In a multicenter Japanese study, Ennishi et al have demonstrated both the benefits and the risks of adding rituximab to CHOP chemotherapy (R-CHOP) in patients with diffuse large B-cell lymphoma (DLBCL) and hepatitis C virus (HCV) infection.
Comparing both toxicity and long-term outcomes with a cohort of contemporaneously treated HCV-negative patients, the HCV-positive group receiving R-CHOP achieved similar long-term progression-free survival but at the cost of an almost 10-fold risk of “severe hepatic toxicity” (specifically, grade III/IV elevation of aspartate aminotransferase [AST] and alanine aminotransferase [ALT]).1 This occurred in association with an increase in HCV RNA viral load, including in patients with undetectable viral load before treatment. Hepatotoxicity was associated with frequent dose modifications and discontinuation of therapy in some patients and a risk of progression of underlying hepatic complications (liver cirrhosis or hepatocellular carcinoma [HCC]). A total of 5% of HCV-positive patients ultimately died from hepatic failure, which occurred most frequently in those with underlying HCC. There was no evidence of concurrent hepatitis B virus (HBV) infection, and omission of prednisone in some patients did not appear to diminish the rate of severe hepatotoxicity.
HCV infection has a prevalence of approximately 1.5% in the United States2 and is associated with an increase incidence of B-cell non-Hodgkin lymphoma (NHL) ranging from 20%-30%3 to almost double that of HCV-negative controls.4 While this is typically low-grade marginal zone B-cell lymphoma, there is also an association with aggressive lymphoma5 and DLBCL is by far the most frequent histologic subtype of NHL. Importantly, Ennishi et al confirm previous reports that that HCV-positive patients with DLBCL appear to have higher risk disease features at presentation, including being older and having more frequent splenic involvement and higher lactate dehydrogenase levels.6,7 Nevertheless, neither HCV infection itself nor the development of “severe hepatic toxicity” was independently correlated with an impact on survival in the present study, suggesting that HCV-positive patients derive a similar antilymphoma benefit from the addition of rituximab. The potential for dose delays and liver failure due to “severe hepatic toxicity” are the likely explanations for the trend toward an inferior late survival (> 2 years) that was observed after therapy (P = .07).
The higher rate of severe hepatotoxicity observed after the addition of rituximab (compared with previous reports with chemotherapy alone) suggests an important role for humoral immunity in the control of viremia in chronic HCV infection, as has been observed previously.8 A critical question then is the role and timing of antiviral therapy in HCV-positive patients. While HCV treatment with ribavirin and interferon-α alone has been reported to induce remission in some cases with indolent histology B-cell lymphoma,9 the strategy has not been uniformly tested in aggressive B-cell lymphoma and is unlikely on its own to achieve long-term remission. In patients with DLBCL with a history of hepatitis B virus (HBV) infection receiving rituximab-combination chemotherapy, the use of early antiviral therapy at the time of HBV reactivation (as measured by detectable HBV DNA viral load) appears to prevent overt hepatitis and allow continuation of therapy.10 In contrast, while not addressed specifically in the present study, antiviral therapy did not prevent an increase in HCV RNA levels in2 patients after the initiation of R-CHOP.1 However, the strategy of antiviral therapy after completion of chemotherapy in the HCV-positive patient is attractive and was associated with improved outcomes in one small retrospective study.11 The optimal timing of HCV antiviral therapy in aggressive lymphoma will require prospective study, and the results from Ennishi et al suggest that further efforts to prevent progressive liver failure after treatment are needed (see figure).
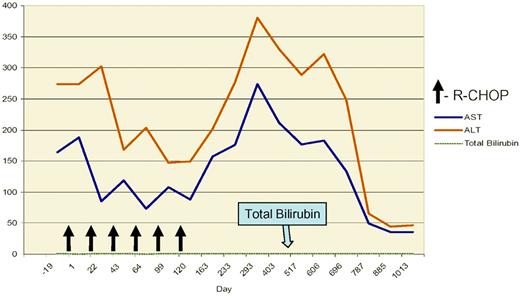
Grade III elevation of transaminases following 6 cycles of R-CHOP for advanced grade III follicular lymphoma in a patient treated at the University of Alabama at Birmingham with chronic HCV infection and elevated HCV viral load (> 107 copies/mL). The albumin and bilirubin remained normal throughout therapy. The patient did not receive further antiviral therapy and the transaminases normalized after approximately 2 years.
Grade III elevation of transaminases following 6 cycles of R-CHOP for advanced grade III follicular lymphoma in a patient treated at the University of Alabama at Birmingham with chronic HCV infection and elevated HCV viral load (> 107 copies/mL). The albumin and bilirubin remained normal throughout therapy. The patient did not receive further antiviral therapy and the transaminases normalized after approximately 2 years.
The greatest advance in the treatment of DLBCL in the past 2 decades has been the addition of rituximab to CHOP (or CHOP-like) chemotherapy, which has increased survival rates significantly in all subgroups with the disease.12 Patients with DLBCL and HCV infection appear to derive similar benefits. Whether R-CHOP has an adverse effect on the long-term natural history of HCV infection and its complications similar to that reported after allogeneic hematopoietic cell transplantation is unclear.13 It is clear that care must be used to monitor for acute and chronic hepatotoxicity associated with therapy, particularly in those with elevated transaminases at presentation or with underlying hepatic complications of HCV infection (including HCC). The role of antiviral therapy in aggressive lymphoma remains undefined and will require focused study.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■