Abstract
The internal tandem duplication (ITD) mutations of the FMS-like tyrosine kinase-3 (FLT3) receptor found in acute myeloid leukemia patients are associated with poor prognosis. Although DNA double-strand breaks (DSBs) are mainly repaired by the DNA-PK–dependent nonhomologous end-joining (NHEJ) pathway in normal mammalian cells, an alternative and less well-defined NHEJ pathway, characterized by microhomology at the repair junctions, play a role in the generation of deletions and translocations leading to cancer progression. Here we report that in FLT3/ITD-expressing cell lines and bone marrow mononuclear cells from FLT3/ITD knock-in mice, end-joining of DSBs occurs at microhomologous sequences resulting in a high frequency of DNA deletions. Strikingly, levels of Ku proteins, key components of the main NHEJ pathway, are decreased in FLT3/ITD+ cell lines and murine FLT3/ITD bone marrow mononuclear cells. Concomitantly, levels of DNA ligase IIIα, a component of ALT NHEJ, are increased in FLT3/ITD-expressing cells. Cells treated with a FLT3 inhibitor demonstrate decreased DNA ligase IIIα and a reduction in DNA deletions, suggesting that FLT3 signaling regulates the pathways by which DSBs are repaired. Thus, therapy to inhibit FLT3/ITD signaling and/or DNA ligase IIIα may lead to repair that reduces repair errors and genomic instability.
Introduction
Several lines of evidence suggest that FMS-like tyrosine kinase-3 (FLT3) plays roles in survival, proliferation, and differentiation.1 Aside from its role in regulating normal hematopoiesis, FLT3 is also highly expressed in several hematologic malignancies.2 Mutations in the receptor, both in the form of internal tandem duplication (ITD) of the juxtamembrane domain and point mutations of the kinase domain, result in constitutive activation.2 These mutations occur in approximately one-third of acute myeloid leukemia (AML) patients, making FLT3 one of the most commonly mutated genes in AML.3,4 Patients with FLT3/ITD mutations have a very poor prognosis.5-7 However, the molecular basis by which FLT3/ITD mutations lead to aggressive disease and poor prognosis in AML is not clearly understood.
We previously demonstrated that FLT3/ITD mutations have the potential to initiate a cycle of genomic instability, creating additional mutations.8 Specifically, cells carrying the FLT3/ITD mutation induce increased production of reactive oxygen species (ROS). Furthermore, cells transformed by FLT3/ITD mutations, primary FLT3/ITD AML cells, and cell lines established from FLT3/ITD AML patients have increased endogenous DNA double-strand breaks (DSBs), as measured by immunostaining for γH2AX foci. FLT3/ITD-expressing cells also demonstrate aberrant repair of DSBs by homologous recombination and nonhomologous end-joining (NHEJ), the 2 major DSB repair pathways in mammalian cells.8,9 In particular, we demonstrated that FLT3/ITD-expressing cells repair DSBs by NHEJ with increased repair infidelity, compared with these same cells treated with an FLT3 inhibitor.8
The major NHEJ pathway in human cells is initiated by binding of the Ku70/86 heterodimer to DSBs, followed by the recruitment of DNA-dependent protein kinase catalytic subunit (DNA-PKCs) to form the active DNA-dependent protein kinase (DNA PK).10-12 In addition to its essential kinase activity, DNA-PKCs are the end-bridging factor responsible for synapsis of DNA ends. After protein-mediated end-bridging, the DNA ends are subsequently ligated by DNA ligase IV in conjunction with XRCC4.13 The majority of DSBs generated by agents, such as ROS and x-ray radiation, rarely have ligatable 5′-P and 3′-OH termini. Therefore, the synapsed DNA ends must be processed before ligation by DNA ligase IV and XRCC4 during NHEJ. Many of these processing events involve a nuclease because repair by NHEJ frequently results in small DNA deletions (up to ∼ 20 bp), resulting from resection of the DNA ends.14,15
Several lines of evidence suggest that alternative and less well-defined backup end-joining (ALT NHEJ) pathways play an important role in physiologic and pathologic DSB repair.16 First, substantial end-joining occurs in response to γ-irradiation even in the absence of major NHEJ factors, such as the DNA ligase IV and Ku or proteins that aggregate on damaged chromatin.17,18 Second, chromosomal abnormalities, including c-myc/IgH translocations, are observed even in the absence of Ku or the ligase complex component XRCC4.19 Third, rare aberrant V(D)J coding joins are found in lymphocytes lacking Ku and the DNA-PKcs.20 Finally, mice lacking either Ku or XRCC4, as well as the p53 tumor suppressor protein, invariably develop pro-B lymphomas that result from translocations between the IgH locus and c-myc.21,22 The hallmark features of the ALT NHEJ pathway are large DNA deletions, insertions, and repair junctions marked by DNA sequence microhomology. We recently demonstrated increased ALT NHEJ activity in BCR-ABL+ chronic myeloid leukemia cells in that increased expression of DNA ligase IIIα ((Lig IIIα) with concomitant down-regulation of the main NHEJ proteins, Artemis and DNA ligase IV, was observed.23 BCR-ABL+ cells were characterized by an increased frequency of DNA deletions and repair of DSBs involving regions of microhomology. Importantly, siRNA knockdown of DNA Lig IIIα in BCR-ABL+ cells resulted in an increased frequency of DSBs, a decreased end-joining efficiency and repair using nucleotide sequence microhomologies.23
Here, we report that both FLT3/ITD-expressing cell lines and bone marrow mononuclear cells (BM MNCs) from knock-in mice expressing an FLT3/ITD mutation generate a high frequency of DNA abnormalities resulting from illegitimate ligation of DSBs characterized by larger DNA deletions in in vivo repair assays, compared with controls. Furthermore, steady-state levels of Ku proteins are significantly decreased, whereas the ALT NHEJ component, DNA Lig IIIα, protein levels are increased. Importantly, treatment of these cells with a FLT3 inhibitor or siRNA knockdown of DNA Lig IIIα leads to not only a decrease in DNA Lig IIIα expression levels but also to a decrease in NHEJ repair abnormalities, suggesting the possible reduction or abrogation of pathways that can lead to genomic instability.
Methods
Cells and reagents
Human AML cell line MOLM-14 (expressing FLT3/ITD mutations), and leukemia REH cell line (expressing wild-type [WT] FLT3) were obtained from ATCC. The BaF3/ITD cell line was generated by stably expressing an FLT3/ITD cDNA in the mouse pro-B lymphocyte line BaF3.24 Cells were maintained at 37°C in the presence of 5% CO2 in RPMI 1640 medium plus 10% heat-inactivated fetal bovine serum (ATCC), 5% penicillin/streptomycin, and 2mM l-glutamine. Growth medium of BaF3 cells was supplemented with interleukin-3 (1 ng/mL).
The FLT3 inhibitor, CEP-701, was purchased from LC Laboratories for in vitro studies. Cepahalon kindly provided the inhibitor for in vivo studies. CEP-701 was stored as a 4mM stock solution in dimethyl sulfoxide at −70°C.
Mouse bone marrow cells
FLT3/ITD knock-in mice were maintained in a C57BL/6 background and housed in microisolator cages in a pathogen-free animal facility. Mouse genotyping analysis was performed as previously described.25 Red blood cell lysis buffer (10mM KHCO3, 155mM NH4Cl, 0.1mM ethylenediaminetetraacetic acid, pH 8.0) was used to lyse red blood cells in the BM before subsequent experiments.
In vivo treatment of mice with FLT3 inhibitor
Mice were administered with 20 mg/kg CEP-701 or vehicle control intraperitoneally every 12 hours for 3 times and killed 4 hours after the last injection. BM was collected and subjected to subsequent experiments.
In vivo DNA NHEJ repair
The in vivo NHEJ assay was based on the reactivation of linearized plasmid. Cells (2-5 × 106) were transfected with 0.5 to 1.5 μg plasmid pUC18 DNA (linearized with EcoRI followed by dephosphorylation), using the nucleofection method (Amaxa). In all transfections, kit V was used, together with program O-017 (MOLM-14), X-001 (BaF3, BaF3/ITD), and T-020 (BM MNCs). Transfected cells were incubated in RPMI 1640 medium overnight. Cells were harvested and washed twice with phosphate-buffered saline. Plasmid DNA was recovered from the cells using QIAprepSpin miniprep kit (QIAGEN) and used to transform competent bacterial DH5α cells (Invitrogen). Bacterial colonies were used to assess several repair parameters: NHEJ repair efficiency was measured by the total bacterial colonies; correct repair and misrepair of DSBs were represented by blue and white bacterial colony, respectively; the misrepair frequency was determined by the number of white colonies as a percentage of total colonies; and the nature of the misrepair was determined by polymerase chain reaction (PCR) and/or DNA sequencing of the breakpoint junction region in individual white colonies.
For statistical analysis, repaired DNA from each transfection was used to perform at least 3 bacterial transformations in parallel. The colony number (in most cases, 100-300 per plate) and percentage misrepair of each transfection were represented by the average values of all the transformations. Transfections were performed at least 3 times, and the mean and error values (expressed as SDs) were calculated based on the values of all transfections.
Colony PCR and plasmid DNA sequencing
Colony PCR was performed using regular Taq DNA polymerase (Invitrogen) and a pair of primers designed to amplify the fragment across EcoRI cutting site of the pUC18 DNA (pUC18: forward, CGGCATCAGAGCAGATTGTA; reverse, TGGATAACCGTATTACCGCC). Randomly selected white bacterial colonies, which contain misrepaired plasmid, were used as the PCR template. PCR products were resolved in 1% agarose gel by electrophoresis. A 628-bp product is expected using correctly repaired template. For sequence analysis, plasmid DNA was prepared using QIAprepSpin miniprep kit (QIAGEN) and sequenced using either pUC18 forward or pUC18 reverse primer (University of Maryland). The Blast program from NCBI web site was used for sequence alignment.
Western blotting analysis
Total cell extracts were prepared by lysing cells in radio immunoprecipitation assay buffer (RIPA; 25mM Tris-HCl, pH 7.5, 0.1% sodium dodecyl sulfate, 1% sodium deoxycholate, 1% NP-40) containing 0.5mM phenylmethylsulfonyl fluoride and Complete protein inhibitor cocktail (Roche Diagnostics). Nuclear extracts were prepared using the kit from Sigma-Aldrich (product code NXTRACT). Extracts were separated in a 10% polyacrylamide gel. After being transferred to polyvinylidene difluoride membrane, proteins were detected with various antibodies: anti-DNA Lig IIIα (Sigma-Aldrich; HPA006723), anti-Ku70 (Santa Cruz Biotechnology; sc-17789), anti-Ku86 (Santa Cruz Biotechnology; sc-1484), and anti-actin (Abcam; ab6276).
siRNA transfection
siRNA oligonucleotides were purchased from Dharmacon RNA Technologies. MOLM-14 cells were transiently transfected with the siRNA oligonucleotides for DNA Lig IIIα by the nucleofection method with kit V (Amaxa). For each transfection, 5 μL of 20μM siRNA was used for 2.5 × 106 cells. Three days after transfection, cells were harvested either to generate protein for Western blotting or to perform the in vivo plasmid DSB repair assay. Scrambled oligonucleotide siRNA was used as a control.
Ligation activity assay
The ligation activity in cell extracts from mouse BM MNCs was assessed by sealing a DNA substrate containing a nick.26 The substrate was prepared by annealing 3 DNA oligos: 15P (CTGCAGCTGATGCGC), pC19 (pCGTACGGATCCCCGGGTAC), and 34G (GTACCCGGGGATCCGTACGGCGCATCAGCTGCAG). The 15P oligo was labeled by 32P at the 5′ end. Ligation between 15P and pC19 results in a 34-nt fragment. Whole cell extracts were prepared by sonicating cells suspended in lysis buffer (50mM Tris-HCl, pH 7.4, 1mM ethylenediaminetetraacetic acid, 1mM dithiothreitol, 10% glycerol, 0.5mM phenylmethylsulfonyl fluoride, and Complete protein inhibitor cocktail, Roche Diagnostics). Ligation reactions were performed at 37°C for 2 hours in buffer (50mM Tris-HCl, pH 7.4, 1mM dithiothreitol, 5% glycerol and 5mM adenosine triphosphate), using 1 pmol nick substrate and 5 μg cell extracts. Reactions were separated in 12% denaturing acrylamide gel, and the products were detected by phosphor imagining.
Results
FLT3/ITD expression confers decreased end-joining efficiency, increased misrepair, and DNA deletions
To molecularly characterize end-joining repair, in vivo plasmid reactivation assays were performed, as we have previously described.23 In this assay, the repair of a single DSB within the LacZα gene of the pUC18 plasmid transfected into cells of interest, is used to quantitatively assess multiple repair parameters. After transformation of Escherichia coli with repaired plasmid DNA, the repair efficiency was determined by the total number of bacterial colonies produced. Correct versus incorrect repair was estimated from the number of blue (correctly repaired LacZα leading to gene expression) versus white (incorrectly repaired LacZα giving no gene expression) colonies produced. The type of repair abnormalities in individual colonies was examined by PCR of the region spanning the DSB in individual repaired plasmids, followed by gel electrophoresis and/or DNA sequencing of the region encompassing the DSB repair junction (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).27
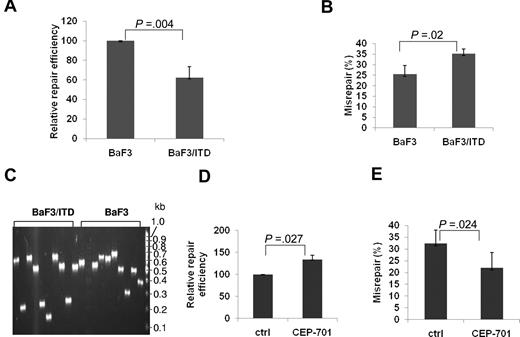
To molecularly characterize NHEJ repair related to FLT3/ITD expression, we examined BaF3 cells that stably express the FLT3/ITD mutation (BaF3/ITD),28 using the plasmid reactivation assay described in this section, and compared results with those from parental BaF3 control cells. As shown in Figure 1A, the NHEJ efficiency is significantly decreased (62%) in BaF3/ITD cells, compared with parental BaF3 cells (P = .004). Moreover, BaF3/ITD cells demonstrate increased repair errors (35%), compared with BaF3 cells (25%; P = .02; Figure 1B). To determine the nature of the repair errors, PCR of the breakpoint junction region was performed in a randomly selected set of 9 or 10 white (misrepaired) bacterial colonies from both BaF3/ITD cells and parental controls per experiment. NHEJ products from BaF3/ITD cells demonstrate a trend of increased deletion size in the repaired products, compared with those colony PCR products from BaF3 cells, as shown in the representative gel image (Figure 1C). Furthermore, when BaF3/ITD cells were treated with the FLT3 inhibitor, CEP-701, the repair efficiency is increased (Figure 1D, P = .027), whereas the misrepair frequency is dramatically decreased (Figure 1E, P = .024). These results suggest that FLT3/ITD expression leads to decreased efficiency and increased errors of NHEJ.
BaF3/ITD cells show decreased NHEJ efficiency and increased repair errors compared with parental BaF3 control cells. Relative NHEJ repair efficiency (A) and percentage of DSBs that are misrepaired (B) in BaF3 and BaF3/ITD cells. (C) An agarose gel image of colony PCR products of the region spanning the repaired DSB, using randomly selected white (misrepaired) colonies as templates. The size of a PCR product from a correctly repaired DSB is 628 bp. PCR products of a smaller molecular weight indicate that deletions have occurred during repair. Molecular size markers are indicated on the right. (D-E) Relative repair efficiency (D) and percentage of DSBs that are misrepaired (E) in BaF3/ITD cells treated with the FLT3 inhibitor CEP-701. P values are shown for all graphs. Vertical lines represent SD.
BaF3/ITD cells show decreased NHEJ efficiency and increased repair errors compared with parental BaF3 control cells. Relative NHEJ repair efficiency (A) and percentage of DSBs that are misrepaired (B) in BaF3 and BaF3/ITD cells. (C) An agarose gel image of colony PCR products of the region spanning the repaired DSB, using randomly selected white (misrepaired) colonies as templates. The size of a PCR product from a correctly repaired DSB is 628 bp. PCR products of a smaller molecular weight indicate that deletions have occurred during repair. Molecular size markers are indicated on the right. (D-E) Relative repair efficiency (D) and percentage of DSBs that are misrepaired (E) in BaF3/ITD cells treated with the FLT3 inhibitor CEP-701. P values are shown for all graphs. Vertical lines represent SD.
CEP-701 treatment of FLT3/ITD-expressing AML cells leads to increased NHEJ efficiency and fidelity
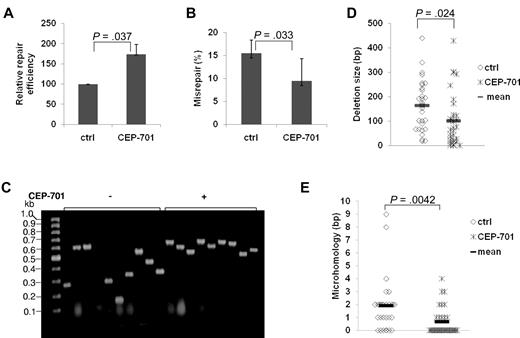
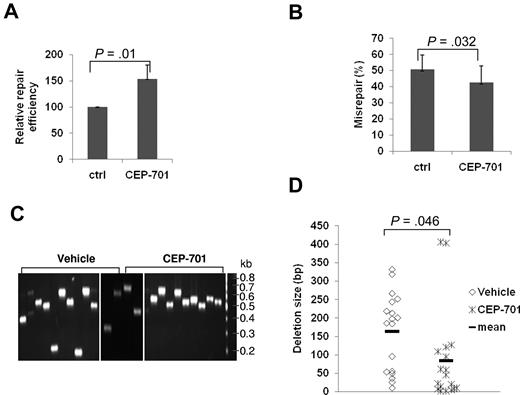
To confirm the effect of FLT3/ITD expression on NHEJ observed in the previous “Results” section and to further analyze the type of misrepair in these cells, we performed plasmid reactivation assays in AML cell line MOLM-14 expressing an FLT3/ITD mutation before and after treatment with the FLT3 inhibitor, CEP-701.8 Treatment of MOLM-14 cells with CEP-701 resulted in an increase (170%) in colony numbers, compared with untreated controls, suggesting a significant increase in the end-joining repair efficiency (Figure 2A; P = .037). Analysis of the colonies from the plasmid reactivation assays for their frequency of repair errors26 revealed that CEP-701 treatment results in a significant decrease (P = .033) in the frequency of misrepaired colonies, compared with untreated controls (Figure 2B).
Treatment of FLT3/ITD AML cell lines with an FLT3 inhibitor increased NHEJ efficiency and decreased misrepair. (A-B) Relative repair efficiency (A) and misrepair frequency (B) in MOLM-14 cells with and without CEP-701 treatment. (C-E) Colony PCR and sequence analysis of randomly selected mirepaired NHEJ products in MOLM-14 cells with and without CEP-701 treatment. (C) An agarose gel image of the colony PCR products. The size of a PCR product from a correctly repaired DSB is 628 bp. PCR products of a smaller molecular weight indicate that deletions have occurred during repair. Molecular size markers are indicated on the left. (D-E) Scatter plots showing the distribution of deletion size (D) and nucleotides of microhomology (E) at DSB repair junctions in randomly selected misrepaired NHEJ products. P values are shown for all graphs. Vertical lines represent SD. The mean is indicated as a solid horizontal line in scatter plots.
Treatment of FLT3/ITD AML cell lines with an FLT3 inhibitor increased NHEJ efficiency and decreased misrepair. (A-B) Relative repair efficiency (A) and misrepair frequency (B) in MOLM-14 cells with and without CEP-701 treatment. (C-E) Colony PCR and sequence analysis of randomly selected mirepaired NHEJ products in MOLM-14 cells with and without CEP-701 treatment. (C) An agarose gel image of the colony PCR products. The size of a PCR product from a correctly repaired DSB is 628 bp. PCR products of a smaller molecular weight indicate that deletions have occurred during repair. Molecular size markers are indicated on the left. (D-E) Scatter plots showing the distribution of deletion size (D) and nucleotides of microhomology (E) at DSB repair junctions in randomly selected misrepaired NHEJ products. P values are shown for all graphs. Vertical lines represent SD. The mean is indicated as a solid horizontal line in scatter plots.
Approximately 40 colonies from 3 or 4 experiments, with or without CEP-701 treatment, were analyzed in detail by colony PCR or sequencing of the region of DNA encompassing the repair junction. Importantly, CEP-701 treatment of the MOLM-14 cells resulted in a general reduction in the size of DNA deletions (Figure 2C-D). Detailed analysis of the DNA sequences at the repair junctions of misrepaired products revealed that the majority of DSBs (77%) were repaired at regions of sequence microhomology in MOLM-14 cells (Figure 2E). After FLT3 inhibitor treatment in the AML cells, repair using microhomologous sequences was significantly reduced (77% untreated vs 34% treated; Figure 2E). Thus, molecular analysis of the type of end-joining in FLT3/ITD-expressing cells suggests that a high percentage of DSBs may be repaired by ALT NHEJ pathways, resulting in abnormal DNA repair characterized mainly by larger DNA deletions, and that FLT3 inhibitors may reduce the frequency of these repair abnormalities. Of note, although the majority of repair abnormalities in FLT3/ITD-expressing AML cell lines were deletions, a minority of oligonucleotide insertions (11%) also occurred. Interestingly, we also observed rare (< 0.1%) translocations of genomic DNA to plasmids (supplemental Figure 2).
Levels of Ku proteins are decreased and DNA Lig IIIα are increased in FLT3/ITD+ cells
Previous studies have demonstrated ALT NHEJ repair when repair using the classic or main NHEJ pathway is absent or down-regulated.16 We previously demonstrated in chronic myeloid leukemia that the steady-state levels of classic NHEJ pathway components Artemis and DNA ligase IV are decreased. In contrast, levels of DNA Lig IIIα protein are increased.23
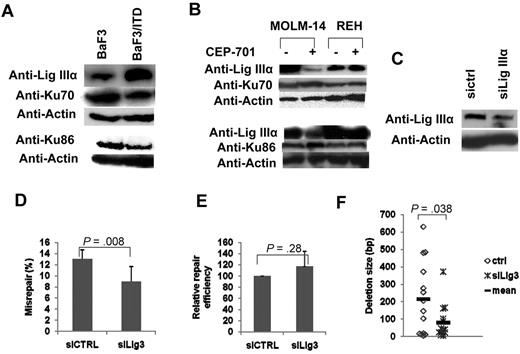
To determine whether the steady-state levels of NHEJ proteins are altered in cells expressing FLT3/ITD mutations, we performed Western blotting analysis of both classic or main NHEJ proteins (Ku70, Ku86, DNA-PKCs, Artemis, and DNA ligase IV) and ALT NHEJ proteins (DNA Lig IIIα and poly(ADP-ribose) polymerase [PARP]) in BaF3/ITD cells, and compared results with that of BaF3 parental controls. Although the levels of the main NHEJ proteins Artemis and DNA ligase IV appear unchanged, both Ku70 and Ku86 are decreased in BaF3/ITD cells, compared with their expression in BaF3 cells (Figure 3A). In contrast, levels of the ALT NHEJ protein DNA Lig IIIα are significantly increased in BaF3/ITD cells, compared with parental controls (Figure 3A). Interestingly, DNA Lig IIIα gene transcripts are similarly increased in BaF3/ITD cells, suggesting that this repair protein may be regulated at the transcriptional level (supplemental Figure 3).
FLT3/ITD cells showed decreased Ku70/86 and increased DNA Lig IIIα levels. (A) Western blotting analysis of Ku70, Ku86, and DNA Lig IIIα in cell extracts of BaF3/ITD and parental BaF3 cells. Actin was used as a loading control. (B) Western blotting analysis of DNA Lig IIIα, Ku70, and Ku86 in cell extracts of MOLM-14 and REH cells with and without CEP-701 treatment. Actin was used as a loading control. (C-E) NHEJ assay in MOLM-14 cells treated with siRNA for DNA Lig IIIα. (C) Western blotting analysis of DNA Lig IIIα in cell extracts. Actin was used as a loading control. (D) Relative NHEJ efficiency. (E) Misrepair frequency. (F) A scatter plot showing the distribution of deletion size. P values are shown for all graphs. Vertical lines represent SD. The mean is indicated as a solid horizontal line in scatter plot.
FLT3/ITD cells showed decreased Ku70/86 and increased DNA Lig IIIα levels. (A) Western blotting analysis of Ku70, Ku86, and DNA Lig IIIα in cell extracts of BaF3/ITD and parental BaF3 cells. Actin was used as a loading control. (B) Western blotting analysis of DNA Lig IIIα, Ku70, and Ku86 in cell extracts of MOLM-14 and REH cells with and without CEP-701 treatment. Actin was used as a loading control. (C-E) NHEJ assay in MOLM-14 cells treated with siRNA for DNA Lig IIIα. (C) Western blotting analysis of DNA Lig IIIα in cell extracts. Actin was used as a loading control. (D) Relative NHEJ efficiency. (E) Misrepair frequency. (F) A scatter plot showing the distribution of deletion size. P values are shown for all graphs. Vertical lines represent SD. The mean is indicated as a solid horizontal line in scatter plot.
To determine whether FLT3/ITD signaling has an effect on the steady-state levels of NHEJ proteins, FLT3/ITD+ MOLM-14 cells were treated with CEP-701 and cell extracts were subjected to Western blotting analysis for NHEJ proteins. Figure 3B shows that CEP-701 treatment leads to a significant reduction in the steady-state level of DNA Lig IIIα in the FLT3/ITD-containing AML cell line MOLM-14, compared with untreated controls. Concomitantly, there appears to be a small increase in Ku70 and Ku86 expression levels in cells treated with the FLT3 inhibitor. In contrast, the leukemia cell line REH that expresses WT FLT3 shows no changes in the expression levels of DNA Lig IIIα, Ku70, or Ku86 after treatment with CEP-701.
As a further confirmation of the role of DNA Lig IIIα in NHEJ of MOLM-14 cells, we performed NHEJ assays after down-regulation of DNA Lig IIIα by siRNA technology. Figure 3C shows that DNA Lig IIIα expression levels were reduced by approximately 50% of the control 3 days after transfection with Lig IIIα siRNA oligonucleotides. Partial down-regulation of DNA Lig IIIα in MOLM-14 resulted in a significant decrease in misrepair frequency compared with results from siRNA control treated cells (Figure 3D), but not in repair efficiency (Figure 3E). Sequencing and colony PCR also revealed an overall reduction in the size of DNA deletions during NHEJ (Figure 3F; supplemental Figure 4).
FLT3/ITD knock-in mice BM MNCs demonstrate lower efficiency and higher errors of NHEJ
Heterozygous FLT3wt/ITD knock-in mice develop myeloproliferative disease, characterized by splenomegaly, leukocytosis, and myeloid hypercellularity, which progresses to fatal myeloproliferative disease by 6 to 20 months. Homozygous FLT3ITD/ITD mice die of fatal myeloproliferative disease by 2 to 8 months. BM MNCs from FLT3wt/ITD mice demonstrate increased ROS and DSBs, compared with WT controls (J.F., L.L., D.S., F.R., unpublished data).
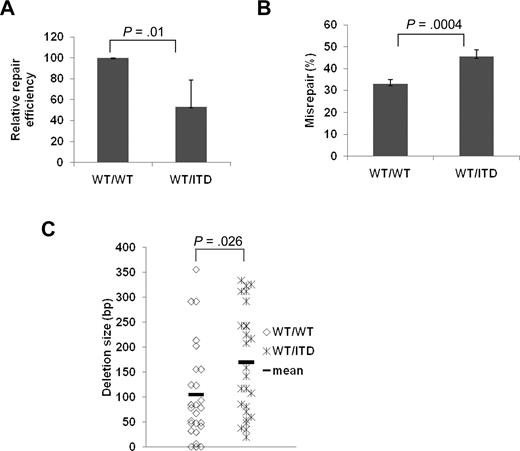
To verify that the end-joining abnormalities observed in cell lines that express FLT3/ITD mutations are also generated in vivo in mouse models with FLT3/ITD mutations, we examined end-joining repair by introducing linearized pUC 18 into BM MNCs isolated from heterozygous FLT3/ITD knock-in mice, and compared the results with those from WT controls.25 Figure 4A shows that BM MNCs from FLT3/ITD knock-in mice demonstrate a dramatic decrease in the efficiency of end-joining, compared with WT controls (P = .01). Furthermore, the frequency of misrepair is concomitantly increased in the BM MNCs of FLT3/ITD mice compared with WT controls (Figure 4B, P = .0004). Analysis of the repair abnormalities in individual colonies suggests that FLT3/ITD knock-in mice BM MNCs demonstrate an increase in the size of DNA deletions (Figure 4C) compared with control cells (P = .026), as was seen previously with the cell lines stably expressing FLT3/ITD.
BM MNCs from WT/ITD mice showed decreased NHEJ efficiency and increased misrepair compared with WT/WT counterparts. Relative repair efficiency (A) and misrepair frequency (B). (C) Scatter plot showing the distribution of DNA deletion size in randomly selected misrepaired products. P values are shown for all graphs. Vertical lines represent SD. The mean is indicated as a solid horizontal line in scatter plot.
BM MNCs from WT/ITD mice showed decreased NHEJ efficiency and increased misrepair compared with WT/WT counterparts. Relative repair efficiency (A) and misrepair frequency (B). (C) Scatter plot showing the distribution of DNA deletion size in randomly selected misrepaired products. P values are shown for all graphs. Vertical lines represent SD. The mean is indicated as a solid horizontal line in scatter plot.
To determine whether end-joining repair abnormalities decrease in cells from FLT3/ITD knock-in mice treated with FLT3 inhibitors, as was seen in the FLT3/ITD-expressing cell lines, plasmid reactivation assays were performed in BM MNCs from heterozygous FLT3/ITD knock-in mice with and without treatment with CEP-701 in vivo. FLT3/ITD-treated animals demonstrated a significant (P = .01) increase in repair efficiency (Figure 5A). Moreover, CEP-701-treated animals showed a decrease both in misrepair frequency and the size of DNA deletions (Figure 5B-D).
Treatment of WT/ITD mice with the FLT3 inhibitor CEP-701 showed an increase in efficiency and a decrease in misrepair frequency and deletion size during NHEJ. Relative repair efficiency (A) and misrepair frequency (B). (C) An agarose gel image of colony PCR products using as templates randomly selected misrepaired products from NHEJ assays. The size of a PCR product from a correctly repaired DSB is 628 bp. PCR products of a smaller molecular weight indicate that deletions have occurred during repair. Molecular size markers are indicated on the right. Vertical lines have been inserted to indicate repositioned gel lanes. (D) Scatter plot showing the distribution of DNA deletion size in randomly selected misrepaired products. P values are shown for all graphs. Vertical lines represent SD. The mean is indicated as a solid horizontal line in scatter plot.
Treatment of WT/ITD mice with the FLT3 inhibitor CEP-701 showed an increase in efficiency and a decrease in misrepair frequency and deletion size during NHEJ. Relative repair efficiency (A) and misrepair frequency (B). (C) An agarose gel image of colony PCR products using as templates randomly selected misrepaired products from NHEJ assays. The size of a PCR product from a correctly repaired DSB is 628 bp. PCR products of a smaller molecular weight indicate that deletions have occurred during repair. Molecular size markers are indicated on the right. Vertical lines have been inserted to indicate repositioned gel lanes. (D) Scatter plot showing the distribution of DNA deletion size in randomly selected misrepaired products. P values are shown for all graphs. Vertical lines represent SD. The mean is indicated as a solid horizontal line in scatter plot.
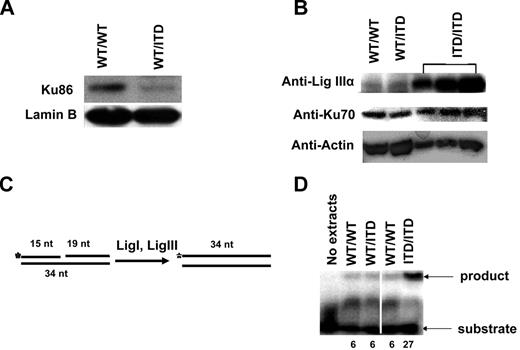
To determine whether altered NHEJ abnormalities found in FLT3/ITD mice correlate with the altered steady-state levels of NHEJ proteins, Western blot analyses were performed in nuclear/cell extracts from BM MNCs of FLT3/ITD knock-in mice, and results were compared with those of WT mice. As with the FLT3/ITD-expressing cell lines examined previously, knock-in mice exhibit decreased levels of protein Ku86 (Figure 6A), but not Ku70 (Figure 6B). Analysis of ALT NHEJ protein levels revealed that DNA Lig IIIα expression is not significantly increased in FLT3/ITD heterozygote mice. However, cell extracts from homozygote ITD mice demonstrate significantly increased DNA Lig IIIα transcript (supplemental Figure 3) and protein levels (Figure 6B) compared with extracts from WT and heterozygote cells.
Extracts from FLT3/ITD-expressing mouse BM MNCs showed decreased Ku86 and increased DNA Lig IIIα. Western blotting analysis of Ku86 in nuclear extracts (A) and DNA Lig IIIα and Ku70 in cell extracts (B) from BM MNCs of WT/ITD and WT/WT mice. Different amounts of cell extracts from a single homozygote ITD/ITD mouse were also examined. Lamin and actin are used as loading controls. (C) A schematic diagram of the DNA nick ligation assay. Annealing of 3 DNA oligonucleotides (15-, 19-, and 34-nt) gives rise to a substrate that contains a nick. DNA ligase I and IIIα in the cell extracts seal the nick. The 5′ end of the 15 mer was radiolabeled so that the product can be detected. (D) A phosphorimage showing the DNA nick ligation activity in cell extracts. The numbers at the bottom of lanes indicate the relative ligation activity. A vertical line has been inserted to indicate repositioned gel lanes.
Extracts from FLT3/ITD-expressing mouse BM MNCs showed decreased Ku86 and increased DNA Lig IIIα. Western blotting analysis of Ku86 in nuclear extracts (A) and DNA Lig IIIα and Ku70 in cell extracts (B) from BM MNCs of WT/ITD and WT/WT mice. Different amounts of cell extracts from a single homozygote ITD/ITD mouse were also examined. Lamin and actin are used as loading controls. (C) A schematic diagram of the DNA nick ligation assay. Annealing of 3 DNA oligonucleotides (15-, 19-, and 34-nt) gives rise to a substrate that contains a nick. DNA ligase I and IIIα in the cell extracts seal the nick. The 5′ end of the 15 mer was radiolabeled so that the product can be detected. (D) A phosphorimage showing the DNA nick ligation activity in cell extracts. The numbers at the bottom of lanes indicate the relative ligation activity. A vertical line has been inserted to indicate repositioned gel lanes.
To confirm that the observed increased DNA Lig IIIα level in FLT3/ITD homozygotes was functional, we performed a DNA ligation assay (Figure 6C).28 In this assay, the DNA substrate consisting of 3 oligonucleotides contains a nick. In the cell extracts, DNA ligase I and IIIα are involved in sealing the nick between the 15-nt and 19-nt oligos, resulting in a 34-nt oligo. As shown in Figure 6D, extracts from homozygous FLT3/ITD mice exhibited significantly higher DNA nick ligation activities (> 4-fold) compared with WT and heterozygous FLT3/ITD mice, correlating with the Western blotting results. Together, these data suggest that FLT3/ITD mutations in knock-in mice lead to reduced repair via main NHEJ pathways and that the remaining repair is more error-prone. Furthermore, overexpression of the ALT NHEJ protein DNA Lig IIIα requires more than one copy of the FLT3/ITD allele.
Discussion
The presence of an FLT3/ITD mutation, at least when present at a high allelic ratio, correlates with aggressive disease and poor prognosis in AML patients.1 However, the mechanisms by which this mutation generates aggressive disease remain elusive. Recently, with data obtained from cell lines and patient samples, we suggested that FLT3/ITD mutations can induce a cycle of events whereby increased ROS production leads to DSBs that are repaired by an error-prone mechanism. This would result in an increased potential for acquisition of genetic alterations, which may, in part, explain aggressive AML in FLT3/ITD patients. In the present study, using both FLT3/ITD cell lines and BM MNCs from FLT3/ITD knock-in mice, we sought to further delineate the potential mechanism of FLT3/ITD mutations causing aberrant DSB repair. Here we report, for the first time, that FLT3/ITD cell lines and primary cells from FLT3/ITD knock-in mice demonstrate reduced levels of Ku proteins, components of the main NHEJ pathway. In addition, FLT3/ITD-expressing cell lines and homozygous knock-in mice show elevated levels of the DNA repair protein, DNA Lig IIIα, which participates in an ALT end-joining pathway. We suggest that this DSB repair pathway, mediated by PARP and DNA Lig IIIα, contributes to repair of numerous ROS-induced DSBs, albeit by a more error-prone pathway. Thus, FLT3/ITD ensures the survival of cells at the cost of increased genomic instability.
The binding of Ku proteins to DSBs is essential to initiate repair via the main NHEJ pathway.10 In FLT3/ITD+ cells, we have shown that the steady-state levels of Ku proteins are reduced, compared with isogenic and normal controls. This suggests that initiation of repair at DSBs using the main NHEJ repair pathway is probably reduced in cells expressing FLT3/ITD mutations. Moreover, this reduced repair is probably reflected in increased levels of DSBs, as we have previously reported.8 It is clear from studies in Ku knock-out mice that abnormal repair of DSBs occurs, leading to chromosomal abnormalities and cancer.21 With Ku protein levels decreased in FLT3/ITD cells, other repair factors, such as PARP that compete with Ku proteins for binding to DSBs, probably initiate alternative and highly error-prone repair, leading to ligation of DNA ends by Lig IIIα.16
One of the standard methods for discriminating between the end-joining pathways used to repair DNA ends is to examine the sequences at the joins; ALT NHEJ repair appears to use microhomology-mediated repair at DNA ends, creating genomic alterations, such as deletions.29-32 In the FLT3/ITD-expressing MOLM-14 cell line, approximately 77% of repair of DNA ends used microhomologies. Treatment of cells with the FLT3 inhibitor CEP-701 leads to a significant reduction in microhomology frequency at repair sites. Furthermore, the average size of microhomology sequences used to facilitate repair appears to decrease with this experimental maneuver. Importantly, the average size of DNA deletions, a consequence of highly error-prone repair, also decreases with FLT3 inhibitor treatment. These data provide additional evidence that the ALT end-joining pathway is operative in FLT3/ITD-expressing cells.
It is clear that FLT3/ITD expression directly or indirectly regulates NHEJ repair. Knock-in mice with a single allele of FLT3/ITD clearly have decreased Ku proteins levels, whereas increased transcript and steady-state levels of DNA Lig IIIα are only evident in cell lines and homozygous knock-in mice. Although the mechanism by which Ku regulation occurs is unclear, increased ROS in FLT3/ITD cells may lead to decreased efficiency of main NHEJ pathway repair, whose constituent proteins (DNA-PKCs, Ku) are particularly sensitive to oxidative damage.33,34 Thus, levels of Ku proteins could be regulated indirectly by the high constitutive ROS. Alternatively, Ku expression may be indirectly regulated by FLT3/ITD through induction of a miRNA(s). For example, Hu et al recently reported that NMyc-induced human microRNA, miR-421, suppresses ATM expression by targeting the 3′-untranslated region of ATM transcripts.35 Although the basis for altered levels of ALT NHEJ component DNA Lig IIIα in cell lines and homozygous FLT3/ITD knock-in mice is not known, this may reflect various levels of FLT3/ITD expression. Increased DNA Lig IIIα expression may require a threshold level of FLT3/ITD expression and/ or copy number. Nevertheless, increased transcript and protein levels of DNA Lig IIIα in FLT3/ITD-expressing cells suggests that this constitutively activated tyrosine kinase receptor may regulate the transcription of DNA Lig IIIα directly or indirectly. This is further evident from the decrease in expression of DNA Lig IIIα observed when FLT3/ITD-expressing cell lines are treated with a FLT3 inhibitor.
In cell line and primary AML blast assays, it appears that more than 85% of FLT3/ITD kinase activity needs to be inhibited to achieve a cytotoxic response, and even fuller inhibition might be more efficacious.36-40 Both from phase I/II monotherapy and phase III combination chemotherapy trials of FLT3 tyrosine kinase inhibitor, this requirement for significant inhibition at trough levels of the drugs seems to hold true.36-40 To date, none of the tyrosine kinase inhibitors being tested is specific for FLT3 kinase activity; and if the concentration of free drug available to inhibit FLT3 is achieved, the tyrosine kinase inhibitor will frequently inhibit several other TKs, resulting in toxicities that often preclude achieving high level inhibition of FLT3. Thus, therapeutic strategies that combine FLT3 inhibitors with other drugs that may enhance its efficacy is a valid approach. Given that FLT3/ITD expression leads to increased ALT NHEJ repair, these DNA repair components constitute attractive targets for the development of novel therapeutic strategies in combination with FLT3 inhibitors. This therapeutic approach may also decrease the acquisition of further genetic alterations that can lead to disease resistance. Notably, small-molecule inhibitors of PARP and DNA ligases, components of the ALT NHEJ pathway, have been identified, with the former already showing activity in cancers with DSB repair defects.41,42
In conclusion, the use of PARP and DNA ligase inhibitors and further characterization of how FLT3/ITD regulates repair of DSBs will not only provide insights into the role of genomic instability and disease resistance but may also lead to the development of novel therapeutic strategies in patients with FLT3/ITD+ AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alisa Thavikulwat for assisting with the RT-PCR experiments.
This work was supported by the University of Maryland Cancer Center (F.R., J.F.) and the National Institutes of Health (grants CA90668 and CA70970; D.S.). D.S. is also supported by the Kyle Haydock Professorship.
National Institutes of Health
Authorship
Contribution: J.F. designed individual experiments, performed the research, analyzed the data, and participated in writing the paper; L.L. performed the research; D.S. contributed reagents and participated in designing some of the experiments and writing the paper; and F.R. was responsible for the overall design of the study, analysis of the data, and writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Feyruz Rassool, University of Maryland School of Medicine, 655 West Baltimore St, BRB 7-023A, Baltimore, MD 21201; e-mail: frassool@som.umaryland.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal