Abstract
In this report, we investigated the mechanism responsible for synergistic induction of myeloma cell apoptosis induced by the combination of tipifarnib and bortezomib. Immunofluorescence studies revealed that bortezomib alone resulted in an accumulation of puncta of ubiquitinated proteins that was further enhanced by the addition of tipifarnib. These data suggest inhibition of the degradation of bortezomib-induced aggresomes; and consistent with this possibility, we also observed an increase in p62SQSTM1 in cells treated with the combination. However, autophagy in these cells appears to be normal as LC3BII is present, and autophagic flux appears to be unaffected as demonstrated by the addition of bafilomycin A1. Together, these data demonstrate that tipifarnib synergizes with bortezomib by inducing protein accumulation as a result of the uncoupling of the aggresome and autophagy pathways.
Introduction
We have previously reported that the farnesyl transferase inhibitor (FTI) lonafarnib (SCH66336) combined with the proteasome inhibitor bortezomib induced synergistic apoptosis in myeloma cell lines and primary patient cells; however, the mechanism responsible for this synergy was unclear.1-5 Whereas many groups have speculated that FTIs induce their effect via ras signaling,6-8 others have suggested alternative pathways by which FTIs exert their anticancer effects.9-11 Marcus et al first reported that FTIs inhibit histone deacetylase 6 expression, resulting in destabilization of the microtubule assembly structure, and impaired cell survival in nonsmall cell lung cancer cell lines.12 Based on published preclinical data demonstrating that histone deacetylase inhibitors synergize with proteasome inhibition via inhibition of aggresome formation, we sought to determine whether the in vitro synergy we have previously observed using the combination of an FTI with bortezomib was a result of dual blockade of the proteasome and aggresome pathways of protein catabolism.
Methods
The MM.1S (provided by S. Rosen, Chicago, IL) and RPMI8226 (ATCC) cell lines were used in this study. Tipifarnib (R115777) was provided by Johnson & Johnson Pharmaceuticals, and bortezomib was provided by Millennium Pharmaceuticals. Methyl-thiazol-tetrazolium assays, annexin V staining, and sodium dodecyl sulfate–polyacrylamide gel electrophoresis were performed according to previously published methods.1,5,13 The antibodies used included antiubiquitin, anticaspase-8, anticaspase-9, anticaspase-3, and anti–poly-adenosine diphosphate or PARP ribose polymerase (Cell Signaling). p62SQSTM1 antibody (MBL International), bafilomycin A1, and LC3B antibody (Sigma-Aldrich) were used to assess autophagy. Confocal microscopy was performed using standard methods with minor modifications to assess aggresome formation.12,13 Vimentin (Sigma-Aldrich) and γ-tubulin (Abcam) antibodies were used in the evaluation of aggresome formation by confocal immunofluorescence microscopy.
Results and discussion
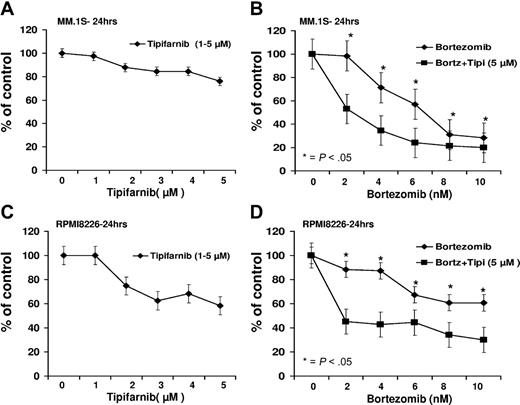
Consistent with our previous findings using lonafarnib in myeloma cells, the combination of tipifarnib and bortezomib resulted in greater growth inhibition than when either agent was used separately in both MM.1S as well as in RPMI8826 with concentrations up to 10nM of bortezomib and 5μM of tipifarnib (Figure 1). Combination therapy with low-dose bortezomib and tipifarnib resulted in enhanced myeloma cell apoptosis, which was demonstrated to be synergistic as evidenced by a low combination index (< 0.6) in these MM cell lines (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Growth inhibition dose-response curves. (A-D) Growth inhibition dose-response curve for MM.1S and RPMI8226 cell lines with bortezomib, tipifarnib, or the combination for 24 hours. *P < .05.
Growth inhibition dose-response curves. (A-D) Growth inhibition dose-response curve for MM.1S and RPMI8226 cell lines with bortezomib, tipifarnib, or the combination for 24 hours. *P < .05.
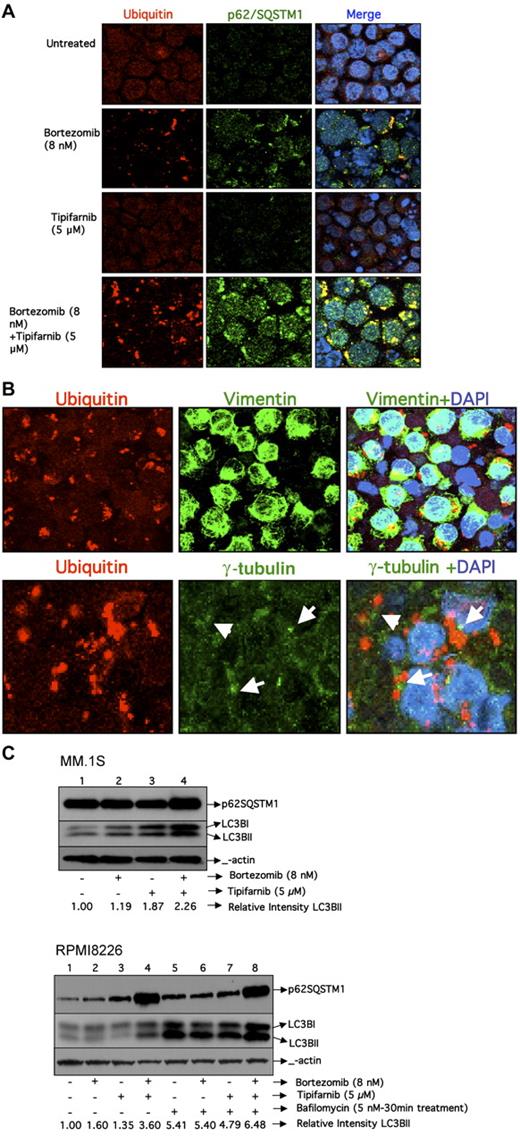
We then determined the effects of single-agent or combination therapy on ubiquitinated proteins in RPMI8226 cells using confocal fluorescence microscopy. Consistent with previous findings, the use of bortezomib results in the accumulation of ubiquitinated protein aggregates in the cytoplasm (Figure 2A), which was not seen with tipifarnib alone, and was enhanced when bortezomib and tipifarnib were given in combination. To further characterize the nature of these ubiquitin aggregates, we investigated the other protein components of the aggregates. p62/SQSTM1 is a ubiquitin-binding protein that is responsible for localizing ubiquitin-containing aggresomes with the autophagy membranes.14-17 As seen in Figure 2A, p62SQSTM1 is colocalized with ubiquitin-containing aggregates, suggesting that these aggregates are aggresomes. To validate these findings, we determined whether other aggresome-related proteins colocalized with the ubiquitin-containing aggregates. Consistent with this possibility, vimentin also colocalized with the ubiquitin aggregate, whereas α-tubulin was not part of the aggregate but was proximal (Figure 2B), suggesting that the aggregates are proximal to the microtubule-organizing center. Taken together, these data suggest that the aggregates that are accumulating are indeed aggresomes. The accumulation of aggresomes observed with combination therapy could result from inhibition of autophagy or the lysosome degradation of the aggresome late in the autophagy pathway. To distinguish between these possibilities, we investigated the effects of tipifarnib with or without bortezomib on the accumulation of LC3BII in the MM.1S and RPMI 8226 cell lines. Activation of autophagy results in lipidation of LC3B, which can be detected by faster migration of the protein in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (LC3BII). As seen in Figure 2C, both p62SQSTM1 and LC3BII are increased when cells are treated with the combination of bortezomib and tipifarnib in MM.1S cells by Western blotting. However, because both LC3BII and p62 are degraded by autophagy, an increase in the steady-state levels could be the result of either activation or inhibition of autophagy. To discriminate between these possibilities, RPMI8226 cells were treated with bafilomycin A1 during the last 30 minutes of incubation. Bafilomycin A1 is a vacuolar type H+-ATPase inhibitor that suppresses acidification and, therefore, lysosomal-dependent protein degradation in the autophagosome.18 The accumulation of LC3BII was noted to occur independent of bortezomib or tipifarnib addition as well as the presence or absence of bafilomycin A1 (Figure 2C). Because the increase in LCB3II occurred in the untreated and treated cells, these data suggest that autophagy is active in these cells and unaffected by exposure to bortezomib, tipifarnib, or the combination. However, among cells treated with bafilomycin A1, p62SQSTM1 expression was increased only in the untreated cells. In aggregate, these data suggest that tipifarnib blocks lysosomal-dependent degradation of bortezomib-induced aggresomes without inhibition of the early steps of autophagy.
Assessment of autophagy. (A) Confocal images of RPMI8226 cell line treated with bortezomib, tipifarnib, or the combination for 24 hours. Images were acquired using a Zeiss LSM 510 confocal mounted on a Zeiss Axioplan 2 microscope. A 63× oil objective (NA 1.4) was used for image acquisition using Zeiss LSM software Version 4.2. All images were acquired with equivalent acquisition settings to allow for intensity measurements between samples. Images were processed in Adobe Photoshop (CS3) with equal contrast expansion for image display. Cells were stained with antibodies against ubiquitin and p62 SQSTM1antibody as well as 4,6-diamidino-2-phenylindole. Colocalization of ubiquitin and p62SQSTM1 in the merged image is visualized in yellow. (B) Confocal images of RPMI8226 cells that were treated with bortezomib and tipifarnib combination for 24 hours. Cells were stained for ubiquitin and then separately stained with vimentin and γ-tubulin. Colocalization of ubiquitin and vimentin in the merged image is visualized in yellow. γ-Tubulin is visualized in the periphery of ubiquitin aggregates distinct from the ubiquitin aggregates. (C) Western blotting for p62SQSTM1 and LC3BII inMM.1S cells and also in RPMI8226 cells in the presence and absence of bafilomycin A1. Relative intensity of LC3BII was calculated by normalizing the untreated control to a relative intensity of 1.0 and then dividing subsequent LC3BII band intensity by actin band intensity in the same treatment group using densitometry.
Assessment of autophagy. (A) Confocal images of RPMI8226 cell line treated with bortezomib, tipifarnib, or the combination for 24 hours. Images were acquired using a Zeiss LSM 510 confocal mounted on a Zeiss Axioplan 2 microscope. A 63× oil objective (NA 1.4) was used for image acquisition using Zeiss LSM software Version 4.2. All images were acquired with equivalent acquisition settings to allow for intensity measurements between samples. Images were processed in Adobe Photoshop (CS3) with equal contrast expansion for image display. Cells were stained with antibodies against ubiquitin and p62 SQSTM1antibody as well as 4,6-diamidino-2-phenylindole. Colocalization of ubiquitin and p62SQSTM1 in the merged image is visualized in yellow. (B) Confocal images of RPMI8226 cells that were treated with bortezomib and tipifarnib combination for 24 hours. Cells were stained for ubiquitin and then separately stained with vimentin and γ-tubulin. Colocalization of ubiquitin and vimentin in the merged image is visualized in yellow. γ-Tubulin is visualized in the periphery of ubiquitin aggregates distinct from the ubiquitin aggregates. (C) Western blotting for p62SQSTM1 and LC3BII inMM.1S cells and also in RPMI8226 cells in the presence and absence of bafilomycin A1. Relative intensity of LC3BII was calculated by normalizing the untreated control to a relative intensity of 1.0 and then dividing subsequent LC3BII band intensity by actin band intensity in the same treatment group using densitometry.
The aggresome/autophagy pathway is an important accessory pathway that is critical for the degradation of many proteins and is even more important when the proteasome/ubiquitin pathway is blocked. The combination of LBH589, vorinostat, or romidepsin with bortezomib has been shown to inhibit aggresome formation and result in apoptosis, probably as a result of accumulation of toxic proteins that are not subsequently packaged for disposal via the aggresome/autophagy pathway.13,19-25 It has been presumed that accumulation of unpackaged misfolded proteins is toxic to cells and that packaging of these proteins into aggresomes results in their disposal and therefore protects the cell from toxic injury. However, the pathway for catabolism of proteins via autophagy may be more complex. Aggresome formation and packaging of proteins for degradation are clearly an important step in the cells' ability to manage misfolded proteins; however, it must be coupled with efficient delivery of the aggresome to the autophagosome for degradation. Our current data support the hypothesis that, in addition to aggresome formation, aggresome disposal is an equally important step in the process, one that can be regulated by agents, such as tipifarnib, and therefore represents a novel drug target. In addition, the packaging of misfolded proteins alone is not sufficient to protect the cell as accumulation of aggresomes without appropriate disposal can also induce apoptosis as demonstrated by our data. Accumulation of aggresomes represents a novel mechanism by which to induce apoptosis, especially among cells where protein production and catabolism are critically important for malignant cell survival, as is the case for malignant and normal immunoglobulin-producing plasma cells. We have demonstrated that, when tipifarnib is combined with bortezomib in malignant plasma cells, aggresome formation is enhanced, yet there is an uncoupling of aggresome formation from autophagic degradation that leads to synergistic apoptosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Johnson & Johnson Pharmaceuticals and Millennium Pharmaceuticals for providing tipifarnib (R115777) and bortezomib, respectively, the Cell Imaging and Microscopy Core, Winship Cancer Institute of Emory University for their support and service, and Dr Jay Debnath for providing helpful advice on measuring autophagy.
S.L. is supported by the Leukemia & Lymphoma Society (Translational Research Grant) and the Multiple Myeloma Research Foundation (Combination Therapy Grant).
Authorship
Contribution: E.D. was involved in experimental design and execution and manuscript preparation; J.L.K., C.R.F., J.C., S.-Y.S., A.I.M., C.T., and K.S.-H. were involved in the experimental design, data interpretation, and manuscript preparation; and L.H.B. and S.L. participated in experimental design, data interpretation, and manuscript preparation.
Conflict-of-interest disclosure: S.L. reports consulting and research support from Millennium, Celgene, Bristol-Myers Squibb, and Novartis. J.L.K. reports consulting with Millennium and Celgene and research support from Merck and Celgene. The remaining authors declare no competing financial interests.
Correspondence: Sagar Lonial, Department of Hematology and Medical Oncology, Winship Cancer Institute of Emory University, 1365 Clifton Rd NE, Atlanta, GA 30322; e-mail: sloni01@emory.edu.