Abstract
The influence of hepatitis C virus (HCV) infection on prognosis and hepatic toxicity in patients with diffuse large B-cell lymphoma in the rituximab era is unclear. Thus, we analyzed 553 patients, 131 of whom were HCV-positive and 422 of whom were HCV-negative, with DLBCL treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP)–like chemotherapy. Survival outcomes and hepatic toxicity were compared according to HCV infection. The median follow-up was 31 and 32 months for patients who were HCV-positive and HCV-negative, respectively. HCV infection was not a significant risk factor for prognosis (3-year progression-free survival, 69% vs 77%, P = .22; overall survival, 75% vs 84%, P = .07). Of 131 patients who were HCV-positive, 36 (27%) had severe hepatic toxicity (grade 3-4), compared with 13 of 422 (3%) patients who were HCV-negative. Multivariate analysis revealed that HCV infection was a significant risk factor for severe hepatic toxicity (hazard ratio: 14.72; 95% confidence interval, 6.37-34.03; P < .001). An exploratory analysis revealed that pretreatment transaminase was predictive of severe hepatic toxicity. HCV-RNA levels significantly increased during immunochemotherapy (P = .006). These results suggest that careful monitoring of hepatic function and viral load is indicated during immunochemotherapy for HCV-positive patients.
Introduction
Many epidemiologic studies have demonstrated an association between hepatitis C virus (HCV) infection and non-Hodgkin lymphoma, suggesting that HCV plays a role in the development of this malignancy.1-8 Fewer data are available for patients who are HCV-positive with diffuse large B-cell lymphoma (DLBCL), as low-grade marginal zone lymphoma is the most common lymphoma subtype associated with HCV infection.3,8 Thus, studies comparing DLBCL outcomes based on HCV infection are extremely rare, and the prognostic value of HCV infection remains controversial, because of heterogeneity in histology and treatment strategies.9,10 Several series have shown good tolerance to standard chemotherapy for lymphoma patients who are infected with HCV.9-11 However, these studies were conducted in the pre-rituximab era.
Although the cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen has been the mainstay treatment for aggressive lymphomas for several decades,12 treatment outcomes have improved significantly since the introduction of rituximab (an anti-CD20 chimeric antibody) in both young and elderly patients.13-15 Since the introduction of rituximab, several prognostic factors have been reevaluated in DLBCL patients,16-18 but the prognostic value of HCV infection in rituximab combination chemotherapy has not been well established. In addition, hepatitis B virus (HBV) reactivation is a well-documented complication that occurs frequently after introduction of rituximab.19,20 However, no large-scale study has investigated the influence of HCV infection on hepatic toxicity in patients with DLBCL treated with rituximab-containing chemotherapy.
We conducted a multicenter retrospective analysis to compare the prognosis and hepatic toxicity of untreated patients with DLBCL uniformly receiving rituximab plus CHOP-like chemotherapy according to HCV infection.
Methods
Patients
We collected medical information from patients who were HCV-positive with DLBCL, who received rituximab plus CHOP (RCHOP)-like therapy as a first-line treatment at 32 participating Japanese institutions between January 2004 and April 2008, and who were followed until March 2009. We also collected medical information from patients who were HCV-negative, treated within 2 months of the treatment start date for each patient who was HCV-positive. This study protocol was approved by the institutional review boards of each participating institute and complied with all provisions of the Declaration of Helsinki. DLBCL was diagnosed by an expert hematopathologist at each institute, based on the World Health Organization classification.21 Patients were included if they were more than 20 years old, and an HCV infection test was performed before treatment. Both de novo DLBCL and transformed DLBCL (t-DLBCL) from low-grade B-cell lymphomas were included. Patients were excluded if they were positive for hepatitis B surface antigen (HBsAg) or for human immunodeficiency virus-1 or -2 antigens. Patients with primary central nervous system lymphoma, primary testicular lymphoma, and intravascular large-cell lymphoma (IVL) were also excluded.
In total, 590 patients, including 136 with and 454 without HCV infection, were registered. Then, 37 patients were excluded for the following reasons: HBsAg-positive (n = 2), non–rituximab-containing regimen (n = 2), absence of final outcome data (n = 1) in patients who were HCV-positive; except the definitive period corresponding to each patient who was HCV-positive (n = 26), regimens other than RCHOP-like (n = 3), HBsAg-positive (n = 2), and IVL (n = 1) in patients who were HCV-negative. In total, 553 patients were eligible; 131 patients (23.7%) were HCV-positive, whereas 422 (76.3%) were HCV-negative.
Clinical and laboratory information, including antibodies to HBsAg (anti-HBs) and hepatitis B core antigen (anti-HBc), was available at the time of diagnosis. HCV infection was defined as the detection of anti-HCV antibodies with commercially available second- or third-generation immunoassay kits (Monolisa anti-HCV Plus, Sanofi Diagnostics Pasteur; and Axsym HCV Version 3.0, Abbott Laboratories).
Treatment and response assessment
All DLBCL patients with HCV infection during the definitive period received immunochemotherapy. Chemotherapy regimens included RCHOP, rituximab plus cyclophosphamide, therarubicin, vincristine, and prednisone, and rituximab plus cyclophosphamide, epirubicin, vincristine, and prednisone. Disease stage was evaluated using the Ann Arbor staging system. Liver and spleen involvement was diagnosed by imaging lymphoma invasion, such as nodular lesions or heterogeneous concentrations. Chemotherapy sensitivity was defined according to standard volume criteria, using computed tomography (CT) and positron emission tomography (PET)/CT with [18F]-fluorodeoxyglucose imaging.22,23
Liver function tests and HCV viral markers
In all patients enrolled, pretreatment levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and their highest levels up to 6 months after completing immunochemotherapy were collected for analysis. Hepatic toxicity was defined by the National Cancer Institute of Canada criteria, and severe hepatic toxicity was defined as an increase in transaminase levels (AST or ALT, grade 3 or 4; > 5.0 × ULN). To assess impaired hepatic synthesis, serum total bilirubin (T-Bil), albumin (Alb), prothrombin time, and platelet counts were collected at the time of DLBCL diagnosis and at 1 and 6 months after treatment. Serum HCV RNA load was determined by quantitative reverse-transcription polymerase chain reaction (detection value of 5-5000 KIU/mL; Amplicor HCV Monitor Test, Version 2.0, Cobas-High-Range assay, Roche Diagnostics).
Statistical analyses
Progression-free survival (PFS) was calculated from the treatment initiation date to the date of documented disease progression, relapse, or the end date of the study. Non–lymphoma-related deaths were censored for PFS. Overall survival (OS) was calculated from the treatment initiation date until death from any cause or the last follow-up. If the stop date was not reached, the data were censored at the date of the last follow-up evaluation. Survival curves were created by the Kaplan-Meier method, and overall differences were compared using the log-rank test. Multivariate analysis was performed using a Cox proportional hazard model to estimate the independent impact of HCV infection on survival and severe hepatic toxicity. In patients who were HCV-positive, risk factors for severe hepatic toxicity were also evaluated by multivariate analysis. Changes in transaminase levels with respect to HCV infection were compared using the Kruskal-Wallis test. Intraindividual changes in serum HCV-RNA levels were assessed using the Wilcoxon signed-rank test. We evaluated impaired hepatic synthesis according to HCV infection using a univariate linear regression model. The basic characteristics and outcomes of each group (HCV-positive and -negative) were compared using the χ2 test, t test, and the Mann-Whitney U test, as appropriate. All statistical tests were 2-sided, and the differences were deemed to be statistically significant if P < .05. Data were analyzed using the Stata software Version 10 (StataCorp LP).
Results
Patient characteristics
Patient characteristics are listed in Table 1. The median age of all patients was 68 years (range, 20-92 years). Before treatment, patients who were HCV-positive were older (P < .001), had more frequently elevated lactate dehydrogenase levels (P = .002), more than 2 extranodal sites (P = .02), spleen involvement (P = .001), and a higher international prognostic index (IPI) (P = .01) than patients who were HCV-negative. There was no difference in the occurrence of t-DLBCL according to HCV infection.
Comparison of HCV-positive with HCV-negative patients
| . | HCV-positive (n = 131), n (%) . | HCV-negative (n = 422), n (%) . | P . |
|---|---|---|---|
| Median age, y (range) | 70.4 (42-86) | 64.3 (20-92) | < .001 |
| Sex, male/female | 79/52 | 228/194 | .21 |
| LDH > normal | 81 (62) | 196 (46) | .002 |
| PS > 1 | 16 (12) | 40 (9) | .47 |
| Stage | .48 | ||
| I | 28 (21) | 92 (22) | |
| II | 39 (30) | 130 (31) | |
| III | 20 (15) | 84 (20) | |
| IV | 44 (34) | 116 (27) | |
| Extranodal sites > 1 | 36 (27) | 75 (18) | .02 |
| IPI: H/I, H | 53 (40) | 139 (33) | .01 |
| BM involvement | 12 (9) | 38 (9) | .96 |
| Spleen involvement | 24 (18) | 35 (8) | .001 |
| Liver involvement | 12 (9) | 25 (6) | .20 |
| t-DLBCL | 5 (4) | 11 (3) | .82 |
| FL | 3 | 5 | |
| MZBCL | 2 | 6 | |
| HBsAb-positive | 7/59 (12) | 13/135 (10) | .24 |
| HBcAb-positive | 11/22 (50) | 9/57 (16) | .03 |
| Treatment | .12 | ||
| RCHOP | 96 (73) | 339 (80) | |
| RTHPCOP | 31 (24) | 71 (17) | |
| RCEOP | 4 (3) | 12 (3) | |
| Baseline transaminase | .48 | ||
| Grade 0-1 | 122 (93) | 415 (98) | |
| Grade 2 | 7 (5) | 3 (1) | |
| Grade 3 | 2 (2) | 4 (1) | |
| Outcome of patients | |||
| Died of lymphoma | 14 (11) | 45 (11) | .87 |
| Died of hepatic failure | 6 (5) | 1 (0.2) | < .001 |
| Died of other causes | 4 (3) | 7 (2) | .76 |
| Hepatic toxicity | |||
| Grade 3-4 | 36 (27) | 13 (3) | < .001 |
| . | HCV-positive (n = 131), n (%) . | HCV-negative (n = 422), n (%) . | P . |
|---|---|---|---|
| Median age, y (range) | 70.4 (42-86) | 64.3 (20-92) | < .001 |
| Sex, male/female | 79/52 | 228/194 | .21 |
| LDH > normal | 81 (62) | 196 (46) | .002 |
| PS > 1 | 16 (12) | 40 (9) | .47 |
| Stage | .48 | ||
| I | 28 (21) | 92 (22) | |
| II | 39 (30) | 130 (31) | |
| III | 20 (15) | 84 (20) | |
| IV | 44 (34) | 116 (27) | |
| Extranodal sites > 1 | 36 (27) | 75 (18) | .02 |
| IPI: H/I, H | 53 (40) | 139 (33) | .01 |
| BM involvement | 12 (9) | 38 (9) | .96 |
| Spleen involvement | 24 (18) | 35 (8) | .001 |
| Liver involvement | 12 (9) | 25 (6) | .20 |
| t-DLBCL | 5 (4) | 11 (3) | .82 |
| FL | 3 | 5 | |
| MZBCL | 2 | 6 | |
| HBsAb-positive | 7/59 (12) | 13/135 (10) | .24 |
| HBcAb-positive | 11/22 (50) | 9/57 (16) | .03 |
| Treatment | .12 | ||
| RCHOP | 96 (73) | 339 (80) | |
| RTHPCOP | 31 (24) | 71 (17) | |
| RCEOP | 4 (3) | 12 (3) | |
| Baseline transaminase | .48 | ||
| Grade 0-1 | 122 (93) | 415 (98) | |
| Grade 2 | 7 (5) | 3 (1) | |
| Grade 3 | 2 (2) | 4 (1) | |
| Outcome of patients | |||
| Died of lymphoma | 14 (11) | 45 (11) | .87 |
| Died of hepatic failure | 6 (5) | 1 (0.2) | < .001 |
| Died of other causes | 4 (3) | 7 (2) | .76 |
| Hepatic toxicity | |||
| Grade 3-4 | 36 (27) | 13 (3) | < .001 |
LDH indicates lactate dehydrogenase; PS, ECOG performance status; H/I, high-intermediate; H, high; BM, bone marrow; t-DLBCL, transformed diffuse large B-cell lymphoma; FL, follicular lymphoma; MZBCL, marginal zone B-cell lymphoma; HBsAb, antibody to hepatitis B surface antigen; HBcAb, antibody to hepatitis B core antigen; RCHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; RTHPCOP, rituximab plus cyclophosphamide, teralbicin, vincristine, and prednisone; and RCEOP, rituximab plus cyclophosphamide, epirubicin, vincristine, and prednisone. Grade was defined by the National Cancer Institute of Canada criteria.
Of the patients who were HCV-positive at the time of DLBCL diagnosis, 57 (43%) and 20 (15%) patients were known to have chronic hepatitis (CH) and liver cirrhosis (LC), respectively. Hepatocellular carcinoma (HCC) complications were detected in 7 patients (5%) before immunochemotherapy. The diagnosis of HCC was made by CT or magnetic resonance imaging in 3 patients and by biopsy in 4 patients. Seven patients with CH and one patient with LC were HB serology-positive. One HB serology-positive patient was given prophylactic lamivudine treatment during immunochemotherapy. Twelve patients (9%) had a history of anti-HCV therapy before DLBCL treatment, 2 patients continued anti-HCV therapy during immunochemotherapy, and 5 patients restarted anti-HCV therapy after immunochemotherapy.
Survival analysis
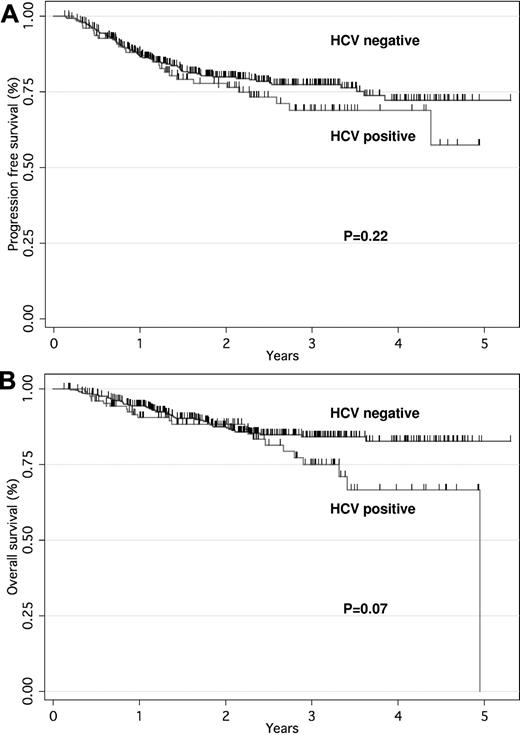
The median follow-up time was 31 months (range, 4-42 months) for patients who were HCV-positive and 32 months (range, 5-51 months) for those who were HCV-negative. Complete remission (CR) or uncertain CR rates were 81% and 83% in HCV-positive and HCV-negative patients, respectively. No significant difference was observed in PFS according to HCV infection (3-year PFS, 69% vs 77%, P = .22). The OS tended to be worse in patients who were HCV-positive than in those who were HCV-negative (3-year OS, 75% vs 84%, P = .07; Figure 1A-B). The PFS and OS rates at 3 years were 56% and 64% for high IPI or high-intermediate and 81% and 88% for low IPI or low-intermediate, respectively. In these cases there were significant differences in the PFS and OS rates (both P < .001). Cox multivariate analysis showed that older age and advanced stage had significant adverse effects on OS. In contrast, HCV infection was not associated with poor PFS or OS (Table 2). According to the cause-specific analysis, survival rates for lymphoma did not significantly correlate with HCV infection (3-year, 84% vs 86%; P = .80).
PFS and OS curves for patients with DLBCL treated with RCHOP according to HCV infection. PFS (A) and OS (B) curves based on patients who were HCV-positive (n = 131) versus HCV-negative (n = 422).
PFS and OS curves for patients with DLBCL treated with RCHOP according to HCV infection. PFS (A) and OS (B) curves based on patients who were HCV-positive (n = 131) versus HCV-negative (n = 422).
Multivariate analysis of survival
| Characteristic . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| HCV infection | ||||||
| Negative | 1.00 | Ref | 1.00 | Ref | ||
| Positive | 0.97 | 0.59-1.60 | NS | 1.22 | 0.69-2.15 | NS |
| Sex | ||||||
| Male | 1.00 | Ref | 1.00 | Ref | ||
| Female | 1.47 | 0.98-2.22 | NS | 1.44 | 0.88-2.37 | NS |
| Age, y | ||||||
| 20-60 | 1.00 | Ref | 1.00 | Ref | ||
| > 60 | 1.02 | 1.01-1.04 | .01 | 1.03 | 1.01-1.06 | .007 |
| LDH | ||||||
| Normal | 1.00 | Ref | 1.00 | Ref | ||
| > Normal | 1.16 | 0.72-1.87 | NS | 1.37 | 0.77-2.43 | NS |
| PS | ||||||
| 0-1 | 1.00 | Ref | 1.00 | Ref | ||
| 2-4 | 1.28 | 1.04-1.59 | .02 | 1.16 | 0.89-1.51 | NS |
| Extranodal sites | ||||||
| 0 or 1 | 1.00 | Ref | 1.00 | Ref | ||
| 2 | 0.77 | 0.45-1.30 | NS | 0.87 | 0.45-1.68 | NS |
| Stage | ||||||
| I, II | 1.00 | Ref | 1.00 | Ref | ||
| III, IV | 1.64 | 1.28-2.11 | < .001 | 1.67 | 1.20-2.32 | .002 |
| Characteristic . | PFS . | OS . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| HCV infection | ||||||
| Negative | 1.00 | Ref | 1.00 | Ref | ||
| Positive | 0.97 | 0.59-1.60 | NS | 1.22 | 0.69-2.15 | NS |
| Sex | ||||||
| Male | 1.00 | Ref | 1.00 | Ref | ||
| Female | 1.47 | 0.98-2.22 | NS | 1.44 | 0.88-2.37 | NS |
| Age, y | ||||||
| 20-60 | 1.00 | Ref | 1.00 | Ref | ||
| > 60 | 1.02 | 1.01-1.04 | .01 | 1.03 | 1.01-1.06 | .007 |
| LDH | ||||||
| Normal | 1.00 | Ref | 1.00 | Ref | ||
| > Normal | 1.16 | 0.72-1.87 | NS | 1.37 | 0.77-2.43 | NS |
| PS | ||||||
| 0-1 | 1.00 | Ref | 1.00 | Ref | ||
| 2-4 | 1.28 | 1.04-1.59 | .02 | 1.16 | 0.89-1.51 | NS |
| Extranodal sites | ||||||
| 0 or 1 | 1.00 | Ref | 1.00 | Ref | ||
| 2 | 0.77 | 0.45-1.30 | NS | 0.87 | 0.45-1.68 | NS |
| Stage | ||||||
| I, II | 1.00 | Ref | 1.00 | Ref | ||
| III, IV | 1.64 | 1.28-2.11 | < .001 | 1.67 | 1.20-2.32 | .002 |
Ref indicates reference; and NS, not significant.
Hepatic toxicity
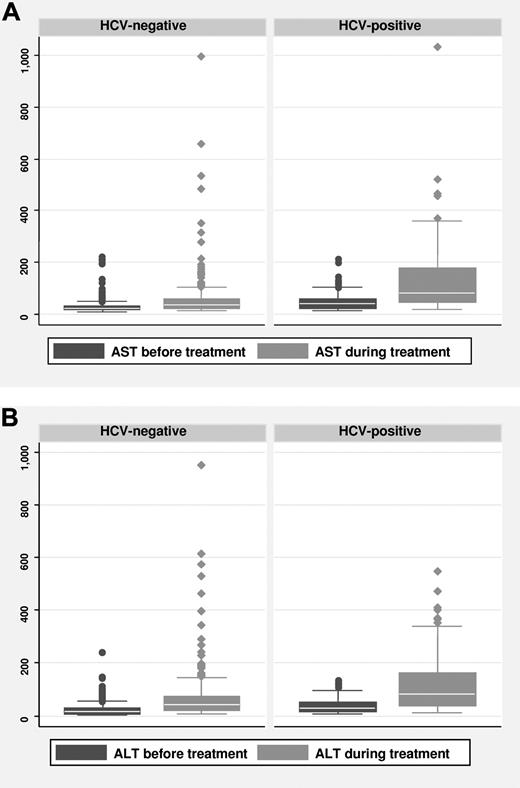
The pretreatment transaminase levels were not significantly different in patients with HCV infection (Table 1). Of the 131 patients who were HCV-positive, 36 (27%) had severe hepatic toxicity, compared with 3% of those who were HCV-negative. Multivariate analysis revealed that HCV infection was a significant risk factor for severe hepatic toxicity (hazard ratio [HR]: 14.72; 95% confidence interval [CI], 6.37-34.03; P < .001; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The changes in AST and ALT levels with respect to HCV infection were significant (both P < .001; Figure 2A-B). The median time from treatment initiation to the development of severe hepatic toxicity was 103 days (range, 8-320 days). In those who were HCV-positive, pretreatment transaminase levels were significant risk factors (HR, 6.42; 95% CI, 1.11-37.12; P < .001) for severe hepatic toxicity among baseline characteristics (supplemental Table 2). In HCV-positive patients, severe hepatic toxicity was observed in 6 (33%) of 18 patients and 30 (27%) of 113 patients with and without omission of prednisone, respectively (P = .82).
The changes of AST and ALT levels between before treatment levels and highest levels up to 6 months after completing immunochemotherapy. The changes in AST (A) and ALT (B) levels with respect to HCV infection were significant (both P < .001).
The changes of AST and ALT levels between before treatment levels and highest levels up to 6 months after completing immunochemotherapy. The changes in AST (A) and ALT (B) levels with respect to HCV infection were significant (both P < .001).
Severe hepatic toxicity was not associated with poor PFS or OS in patients who were HCV-positive (P = .56 and P = .51, respectively). However modification of the scheduled dose was required for 16 patients because of severe hepatic toxicity, and chemotherapy was withdrawn for 6 patients, including 2 who died because of disease progression (Table 3). The remaining 4 patients stopped immunochemotherapy; however, they received radiation therapy after their liver function normalized or they discontinued treatment, and they were alive and in remission.
Details and outcomes of 6 patients withdrawn from immunochemotherapy due to severe hepatic toxicity
| Case . | Age, y . | Sex . | Stage . | Hepatic disease at baseline . | AST/ALT at baseline, IU/L . | AST/ALT peak level, IU/L . | No. of cycles of treatment before withdrawal . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1 | 76 | F | III | LC | 101/73 | 236/114 | 4 | Died of lymphoma progression |
| 2 | 78 | F | III | CH | 38/25 | 1033/340 | 1 | Died of lymphoma progression |
| 3 | 71 | F | I | LC | 90/82 | 289/128 | 1 | Alive |
| 4 | 59 | M | IV | CH | 31/17 | 211/284 | 4 | Alive |
| 5 | 74 | F | IV | Normal | 50/29 | 274/302 | 3 | Alive |
| 6 | 76 | M | II | Normal | 19/25 | 522/550 | 3 | Alive |
| Case . | Age, y . | Sex . | Stage . | Hepatic disease at baseline . | AST/ALT at baseline, IU/L . | AST/ALT peak level, IU/L . | No. of cycles of treatment before withdrawal . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1 | 76 | F | III | LC | 101/73 | 236/114 | 4 | Died of lymphoma progression |
| 2 | 78 | F | III | CH | 38/25 | 1033/340 | 1 | Died of lymphoma progression |
| 3 | 71 | F | I | LC | 90/82 | 289/128 | 1 | Alive |
| 4 | 59 | M | IV | CH | 31/17 | 211/284 | 4 | Alive |
| 5 | 74 | F | IV | Normal | 50/29 | 274/302 | 3 | Alive |
| 6 | 76 | M | II | Normal | 19/25 | 522/550 | 3 | Alive |
Changes in serum T-Bil, Alb, platelet counts, and prothrombin time showed no difference with regard to HCV infection status (supplemental Figure 1). Progression of hepatic complications during treatment occurred in 4 cases; progression from CH to LC, and from LC to HCC, occurred in 2 patients each.
Of the 24 deaths in patients who were HCV-positive, 14 were caused by disease progression, and 6 were caused by hepatic failure (Table 4). The 4 remaining deaths were caused by 2 cardiovascular and 2 brain vascular events. Among HCV-positive patients, HCC was predictive of death from hepatic failure (supplemental Table 3).
Details of 6 patients who died due to hepatic failure
| Case . | Age, y . | Sex . | Stage . | Treatment . | Hepatic disease at baseline . | AST/ALT, IU/L . | HCV-RNA, KIU/mL . | ||
|---|---|---|---|---|---|---|---|---|---|
| At baseline . | Peak level . | At baseline . | Peak level during treatment . | ||||||
| 1 | 75 | M | I | RCEOP | LC | 91/63 | 136/102 | ND | 1000 |
| 2 | 74 | F | IV | RTHPCOP | HCC | 29/16 | 58/49 | 4400 | 5000 |
| 3 | 68 | F | II | RTHPCOP | CH | 118/76 | 203/113 | 510 | 1600 |
| 4 | 71 | F | III | RCHOP | HCC | 34/41 | 242/139 | 470 | ND |
| 5 | 64 | M | IV | RCHOP | HCC | 27/10 | 97/32 | ND | ND |
| 6 | 76 | M | III | RTHPCOP | HCC | 46/40 | 111/107 | 434 | 21 300 |
| Case . | Age, y . | Sex . | Stage . | Treatment . | Hepatic disease at baseline . | AST/ALT, IU/L . | HCV-RNA, KIU/mL . | ||
|---|---|---|---|---|---|---|---|---|---|
| At baseline . | Peak level . | At baseline . | Peak level during treatment . | ||||||
| 1 | 75 | M | I | RCEOP | LC | 91/63 | 136/102 | ND | 1000 |
| 2 | 74 | F | IV | RTHPCOP | HCC | 29/16 | 58/49 | 4400 | 5000 |
| 3 | 68 | F | II | RTHPCOP | CH | 118/76 | 203/113 | 510 | 1600 |
| 4 | 71 | F | III | RCHOP | HCC | 34/41 | 242/139 | 470 | ND |
| 5 | 64 | M | IV | RCHOP | HCC | 27/10 | 97/32 | ND | ND |
| 6 | 76 | M | III | RTHPCOP | HCC | 46/40 | 111/107 | 434 | 21 300 |
ND indicates not done.
In total, 36 HCV-positive patients developed severe hepatic toxicity. At the time they developed severe hepatic toxicity, 29 patients were negative for either HBsAg or HBV-DNA, and 7 patients had no data about HBsAg or HBV-DNA. None of the 36 patients received any anti-HBV viral therapy, such as lamivudine.
HCV viral load
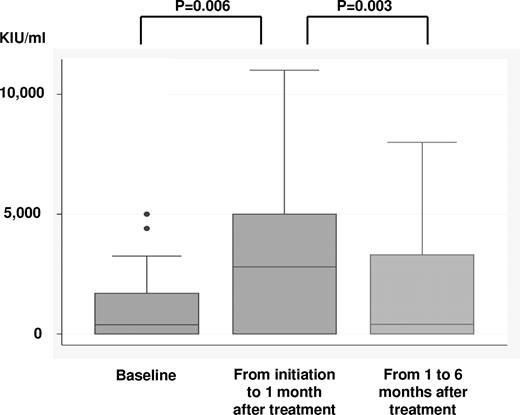
HCV-RNA levels were collected during pretreatment (n = 79), at the highest levels during the period of treatment initiation to 1 mo after treatment completion (n = 47), and 1-6 months after treatment (n = 44). HCV-RNA levels for all 3 time points were available from 34 patients. Mean HCV-RNA levels at the 3 time points as determined from those samples were 1001 (95% CI, 522-1481), 3187 (2148-4232), and 1986 (1145-2827) KIU/mL, indicating that HCV-RNA increased significantly during immunochemotherapy (P = .006) but then decreased afterward (P = .003; Figure 3). In 2 patients who received antiviral therapy during immunochemotherapy, HCV-RNA levels increased after the initiation of immunochemotherapy. In 3 cases where HCV-RNA was under the threshold level (< 5 KIU/mL) before treatment, HCV-RNA increased, more than 100-fold, and 2 patients developed severe hepatic toxicity (Table 5).
HCV-RNA levels at 3 points were available in 34 patients; pretreatment level, highest levels from initiation of treatment to 1 month after completing treatment, and from 1 to 6 months after treatment. HCV-RNA significantly increased during immunochemotherapy (P = .006) and then decreased after treatment (P = .003).
HCV-RNA levels at 3 points were available in 34 patients; pretreatment level, highest levels from initiation of treatment to 1 month after completing treatment, and from 1 to 6 months after treatment. HCV-RNA significantly increased during immunochemotherapy (P = .006) and then decreased after treatment (P = .003).
Details and outcomes of the 3 DLBCL patients with HCV-RNA below threshold level before immunochemotherapy
| Case . | Age, y . | Sex . | HCV-RNA level, KIU/mL . | AST/ALT, IU/L . | Outcome/follow-up time, (mo) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| At baseline . | Peak level during treatment . | At last evaluation . | At baseline . | Peak level during treatment . | At last evaluation . | ||||
| 1 | 59 | M | < 5 | 3960 | 79 | 19/12 | 212/71 | 44/18 | Alive/6 |
| 2 | 77 | F | < 5 | 1200 | < 5 | 40/15 | 156/125 | 33/34 | Alive/28 |
| 3 | 74 | F | < 5 | 590 | 270 | 50/29 | 274/302 | 34/29 | Alive/28 |
| Case . | Age, y . | Sex . | HCV-RNA level, KIU/mL . | AST/ALT, IU/L . | Outcome/follow-up time, (mo) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| At baseline . | Peak level during treatment . | At last evaluation . | At baseline . | Peak level during treatment . | At last evaluation . | ||||
| 1 | 59 | M | < 5 | 3960 | 79 | 19/12 | 212/71 | 44/18 | Alive/6 |
| 2 | 77 | F | < 5 | 1200 | < 5 | 40/15 | 156/125 | 33/34 | Alive/28 |
| 3 | 74 | F | < 5 | 590 | 270 | 50/29 | 274/302 | 34/29 | Alive/28 |
Discussion
This study showed that prognosis did not differ according to HCV infection. In sharp contrast, the incidence of severe hepatic toxicity was high in HCV-positive patients, and HCV infection was determined to be a strong risk factor for this adverse effect. These findings were not consistent with previous reports demonstrating good tolerance for HCV infection in the pre-rituximab era.8,10,11,24 This is the first large study to show the influence of HCV infection on prognosis and hepatic toxicity in patients with DLBCL in the rituximab era.
A previous report showed that HCV-positive patients with DLBCL exhibited worse OS, but not event-free survival; however, this study included a very small number of HCV-positive patients (n = 23) and was based on short-term follow-up.9 Another large scale study of DLBCL demonstrated that the 5-year PFS was 51% and OS was 72%, but the relationship between HCV infection and outcome in this study remained unclear, because of the lack of an HCV-negative control group.10 In addition, these studies were performed in the pre-rituximab era, and no large-scale study has compared the outcome for DLBCL treated with rituximab according to HCV infection. The present study found that HCV-positive patients exhibited more aggressive baseline behavior, consistent with previous reports. These patients also exhibited borderline poor OS by univariate analysis. However, it is important to note that no significant difference was observed in CR rates or PFS according to HCV infection, and multivariate analysis revealed that HCV infection was not a significant risk factor for prognosis.
In the pre-rituximab era, several series showed good tolerance to standard chemotherapy for HCV-infected patients with lymphoma or other hematologic malignancies, with greater than grade 3 liver dysfunction in 10%-18% of patients, and grades 1-4 liver dysfunction in 24% of patients.10,11,25 In contrast, Besson et al found a higher incidence of toxic deaths among HCV-positive DLBCL patients compared with HCV-negative patients, although this study was based on patients treated with more aggressive chemotherapy than the standard CHOP regimen.9 A recent analysis also showed an increase in hepatic toxicity in HCV-positive patients with B-cell lymphoma.26 However, this study included no control group of HCV-negative patients and included heterogeneous treatment strategies and several lymphoma subtypes. In addition, previous series did not exclude HBsAg-positive patients, known to be a high-risk population for severe hepatic injury or fulminant hepatitis.9-11,25,26 Our study showed that the incidence in severe hepatic toxicity in HCV-positive patients was significantly higher than that of HCV-negative patients (27% vs 3%; P < .001), and that these hepatic toxicities led to modification and discontinuation of immunochemotherapy, resulting in lymphoma progression. Careful monitoring of hepatic function should, thus, be recommended for HCV-positive patients, particularly those with high levels of pretreatment transaminase.
Recent case reports have suggested that combined use of rituximab and chemotherapy poses an additional risk for exacerbation of HCV infection.27-29 However, HCV replication during chemotherapy has not been well characterized. In the present study, monitoring of HCV viral load demonstrated a marked enhancement in HCV replication, and it is suggested that increased HCV expansion results in severe hepatic toxicity. Thus, HCV viral load should also be carefully monitored in HCV-positive patients who receive immunochemotherapy, although this finding must be considered with caution because of the small sample size. In addition, this study revealed that HCV-RNA levels could increase during immunochemotherapy, even though HCV-RNA levels were extremely low (< 5 KIU/mL) before treatment.
Recent reports have indicated that HBsAg-negative/anti-HBc or HBs-positive patients could also develop HBV reactivation after rituximab treatment.30,31 Among the HCV-positive patients analyzed in the present study for HBV-DNA and HBsAg at the time of hepatic dysfunction, none was positive, and in all patients, severe hepatic toxicity improved with no anti-HBV treatment. In addition, neither anti-HBs– nor anti-HBc–positive patients were found to be in a significant risk group for severe hepatitis (anti-HBs; HR, 0.42; 95% CI, 0.03-5.81, anti-HBc; HR, 0.23; 95% CI, 0.01-1.98). Although we could not completely rule out the possibility of HBV reactivation, we suggest that HCV rather than HBV contributed to severe hepatic toxicity in the present cohort infected with HCV.
In accordance with previous reports,9-11 HCV-positive patients in the present unmatched study had more aggressive tumor behavior at baseline and more frequent spleen involvement. The mechanisms underlying the association of HCV infection with aggressive tumor behavior are not well understood. Further studies of the biological features of HCV-infected DLBCL, such as germinal center phenotype, are necessary.
HCV infection results in a long-term risk for progression to LC and HCC. One study reported a rapid progression of hepatitis C in patients with humoral immunodeficiency disorders,32 and an increased rate for liver fibrosis progression was observed in HCV-infected patients who received an allogeneic bone marrow transplant.33 A possible explanation for the genesis of cirrhosis could be an immune imbalance or impaired regulation of B and T cells.32,33 We showed that hepatic synthesis after treatment was not affected by HCV infection. However, hepatic disease progressed in 4 patients, and HCC was found to significantly increase the risk of death of hepatic failure, even during short-term observation. Further studies are necessary to clarify the contribution of rituximab to the risk of progressive liver damage.
Although we believe that our data provide novel information about rituximab-treated patients who are HCV-positive with DLBCL, some limitations of these findings should be discussed. First, because this retrospective study included enrollment from many institutions, unrecognized biases might have been introduced. Second, we did not register all patients who were HCV-negative during the study period, but only those who were treated over the same time period as patients who were HCV-positive at each institution. This might have caused case-selection bias. Finally, we did not define the timing of immunochemotherapy withdrawal because of hepatic toxicity as we believe that the decision to terminate immunochemotherapy must be made by the treating physician.
In conclusion, our study highlighted a high incidence of severe hepatic toxicity in patients who were HCV-positive, so hepatic function should be carefully monitored in patients who are HCV-positive and receive immunochemotherapy. Well-designed studies will be necessary to determine whether early detection and prevention of HCV replication would provide improved disease management for HCV infected patients receiving immunochemotherapy.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
This study was presented in part at the 51st annual meeting of the American Society of Hematology, New Orleans, LA, December 5-8, 2009.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all of the clinicians and pathologists in the participating institutes for their invaluable contributions to this multicenter study.
This study was supported by a Grant-in-Aid (21-6-3) for Cancer Research from the Ministry of Health, Labor and Welfare of Japan.
Authorship
Contribution: D.E. and Y.M. designed research, performed research, analyzed data, and wrote the paper; N.N, M.K, K.I, J.T, S.K, M.O, M.Y., and A.T. designed and performed research; Y.T, K.S, A.M, K.M, A.S, M.M., and K.S. performed research; K.M. designed research, analyzed data, and wrote the paper; and T.K and T.M. designed research and gave final approval.
Conflict-of-interest disclosure: S.K. received research funding from Chugai Pharmaceutical Co Ltd; M.Y. is a medical advisor to Chugai Pharmaceutical Co Ltd; T.K. received honoraria from Chugai Pharmaceutical Co Ltd and Zenyaku Kogyo Co Ltd, and research funding from Zenyaku Kogyo Co Ltd. The remaining authors declare no competing financial interests.
Correspondence: Daisuke Ennishi, MD, Department of Hematology and Oncology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Science, 2-5-1 Shikata-cho, Okayama City, Okayama 700-8558, Japan; e-mail: daisukeennishi@yahoo.co.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal