 and we agree with the authors that discrepancies in published data on this topic may be due to differences in methods, reagents, and experimental conditions used by different investigators. We believe that the opportunity to use reagents and methods already validated in other laboratories might help in reproducing data and solving such important issues as the present one.
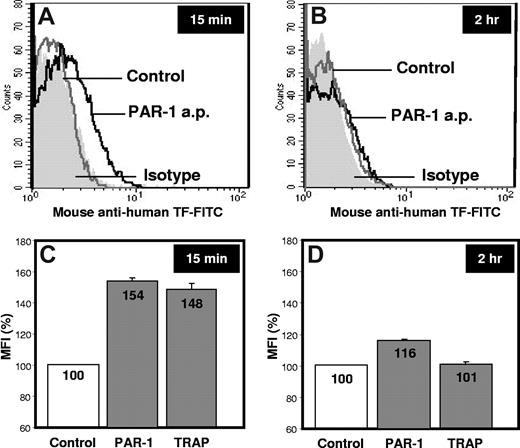
Results reported by Bouchard et al indicate that human platelets stimulated with protease-activated receptor (PAR)–1 and PAR-4 agonist peptides do not express TF at 2 hours.1 This evidence and our experience on this topic prompted us to carry out experiments in the same conditions described by the authors. Our results confirm that the amount of TF present on the platelet surface after 2 hours stimulation with PAR-1 agonist peptide and thrombin receptor-activating peptide (TRAP)–6 is negligible (1.5 ±0.2, 1.7 ± 0.1, 1.5 ± 0.2 MFI for control, PAR-1 and TRAP-6 stimulation, respectively; Figure 1). After a 15-minute stimulation, however, PAR-1 and TRAP-6 significantly increase platelet TF expression (2.7 ± 0.2 and 2.6 ± 0.3 MFI, respectively; P < .01) compared with resting platelets (1.8 ± 0.2 MFI). This indicates that TF expression by platelets in response to certain agonists is an early event, confirming data already published by our group.2
Platelet-associated TF expression. Washed platelets from healthy subjects were left untreated (Control) or stimulated with PAR-1 agonist peptide (PAR-1 a.p.) or TRAP-6 for 15 minutes or 2 hours at 37°C. Samples were then incubated for 15 minutes with saturating concentration of mouse anti–human TF–fluorescein isothiocyanate (American Diagnostica, catalog no 4507CJ) and mouse anti–human CD41-phycoerythrin monoclonal antibodies (Instrumentation Laboratories, catalog no A07781).2 Fluorescein isothiocyanate– and phycoerythrin-conjugated isotype controls were used in all the experiments to quantify the background labeling. TF-positive platelets were determined in 10 000 CD41-positive events per sample. Mean fluorescence intensities (MFI) were calculated from fluorescence histograms for the gated population, and data were analyzed with CellQuest Pro 5.2.1 software (Becton Dickinson). (A-B) Representative fluorescence histograms of 3 independent experiments performed in triplicate. (C-D) The mean value of the results, reported as %MFI ± SD.
Platelet-associated TF expression. Washed platelets from healthy subjects were left untreated (Control) or stimulated with PAR-1 agonist peptide (PAR-1 a.p.) or TRAP-6 for 15 minutes or 2 hours at 37°C. Samples were then incubated for 15 minutes with saturating concentration of mouse anti–human TF–fluorescein isothiocyanate (American Diagnostica, catalog no 4507CJ) and mouse anti–human CD41-phycoerythrin monoclonal antibodies (Instrumentation Laboratories, catalog no A07781).2 Fluorescein isothiocyanate– and phycoerythrin-conjugated isotype controls were used in all the experiments to quantify the background labeling. TF-positive platelets were determined in 10 000 CD41-positive events per sample. Mean fluorescence intensities (MFI) were calculated from fluorescence histograms for the gated population, and data were analyzed with CellQuest Pro 5.2.1 software (Becton Dickinson). (A-B) Representative fluorescence histograms of 3 independent experiments performed in triplicate. (C-D) The mean value of the results, reported as %MFI ± SD.
Specifically, in 2003 we showed that platelet activation by adenosine diphosphate (ADP), TRAP, epinephrine, thromboxane analog U46619, and calcium ionophore A23187 resulted in a concentration-dependent expression of TF on the cell surface.2 The kinetics of this event was very rapid, because TF was detected on the platelet surface as early as 3 minutes after stimulation; a constant increase was observed up to 30-60 minutes, then a return to basal levels within 2-4 hours. Interestingly, at 2 hours, platelet-associated TF did not significantly differ from that observed in resting conditions. To ensure the identity of the platelet-associated TF, flow cytometric analyses were performed using 3 different monoclonal antibodies against TF (2 of them were commercially available, and one was kindly provided by Prof Yale Nemerson, Mt Sinai School of Medicine, New York, NY [deceased, 2009]) and a fluorescein isothiocyanate–labeled recombinant factor VIIa. Moreover, the exposed TF was confirmed to be functionally active because it was able to generate factor Xa as demonstrated by chromogenic assay. More recently, we also demonstrated that acute coronary syndrome patients had a significantly higher amount of TF-positive platelets than stable angina patients or controls and that the functional activity of platelet-associated TF was significantly higher in acute coronary syndrome patients than in stable angina patients and controls,3 thus suggesting that TF exposed on the platelet surface might have a pathophysiologic relevance in arterial thrombosis.
In conclusion, we agree with Bouchard et al that platelets do not express detectable TF after 2 hours' stimulation. Nevertheless, our data support the evidence that platelets do express TF as a result of a rapid and dynamic process. Thus, its detection may considerably vary if observed at different time points after in vitro cell stimulation.
Authorship
Contribution: M.C. conceived of research, analyzed data, and wrote the paper; M.B. conceived of research, designed experiments, analyzed data, and reviewed the manuscript; and V.T. and E.T. conceived of research and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Camera, PhD, Department of Pharmacological Sciences, Università degli Studi di Milano, Via Balzaretti, 9; 20133 Milan, Italy; e-mail: marina.camera@unimi.it or marina.camera@ccfm.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal