Abstract
Adenovirus (AdV) infections are very common in the general pediatric population. The delayed clearance in young persons imposes a threat to immunocompromised patients after hematopoietic stem cell transplantation (HSCT), who can reactivate the virus, resulting in life-threatening disseminated disease. Although a definitive cure requires adequate immune reconstitution, 2 approaches appear to be feasible and effective to improve the outcomes of AdV infections. Strict monitoring with AdV quantitative polymerase chain reaction followed by preemptive treatment with low-dose (1 mg/kg) cidofovir 3 times a week, is effective in most cases to bridge the severely immunocompromised period shortly after HSCT, with acceptable toxicity rates. For centers who have the access, AdV-specific cytotoxic T cells can be the other important cornerstone of anti-AdV therapy with promising results so far. Methods to positively influence the reconstitution of the immune system after HSCT and optimizing new and currently available cellular immunotherapies will make HSCT safer against the threat of AdV infection/reactivation and associated disease.
Introduction
Hematopoietic stem cell transplantation (HSCT) is the last treatment option for a variety of diseases, including certain hematologic malignancies, inborn errors of metabolism, immune deficiencies, and bone marrow failure syndromes. Although HSCT has become much safer over the past decade, the main limitations remain transplantation-related mortality (TRM) and relapse (in malignancies). In addition to the development of graft-versus-host disease (GVHD), infections are an important complication during HSCT procedures and contribute significantly to morbidity and TRM. They occur during the immunosuppressed period after HSCT which is a consequence of both the preparative regimen (including serotherapy), a donor-derived reconstituting immune system, and the administration of immune-suppressive agents. Besides the risk of developing bacterial and fungal infections, there is a substantial risk of viral infection and reactivation. Of the latter, herpes viruses (varicella-zoster virus, herpes simplex virus, human herpes virus 6 [HHV-6], Epstein Barr virus [EBV] cytomegalovirus [CMV]) and adenovirus (AdV) are the most important. In this review we address the problem of AdV infections that can lead to lethal multiorgan involvement in the immunocompromised host, and we present a practical guideline for how we treat AdV-infections in HSC transplant recipients.
AdV infections and host defense
AdV, belonging to the Adenoviridae family of DNA viruses, has 51 subtypes, of which 1, 2, 5, 7, and 14 are common causes of infections in the general population. In immunocompromised patients, mainly in patients after HSCT, AdV disease may be life threatening.1
For normal host defense against AdV both humoral and cellular responses seem to be important. In the first 6 months of life primary infections with AdV are uncommon, which is attributed to the presence of demonstrable levels of maternally derived serum immunoglobulin G antibodies against several AdV subtypes in most infants at birth.2 Subtype-specific antibodies are formed against capsid as well as fiber proteins, which offer lifelong protection in the immunocompetent host. For cellular immunity directed against AdV, in vitro studies have shown that AdV elicits both CD4+ and CD8+ T-cell responses. However, in vitro removal of CD4+ T cells (but not CD8+ T cells) from a peripheral blood mononuclear cell population incubated with AdV abrogates the lymphoproliferative response.3 In support of this finding, with the use of major histocompatibility complex class II–blocking antibodies, this lymphoproliferative response was shown to be major histocompatibility complex class II restricted. AdV-activated CD4+ T cells have been shown to produce interferon-γ (IFN-γ) and can be cytotoxic, being able to lyse AdV-infected cells in vitro.3,4 Approximately one-third of the AdV genome is devoted to counteract innate and adaptive immune defenses compromising the development of a protective immune response.5 The frequent reinfections and persistence of the virus in children on the one hand and the presence of AdV-specific CD4+ T cells in asymptomatic adults on the other hand are a sign that the development of an adequate specific cross-reactive immune protection against AdV takes many years to develop.2,3,6 AdV persists within lymphoreticular tissue: in macrophages of tonsils, adenoids, and intestines of infected hosts.7 Shedding can occur for months or years even in healthy children, making acquisition of the virus by horizontal transmission a main risk to immunocompromised patients. Although subtype-specific responses to various fiber epitopes have been detected, most immunogenic epitopes are in the hexon protein of the capsid and appear to be conserved between several subtypes.8-12 Some of these have been shown to trigger both CD4+ and CD8+ T-cell responses, which proved to be of great importance for the development of AdV-targeting T-cell immunotherapy.9,12
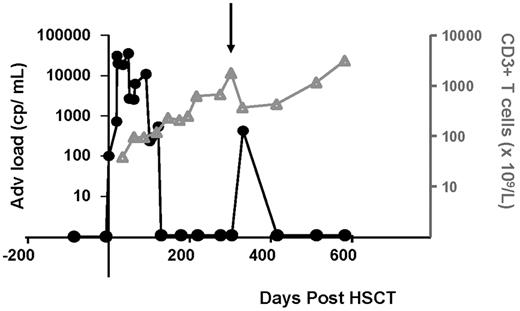
Permanent circulation of the virus among children explains why AdV infections are much more of a problem in pediatric patients (20%-26%) undergoing HSCT than in adults (9%).2 Although AdV causes mild respiratory or gastrointestinal disease in healthy persons, in the severely immunocompromised patient they are the cause of severe respiratory disease, hepatitis, and colitis. Other complications of the disease may involve hemorrhagic cystitis and adenoviral keratoconjunctivitis. AdV primo-infected or reactivating patients can be divided into patients with subclinical viremia, viremia with disease symptoms, and disseminated disease. The incidence of disseminated disease is 1%-7% with a reported mortality of 8%-26% (Table 1). High rates of mortality for disseminated disease were the reason for development of sensitive detection “monitoring” tools of subclinical AdV infections. Rapidly increasing or sustained adenoviremia is associated with the occurrence of severe disease both in children and in adults.13-15 Further complicating things, the antiviral treatment options currently available have a considerable toxicity, which makes it particularly important to identify those patients most at risk of developing AdV disease. Monitoring of the adenoviral load by quantitative AdV polymerase chain reaction (PCR) in the peripheral blood is far superior to other methods such as viral culture and the direct fluorescence assay, both in sensitivity and speed.16,17 Weekly quantitative PCRs (qPCRs) to monitor the AdV, CMV, EBV, and HHV-6 DNA load, as well as immune-reconstitution monitoring (CD3 counts), after HSCT are now widely used methods in many bone marrow transplantation units.14,15 Weekly monitoring appears to be important because of the kinetics of AdV replication, which can be rapid (Figure 1).14,15 Furthermore, AdV qPCR monitoring is used to evaluate therapeutic efficacy during treatment with antivirals. In the University Medical Center (UMC) Utrecht we use an in-house developed real-time PCR-based AdV qPCR test, but commercial tests are also available. The quality of these tests is similar and can be assured by joining the quality control for molecular diagnostics panel testing.18,19 Panels with 3 concentrations of DNA are sent to different laboratories; results are submitted, analyzed, and compared; and the quality is reported to the originating laboratories.18
Cidofovir for the treatment of adenovirus disease in HSCT patients
| Author (year of publication) . | No. of patients . | P/R . | Indication . | Drug therapy . | Nephrotoxicity, n % . | Success AdV clearance, n % . | Mortality, n (%) or direct AdV-related mortality, n (%) . |
|---|---|---|---|---|---|---|---|
| Preemptive treatment | Preemptive treatment | ||||||
| Anderson (2008) | 7 Children | P | 7 patients with AdV isolation from plasma, or from 2 other sites evaluated by PCR | CDV 1 mg/kg intravenously 3 times a week | 0 (0) | 2 (28) | 2 (29) |
| 5 still had isolation of AdV from any of the peripheral sites after CDV discontinuation, all resolved spontaneously. | 0 (0) | ||||||
| Bhadri (2009) | 20 Children | R | Proven AdV in stool, urine, NPA, CSF. | Induction with 5 mg/kg intravenously, followed by 3 mg/kg | 7 (35) | 17 (85) | 14 (70) |
| Routine stool screening rapid culture, antigen detection | When prolonged treatment was needed 1 mg/kg 3 times a week | 2 (10) | |||||
| Screening for AdV in other sites as clinically indicated | |||||||
| Greil et al (2006) | 10 Children | R | AdV detection in 10 of 34 pediatric patients treated with ribavirin prophylaxis | Ribavirin prophylaxis | No data | No data | 1 (10) |
| CDV preemptive | 0 (0) | ||||||
| Ljungman (2003) | 16 Adults and children | R | Asymptomatic patients (n = 16) of a total of 45 patients with any detection of AdV from stool, upper airway specimens, blood, urine (detection by culture, antigen detection, PCR) | CDV 5 mg/kg intravenously weekly (n = 39) | No data for this group; overall in 45 patients, 17 (38%) | (75) | 6 (37) |
| 1-4 mg/kg (n = 6) | 2 (13) | ||||||
| Yusuf (2006) | 14 Children | R | 14 asymptomatic pediatric patients (of a total number of 57 patients), positive AdV detection by PCR | CDV 5 mg/kg intravenously weekly, for 2 weeks, followed by once every 2 weeks until viral clearance with 3 negative samples | 0 | (100) | No data for this group |
| Median 5 doses (range, 1-22) | None of which developed reactivation | 29 of 57 (51) for the whole group | |||||
| 1 of 57 (8) | |||||||
| Therapeutic treatment | Therapeutic treatment | ||||||
| Robin (2007) | 25 Adults and children | R | Disseminated AdV disease | CDV 5 mg/kg intravenously weekly | No data | 6 (24) | 21 (84) |
| Symeonidis (2006) | 11 Adults and children | R | Positive AdV cultures and severe or persistent symptoms | CDV 5 mg/kg intravenously weekly | 2 (18) | No data | 6 (54) |
| OR | 6 (54) | ||||||
| CDV 1 mg/kg every other day | |||||||
| Ouachee-Chardin (2004) | 16 children | R | AdV disease | CDV 3-5 mg/kg intravenously weekly | 5 (31) reversible tubulopathy | 14 (87) | 3 (18) |
| PCR for detection of AdV | 2 (13) | ||||||
| Yusuf (2006) | 43 Children | R | 43 Patients with clinical symptoms attributable to AdV infection | CDV 5 mg/kg intravenously weekly for 2 weeks, followed by once every 2 weeks until viral clearance with 3 negative samples | 0 | 42 (97) | No data for this group |
| AdV detection by PCR (of a total number of 57 patients with positive AdV PCR) | Median 5 doses (range, 1-22) | 29/57 (51) for the whole group | |||||
| 1 of 57 (8) | |||||||
| Hoffman (2001) | 8 Children | P | Of 17 patients with positive AdV cultures, 8 were enrolled in phase 2 trial of CDV treatment | CDV 1 mg/kg intravenously 3 times a week | 1 (13) | 8 (100) | 2 (25) |
| Detection with shell vial culture method | Not leading to discontinuation | 0 (0) | |||||
| Ljungman (2003) | 29 Adults and children | R | Patients with probable and definite AdV disease (n = 29) of a total of 45 patients with any detection of AdV from stool, upper airway specimens, blood, urine (detection by culture, antigen detection, PCR) | CDV 5 mg/kg intravenously weekly (n = 39) | No data of this group. | 20 (69) | 10 (34) |
| 1-4 mg/kg (n = 6) | Overall in 45 patients: 14 (31%) | 5 (17) | |||||
| Muller (2005) | 10 Children | R | AdV isolation from > 1 site and clinical symptoms, or isolation from 1 site with severe clinical symptoms | CDV 5 mg/kg intravenously weekly during 6 weeks, followed by once every 2 weeks for total of 9 doses | 3 (30) (with > 50% serum creatinine elevation) | 9 (90), 1 required additional CDV therapy to clear the virus | 0 (0) |
| Kampmann (2005) | 11 Children | R | Patients in whom AdV was detected (n = 26) were on ribavirin. In case of persistent adenoviremia and AdV disease CDV was started (n = 11). | CDV 5 mg/kg intravenously weekly for 2 weeks, followed by 5 mg/kg every 2 weeks | ? | No data in this group | 9 (81) |
| In case of persistent adenoviremia and AdV disease CDV was started (n = 11). | 5 (46) | ||||||
| Nagafuji (2004) | 16 Adults | P | Patients after HSCT with hemorrhagic cystitis and a urine sample positive for viral culture, Adv PCR and immunochromatograph | CDV 1 mg/kg intravenously 3 times a week, for 3 weeks | 3 (19) | 12 (75) | 2 (13) |
| Author (year of publication) . | No. of patients . | P/R . | Indication . | Drug therapy . | Nephrotoxicity, n % . | Success AdV clearance, n % . | Mortality, n (%) or direct AdV-related mortality, n (%) . |
|---|---|---|---|---|---|---|---|
| Preemptive treatment | Preemptive treatment | ||||||
| Anderson (2008) | 7 Children | P | 7 patients with AdV isolation from plasma, or from 2 other sites evaluated by PCR | CDV 1 mg/kg intravenously 3 times a week | 0 (0) | 2 (28) | 2 (29) |
| 5 still had isolation of AdV from any of the peripheral sites after CDV discontinuation, all resolved spontaneously. | 0 (0) | ||||||
| Bhadri (2009) | 20 Children | R | Proven AdV in stool, urine, NPA, CSF. | Induction with 5 mg/kg intravenously, followed by 3 mg/kg | 7 (35) | 17 (85) | 14 (70) |
| Routine stool screening rapid culture, antigen detection | When prolonged treatment was needed 1 mg/kg 3 times a week | 2 (10) | |||||
| Screening for AdV in other sites as clinically indicated | |||||||
| Greil et al (2006) | 10 Children | R | AdV detection in 10 of 34 pediatric patients treated with ribavirin prophylaxis | Ribavirin prophylaxis | No data | No data | 1 (10) |
| CDV preemptive | 0 (0) | ||||||
| Ljungman (2003) | 16 Adults and children | R | Asymptomatic patients (n = 16) of a total of 45 patients with any detection of AdV from stool, upper airway specimens, blood, urine (detection by culture, antigen detection, PCR) | CDV 5 mg/kg intravenously weekly (n = 39) | No data for this group; overall in 45 patients, 17 (38%) | (75) | 6 (37) |
| 1-4 mg/kg (n = 6) | 2 (13) | ||||||
| Yusuf (2006) | 14 Children | R | 14 asymptomatic pediatric patients (of a total number of 57 patients), positive AdV detection by PCR | CDV 5 mg/kg intravenously weekly, for 2 weeks, followed by once every 2 weeks until viral clearance with 3 negative samples | 0 | (100) | No data for this group |
| Median 5 doses (range, 1-22) | None of which developed reactivation | 29 of 57 (51) for the whole group | |||||
| 1 of 57 (8) | |||||||
| Therapeutic treatment | Therapeutic treatment | ||||||
| Robin (2007) | 25 Adults and children | R | Disseminated AdV disease | CDV 5 mg/kg intravenously weekly | No data | 6 (24) | 21 (84) |
| Symeonidis (2006) | 11 Adults and children | R | Positive AdV cultures and severe or persistent symptoms | CDV 5 mg/kg intravenously weekly | 2 (18) | No data | 6 (54) |
| OR | 6 (54) | ||||||
| CDV 1 mg/kg every other day | |||||||
| Ouachee-Chardin (2004) | 16 children | R | AdV disease | CDV 3-5 mg/kg intravenously weekly | 5 (31) reversible tubulopathy | 14 (87) | 3 (18) |
| PCR for detection of AdV | 2 (13) | ||||||
| Yusuf (2006) | 43 Children | R | 43 Patients with clinical symptoms attributable to AdV infection | CDV 5 mg/kg intravenously weekly for 2 weeks, followed by once every 2 weeks until viral clearance with 3 negative samples | 0 | 42 (97) | No data for this group |
| AdV detection by PCR (of a total number of 57 patients with positive AdV PCR) | Median 5 doses (range, 1-22) | 29/57 (51) for the whole group | |||||
| 1 of 57 (8) | |||||||
| Hoffman (2001) | 8 Children | P | Of 17 patients with positive AdV cultures, 8 were enrolled in phase 2 trial of CDV treatment | CDV 1 mg/kg intravenously 3 times a week | 1 (13) | 8 (100) | 2 (25) |
| Detection with shell vial culture method | Not leading to discontinuation | 0 (0) | |||||
| Ljungman (2003) | 29 Adults and children | R | Patients with probable and definite AdV disease (n = 29) of a total of 45 patients with any detection of AdV from stool, upper airway specimens, blood, urine (detection by culture, antigen detection, PCR) | CDV 5 mg/kg intravenously weekly (n = 39) | No data of this group. | 20 (69) | 10 (34) |
| 1-4 mg/kg (n = 6) | Overall in 45 patients: 14 (31%) | 5 (17) | |||||
| Muller (2005) | 10 Children | R | AdV isolation from > 1 site and clinical symptoms, or isolation from 1 site with severe clinical symptoms | CDV 5 mg/kg intravenously weekly during 6 weeks, followed by once every 2 weeks for total of 9 doses | 3 (30) (with > 50% serum creatinine elevation) | 9 (90), 1 required additional CDV therapy to clear the virus | 0 (0) |
| Kampmann (2005) | 11 Children | R | Patients in whom AdV was detected (n = 26) were on ribavirin. In case of persistent adenoviremia and AdV disease CDV was started (n = 11). | CDV 5 mg/kg intravenously weekly for 2 weeks, followed by 5 mg/kg every 2 weeks | ? | No data in this group | 9 (81) |
| In case of persistent adenoviremia and AdV disease CDV was started (n = 11). | 5 (46) | ||||||
| Nagafuji (2004) | 16 Adults | P | Patients after HSCT with hemorrhagic cystitis and a urine sample positive for viral culture, Adv PCR and immunochromatograph | CDV 1 mg/kg intravenously 3 times a week, for 3 weeks | 3 (19) | 12 (75) | 2 (13) |
Listed are all clinical studies that used cidofovir in patients who received HSCT either for preemptive treatment when symptoms are not yet present or for therapeutic treatment when disease symptoms are present. Only studies with n > 5 were presented.
P indicates prospective study; R, retrospective study; AdV, adenovirus; CDV, cidofovir; NPA, nasopharyngeal aspirate; CSF, cerebrospinal fluid; PCR, polymerase chain reaction.
Adenoviral load in relation to T-cell numbers. A 2-year-old girl was treated with unrelated cord blood HSCT for Hurler syndrome (nonmalignant, MPS1). She developed an AdV primo infection or reactivation, which was detectable by AdV qPCR (100 cp/mL) at day 19. Within a period of 4 days the viral load increases by 2 log, and cidofovir is started. There are no signs of disease. Only as CD3 numbers (gray line) are increasing, adenoviral load (black line) goes down. The downward pointing arrow indicates the timing of intensification of therapy with immunosuppressants when the period after HSCT is complicated by steroid refractory autoimmune cytopenia; she receives mycophenolate mofetil 45 mg/kg/d, prednisone 2 mg/kg/d, anti-CD20 therapy, and fludarabine infusions from day 314. When symptoms stabilize, immunosuppressants are tapered over time and can be stopped at day 455 after HSCT. Tapering of immunosuppressants is associated with CD3+ T-cell recovery and clearance of advenoviremia.
Adenoviral load in relation to T-cell numbers. A 2-year-old girl was treated with unrelated cord blood HSCT for Hurler syndrome (nonmalignant, MPS1). She developed an AdV primo infection or reactivation, which was detectable by AdV qPCR (100 cp/mL) at day 19. Within a period of 4 days the viral load increases by 2 log, and cidofovir is started. There are no signs of disease. Only as CD3 numbers (gray line) are increasing, adenoviral load (black line) goes down. The downward pointing arrow indicates the timing of intensification of therapy with immunosuppressants when the period after HSCT is complicated by steroid refractory autoimmune cytopenia; she receives mycophenolate mofetil 45 mg/kg/d, prednisone 2 mg/kg/d, anti-CD20 therapy, and fludarabine infusions from day 314. When symptoms stabilize, immunosuppressants are tapered over time and can be stopped at day 455 after HSCT. Tapering of immunosuppressants is associated with CD3+ T-cell recovery and clearance of advenoviremia.
In some cases, AdV can be detected significantly earlier at local sites, such as in stool or nasopharyngeal aspirate (NPA). Lion et al20 demonstrated that detection of AdV by PCR technology in stool is associated with disease and precedes AdV DNAemia by 11 days. In addition, we have recently shown that AdV DNA positivity in NPA preceding HSCT is a strong predictor for AdV DNAemia in pediatric patients receiving an unrelated donor; therefore, we perform screening as part of the standard workup before HSCT. Presence of AdV in the NPA or stool might therefore be a decisive factor in postponement of an HSCT in pediatric patients with more elective nonmalignant indications.21
Risk factors and prevention of transmission
Within the HSCT setting, several factors increase the risk of AdV infection which are almost all related to a lack of cellular antiviral activity that is inherent to the first 100 days after transplantation. Feuchtinger et al22 demonstrated that the recurrence of AdV-specific T cells was crucial for clearance of infection, which was confirmed by others.23 In general, the number of CD3+ T cells, which is much more practical to determine, has been shown to be valuable in the context of developing disease in case of viral reactivation and for the ultimate clearance of the virus.24-26 Absence of T cells (CD3+ < 25/μL) or the failure of an AdV response shortly after AdV detection (CD3+ T cells < 300/μL within 2 weeks of AdV detection) has been associated with a poor outcome and was therefore used in the presented guideline (Figure 2).
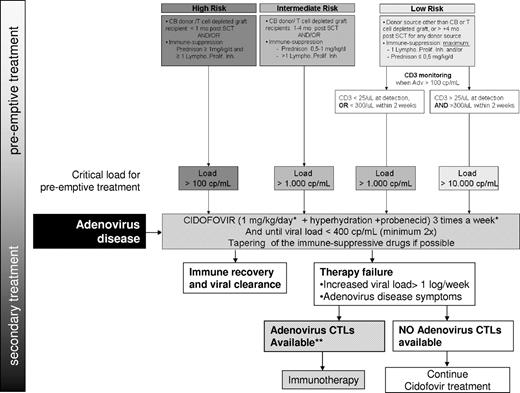
How I treat AdV in HSCT recipients: treatment guideline. Refer to the text for a detailed explanation. Lympho. Prolif. Inh. Indicates lymphocyte proliferation inhibitor (eg, cyclosporin A, CsA); *alternative cidofovir 5 mg/kg intravenously weekly. **For centers that have the AdV CTLs readily available, CTLs are immediately initiated for all high-risk patients and for all patients with AdV symptoms before awaiting cidofovir effect.
How I treat AdV in HSCT recipients: treatment guideline. Refer to the text for a detailed explanation. Lympho. Prolif. Inh. Indicates lymphocyte proliferation inhibitor (eg, cyclosporin A, CsA); *alternative cidofovir 5 mg/kg intravenously weekly. **For centers that have the AdV CTLs readily available, CTLs are immediately initiated for all high-risk patients and for all patients with AdV symptoms before awaiting cidofovir effect.
Graft-versus-host disease.
GVHD and the associated use of immunosuppressive agents (cyclosporine-A, methotrexate, steroids, mycophenolate mofetil).27 However, we and others have shown that AdV DNAemia can also precede the occurrence of acute GVHD (aGVHD). The hypothesis is that virus-induced tissue damage triggers alloreactivity.21,28 Whether preemptive treatment of reactivating AdV will have an effect on the incidence or severity of aGVHD remains to be investigated.
Use of serotherapy.
The use of serotherapy in conditioning regimens can include agents such as antithymocyte globulin (ATG) or alemtuzumab (anti-CD52+). Serotherapy is used to deplete the recipient's T cells in vivo, preventing rejection of the graft, as well as to reduce the risk of aGVHD. However, ATG has a depletory effect on the (antiviral) T cells in the graft, because of the long half-life of the polyclonal antibodies. Alemtuzumab (Campath-1H; anti-CD52), an alternative to ATG, holds an even higher risk of AdV disease, probably because of deeper in vivo T- and natural killer–cell depletion.29
Ex vivo T cell–depleted grafts and cord blood.
Ex vivo T cell–depleted grafts (eg, CD34+ selection) and cord blood are associated with delayed formation of memory T cells and withhold a risk to all viral complications after HSCT.30,31 In cord blood protocols, the use of similar doses of serotherapy as used in the matched unrelated donor setting may result in even further in vivo depletion of T cells (from the graft) and thus an even more delayed T-cell immune reconstitution, which, as a consequence, then mainly relies on new thymic output.
Furthermore, all other donor sources are considered to be seropositive for AdV and are associated with a lower risk of AdV reactivation, because of protection by adoptive immunity from AdV-specific T cells in the graft.
The presence of these risk factors determines individual susceptibility to develop AdV disease in the case of reactivation. Poor results of therapeutic interventions and severe side effects of the therapeutic options available in case of disease have motivated definition of specific risk groups, each with a different stringency of monitoring and treatment (Figure 1). Furthermore, in the prevention of de novo infections from carrying contacts, protective isolation measures and building characteristics of the transplantation units may be crucial. It is not seldom that an HSCT unit faces nosocomial spread or even an AdV outbreak, resulting in substantial rates of morbidity and mortality.2,32 For us (UMC Utrecht) this was one of the main reasons for rebuilding the SCT unit (high-efficiency particulate arresting–-filtered rooms) with each having a separate front room. Thus, hygienic rules (parents, nurses, and visitors), plus active surveillance (stool, NPAs) have prevented nosocomial spread or an outbreak so far (for the past 3 years).33,34
Treatment options for AdV infection and disease in patients receiving HSCT
Antiviral drugs
Ribavirin and cidofovir are agents used in the treatment of AdV. Most evidence for efficacy against AdV, however, is present for cidofovir (Table 1).35,36 Cidofovir is a monophosphate nucleotide analog of cytosine that is phosphorylated intracellularly to a diphosphate that can inhibit viral DNA polymerase and thus viral replication.37 It was first approved by the Food and Drug Administration in 1996 for the treatment of CMV retinitis. The antiviral selectivity of the acyclic nucleotide/nucleoside phosphonates is based on their higher affinity for the viral DNA polymerase compared with cellular DNA polymerases. Diphosphates of cidofovir compete with nucleoside triphosphates and are more efficiently incorporated in DNA strands, thereby inhibiting viral replication.38 Sustained effect of cidofovir in vitro has been shown to be present against all AdV subtypes. Cidofovir-resistant mutants have been described after serial passages in vitro and could be linked to mutations that affect nucleotide binding of viral DNA polymerase.38 A main disadvantage of cidofovir is that bioavailability is low and that the antiviral effect depends on concentrations of the active phosphorylated metabolites present within infected cells. Pharmacologic effects therefore do not correlate well with the prescribed dose, and > 90% of the drug is excreted unchanged in the urine.39
The problem of most other antivirals that have been investigated, such as acyclovir, is that they are nucleoside analogues that depend on viral kinases for their phosphorylation into their active form (nucleoside-diphosphate). They are much less efficiently phosphorylated by human intracellular kinases. Because AdVs, unlike herpesviruses such as CMV and herpes simplex virus, do not encode a kinase themselves, they are relatively insensitive to classical acyclic nucleoside analogues. Of these nucleoside analogues ribavirin is an agent for which in vitro anti-AdV activity differs widely against different subtypes (most active against group C, subtype 1, 2, or 5).40 In contrast with what may be expected, there are several case reports suggesting therapeutic benefit for some patients with no response reported in others.41-43 Because of availability of the more effective cidofovir, it is not included in our guideline. Although less efficient than nucleotide analogues, ganciclovir can be tri-phosphorylated by cellular kinases which results in interference with the function of AdV DNA polymerase, thus inhibiting viral replication in vitro.44 In retrospective studies lower incidences of AdV infections appeared to be reported in patients treated with ganciclovir as CMV prophylaxis.45 However, after adjusting for type of donor and age, ganciclovir administration did not convincingly lead to a reduction of adenoviral disease. Reports of positive outcome in patients with AdV infection treated with ganciclovir are fragmentary and do not justify incorporation in standard treatment protocols.46
So, although cidofovir appears to be the most effective of the agents described, it is also the most toxic. Cidofovir is excreted in the urine by proximal tubule cells. The rate of drug uptake from the blood by organic anion transporters at the antiluminal (basolateral) membrane of renal tubular cells and the slower efflux into the tubule lumen is believed to be responsible for the intracellular accumulation of cidofovir to toxic levels, with the consequence of substantial tubular necrosis. Hyperhydration together with coadministration of the drug probenecid has been shown to have a nephroprotective effect.47,48 Probenecid is an organic acid that competes for the kidney's organic anion transporter and thereby protects tubule cells and increases cidofovir plasma level.
Prophylaxis, preemptive treatment, and therapeutic treatment
Antiviral treatment can be used as prophylaxis, as preemptive treatment led by viral load cutoff values, or as therapeutic treatment in case of AdV disease. Rationale for the first 2 strategies is to bridge the immunosuppressed period after HSCT until the antiviral defense is reconstituted from the graft. Prophylactic treatment has only been described by Greil et al49 in an abstract presented at the 2006 European Study Group for Blood and Marrow Transplantation. Over a 2-year period all pediatric stem cell patients received ribavirin prophylaxis followed by preemptive cidofovir treatment when viral loads were detected. Comparing the outcome with historic controls in which no prophylaxis and cidofovir alone was given as a preemptive treatment, the combined strategy with ribavirin prophylaxis resulted in a significantly lower incidence of AdV infection (29% vs 66%) and AdV-associated mortality (0% vs 14%).49
Although the documented studies with patients who received a SC transplant and experienced AdV disease vary in their detection methods and their definition of infection and disease, it appears that ribavirin, but even cidofovir, have only limited efficacy when started as therapeutic treatment for AdV disease.30,50 This underlines the importance of early detection of AdV viremia to be able to initiate therapy within a certain timeframe before the occurrence of disease symptoms associated with a significant increase in mortality.27,51 This window of opportunity may differ for individual patients, depending on the presence of other risk factors. Our treatment guideline was designed from a pediatric perspective but may be as relevant to the less common adult patient with risk factors for AdV disease (Figure 1).13 The guideline takes these risk factors of patients in consideration, dividing them in a low-, intermediate-, and a high-risk group, each with a different treatment approach.
The observed dose-limiting nephrotoxicity of cidofovir, when given at the recommended dose of 5 mg/kg once weekly, has discouraged practitioners from using cidofovir as a preemptive treatment.52-54 Hyperhydration and coadministration of the drug probenecid (2 g, administered 3 hours before cidofovir infusion) decreases nephrotoxicity. Of the very limited prospective studies on preemptive cidofovir available, it has been shown prospectively that cidofovir 1 mg/kg 3 times weekly in combination with probenecid is effective in the prevention of progression to end-stage organ disease and in the prevention of AdV disease when used as a preemptive treatment at the time the virus is detected by routine screening methods.55-57 Only one of those studies used screening by plasma PCR in the absence of disease symptoms to initiate preemptive treatment (Table 1).56 However, regardless of their detection mode and thus timing of therapy, these studies showed acceptable toxicity in a pediatric HSCT setting despite concomitant use of other nephrotoxic agents such as ciclosporin, which is in line with data from our pediatric transplantation center in the Netherlands.52,56,57 In > 25 patients treated (also for a duration > 3 weeks) over the past 6 years within our center, no nephrotoxicity, except some mild tubulopathy, was observed (data not shown). For the necessity of cotreatment with the nephroprotective probenecid during low-dose cidofovir (1 mg/kg 3 times weekly), no data are currently available. Because of the limited side effects, we recommend using it, although the risk of increasing plasma levels of other drugs (methotrexate, nonsteroidal anti-inflammatory drugs) should be taken into account.
In the UMC Utrecht, we do not limit cidofovir treatment to a 3-week period as reported by others,55 but prolong treatment until viral load has fallen < 400 copies (cp)/mL or until the CD3 count is > 300/mL in high-risk patients receiving preemptive treatment.24,31 However, in line with others, our experience is that clearance of the virus only occurs when T cells reconstitute after HSCT.21 With the use of preemptive cidofovir the viral load usually stabilizes, buying time (weeks to months) for T cells to reconstitute and form AdV-specific T cells to clear the virus. It has become apparent that in case of AdV disease, the effect of cidofovir is limited, resulting in a significant mortality (Table 1). Not all persons die of AdV disease itself; the presence of aGVHD and a poor immune reconstitution, which are often the case, contribute significantly to transplant-related mortality in this group.13,30
Immunotherapy
As for many other virus infections in the posttransplantation setting, an increased risk of AdV infection is clearly associated with the lack of recovery of endogenous virus-specific T cells. First, one should try to taper immunosuppressant therapy to help immunorecovery.24 However, in a number of cases this is not possible because of aGVHD or does not prevent disease symptoms from developing. Because the efficacy of antiviral drugs for the treatment of AdV disease is limited, cellular immunotherapeutic approaches to provide physiologic protection against AdV infection by adoptively transferring T cells with AdV specificity have been investigated. Donor lymphocyte infusions (DLIs) and adoptive immunotherapy have been developed and used.
Donor lymphocyte infusions.
The first adoptive T-cell transfer protocols in the allogeneic HSCT setting were based on the premise that donor peripheral blood contained T cells that were able to mediate antitumor or antiviral activity or both in the HSC transplant recipient. Accordingly, DLIs have been used to provide antiviral immunity. As a proof of principle, Hromas et al58 reported a case of a 19-year-old man who underwent a T cell–depleted allogeneic donor HSCT for T-cell lymphoblastic lymphoma. After presenting with hemorrhagic cystitis secondary to AdV infection and failing to respond to antiviral drugs, he was given donor leukocytes (106 CD3 cells/kg) and subsequently cleared the virus.58 This initial success has been followed by a number of other case studies in which patients were infused with cell doses ranging from 1 × 105 to 3 × 107 CD3 cells/kg with similar positive outcomes.24,59-61 Despite this, DLI is often the last treatment of choice for clinicians because the efficacy of this approach is limited by the low frequency of T cells specific for many common “acute” viruses (such as AdV) and the relatively high frequency of alloreactive T cells that are associated with significant toxicity.
To enhance the safety of DLIs, the infusion of T-cell products from which the alloreactive cells have either been inactivated62,63 or selectively removed ex vivo64-67 or genetically modified with suicide genes to ensure that the high T-cell doses required to provide protection against viruses with a low frequency of reactive cells can be safely administered and eliminated in the event of adverse in vivo effects has also been investigated.68,69
Adoptive immunotherapy.
An alternative, safe, and effective adoptive transfer approach to DLI involves the infusion of AdV-specific T cells that can be selected directly from peripheral blood or selectively expanded in vitro. To date this strategy has been investigated by 2 groups, each with a different approach.
Feuchtinger et al70 were able to directly identify and isolate donor peripheral blood T cells that secreted IFN-γ in response to stimulation with AdV antigen (Miltenyi Gamma Catch). The isolated T cells were transferred into 9 pediatric recipients of allogeneic SC transplants with systemic AdV infection, despite conventional therapy. Donor peripheral blood mononuclear cells were stimulated for 16 hours with AdV lysate and then labeled with an anti–IFN-γ monoclonal antibody conjugated to a CD45 antibody. Thereafter, cells were magnetically labeled and selected with anti–IFN-γ microbeads (Miltenyi Gamma Catch) and infused without further in vitro expansion.70 The frequency of AdV-specific T cells in donor peripheral blood increased from 1.1% ± 1% to 45.7%± 24% after selection, and the cells were polyclonal with a mixture of CD4+ and CD8+ T cells. None of the infusions (range, 1200-50 000 CD3+ cells/kg) were associated with toxicity in vivo, and of 6 evaluable patients 5 showed a significant decrease of adenoviral DNA in peripheral blood and stool with a corresponding increase in the frequency of AdV-specific T cells in vivo. In this small number of patients there is a suggestion that the T-cell efficacy was independent of the infused cell dose and that even low numbers of transferred AdV-specific T cells can expand sufficiently in vivo to reconstitute antiviral immunity in the presence of antigen.70
Our group (Baylor College of Medicine) has achieved similar success in recipients of HLA-matched related, matched unrelated, and haploidentical HSC transplants after the adoptive transfer of in vitro–expanded AdV-specific T cells. To date we have performed 2 clinical studies that used virus-specific T cells containing an AdV-specific T-cell component. Both trials used cytolytic T-cell (CTL) lines produced with antigen-presenting cells transduced with AdV vectors. The AdV component was activated by virion proteins of the vector that were processed and presented to CD4+ and CD8+ T cells. Monocytes and EBV-transformed lymphoblastoid cell lines were used as antigen-presenting cells so that EBV antigens were also presented. In one trial we infused bivirus CTLs targeting AdV and EBV.71 and in the other trial the introduction of a CMV antigen into the AdV vector resulted in CTLs with specificity for all 3 viruses.72 Cells were infused from day 30 after transplantation in patients with ≤ grade II aGVHD. The infused doses ranged from 1.7 × 105 to 4.5 × 106 cells/kg, reconstituted immune responses to all 3 viruses, and were able to control ongoing drug-resistant virus infections. No toxicity or aGVHD was observed.
Of note, although we were routinely able to detect an increase in the frequency of T cells reactive against the latent viruses EBV and CMV (in patients treated with trivirus CTLs) independent of detectable viral reactivation, AdV-specific T cells were detectable only in patients with recent or concurrent adenoviral infection. However, none of the treated patients developed a de novo AdV infection compared with an expected incidence of 68% in pediatric subjects receiving similar transplants in the absence of CTLs.23 We demonstrated evidence of AdV-specific T cells for at least 8 weeks, even in CTL recipients without viral infection, implying that transferred cells were able to persist in sufficient numbers to mediate antiviral protection in the lymphopenic host.71 All patients with detectable AdV in blood, stool, or tracheal aspirate (7 of 24) had a marked reduction in adenoviral load coincident with the rise in their AdV-specific T cells irrespective of infection serotype. This included one patient with progressive adenoviral pneumonia, requiring maximal ventilatory support, who after infusion had a progressive rise in AdV-specific T cells with a reduction in viral DNA load and was completely weaned from ventilatory support within 10 days of receiving cells.
Although both systems described produce T cells that are safe in vivo and can effectively control active infections and provide broad-spectrum antiviral protection in vivo, there are associated limitations. The Miltenyi Gamma Catch system is expensive, requires large starting blood volumes, and requires access to clinical grade antigen as a T-cell stimulus, whereas the in vitro expansion system requires the production of clinical grade viral vectors for antigen presentation and a prolonged period of culture (10- to 12-week manufacturing process) with its attendant demands on technical skill and time. Thus, neither is ideal. Ultimately, the broader implementation of this therapeutic option requires the development of novel production processes that rapidly (< 2 weeks) and cost-effectively ensure the availability of T-cell products that can be generated from small starting blood volumes, and this area is an area of intensive interest for many groups, particularly given the poor efficacy of conventional antiviral therapeutics for AdV. In the future, development of such a system will serve to move T-cell immunotherapies beyond highly specialized centers to a standard-of-care therapy available to all.
Future perspectives
Boosting immune recovery.
In our setting (UMC Utrecht), rabbit polyclonal ATG (Thymoglobulin; Genzyme) is given before HSCT to prevent both graft rejection and aGVHD. The long half-life and unpredictable pharmacokinetics of active-ATG make ATG an extremely important but uncertain variable influencing immune reconstitution after HSCT. A future development might be tailor-made ATG dosing before HSCT, which could be especially important for the cord blood setting, which already contains lower numbers of T cells in the graft. Therefore, more insight in the pharmacokinetics-pharmacodynamics of (active)-ATG is warranted, and these studies are currently being evaluated in our center. A more predictable immune reconstitution may not only have a direct effect on the prevention of viral complications but also is of utmost importance for the efficacy of adjuvant immunotherapies.
Other experimental strategies that may boost immune reconstitution are sex hormone blocking to improve thymic function by increased expression of CCL25 (chemokine important for the immigration of thymocyte precursors from bone marrow into thymus) and thus enhancing the rate of T-cell regeneration,73,74 or by the administration of interleukin-7 and keratinocyte growth factor which also enhance thymopoiesis and the output of recent thymic emigrants.74,75
Antiviral drug development.
Novel drugs are being developed to circumvent the current problems associated with available drugs, such as nucleoside/nucleotide analogues that either do not need to be phosphorylated any further and are already in its active form or that can be adequately phosphorylated by intracellular kinases. Investigated candidates include ganciclovir triphosphate, which does not need further phosphorylation, and the S-2242 compound (N7-isomer of 6-deoxy-ganciclovir) which is adequately phosphorylated by cellular kinases.76 Furthermore, lipid esters of cidofovir are already available to some centers, for compassionate use only. They have been developed because they have a higher antiviral activity and particularly because they have a higher oral bioavailablity and less nephrotoxicity at the median effective concentration of the drug.77,78
Dendritic cell vaccination.
Besides ex vivo generation of specific antiviral cytotoxic T cells, in vivo induction of specific CD4+ and CD8+ T-cell responses to AdV with the use of virus peptide/lysate-loaded dendritic cell (DC) vaccination has been proposed as an elegant and potentially more rapid strategy to be used early after transplantation. The method has already been performed in antitumor vaccination strategies. Development of an immunostimulatory adjuvant antiviral immunotherapy that is based on viral peptide/lysate-pulsed DC vaccination might be especially of interest in seronegative donors (such as cord blood). DCs generated from the graft can be pulsed with viral lysate, can be generated rapidly (< 2 weeks), introduced into the patient by vaccination, and may potentially induce a more polyclonal virus-specific T-cell response. This response is generated in vivo, and the treatment is not HLA restricted. For CMV, a proof-of-principle study showed that DC vaccination in allo-HSCT resulted in induction of human CMV-specific CTL responses in approximately one-half of the evaluable patients (n = 17) and control of CMV in almost all.79 In this study DCs were generated from peripheral blood monocytes from CMV-negative donors.
Treatment guideline: AdV infections in pediatric HSCT recipients
The treatment guideline (Figure 1) is applicable to patients within 4 months after HSCT and patients after HSCT on immune suppression. Although it was developed as a guideline in the pediatric HSCT setting, it can be applicable to high-risk adult patients as well, such as cord blood or haplo-graft recipients and individuals with severe aGVHD.13,30
Main pillars of this treatment protocol
No prophylaxis.
However, postponement of HSCT (eg, in case of DNA positivity in NPA or stool) may be an option for elective HSCTs (eg, nonmalignant indications).
Definition of risk groups and assignment of each patient to the low-, intermediate-, or high-risk group.
The high-risk group has been defined as cord blood and T cell–depleted (< 5 × 10e4/kg) graft recipients within the first month and patients on prednisone with a dose of > 1 mg/kg/d (for any indication) next to ≥ 1 lymphocyte proliferation inhibitor(s).
The intermediate-risk group consists of recipients of cord blood and T cell–depleted grafts > 1 month to < 4 months after HSCT, patients with immune suppression consisting of ≥ 2 lymphocyte proliferation inhibitors (eg, cyclosporine-A and mycophenolate mofetil) or 1 lymphocyte proliferation inhibitor + prednisone > 0.5 and < 1 mg/kg.
The low-risk group consists of recipients of all other transplant sources within the first 4 months after HSCT, without GVHD, and with maximum immune suppressive therapy of 1 proliferation inhibitor and/or prednisone ≤ 0.5 mg/kg/d.
Weekly monitoring of adenoviral load with qPCR to aim for a preemptive treatment.
The frequency of monitoring is based on our experience with the quick rise in adenoviral load that can be seen early after detection (Figure 1). A 1-3 log rise in load within the timeframe of a week is not uncommon. Weekly monitoring is also consistent with others.14,25 If AdV is detected (> 100 cp/mL), we perform PCR twice weekly to monitor response to antiviral medication. Unfortunately, there are no definite data available on how often and which groups of adults after HSCT should be monitored. We recommend that high-risk adults are monitored weekly as well.
Preemptive treatment.
We start preemptive treatment with cidofovir 1 mg/kg 3 times a week (5 mg weekly as an alternative) when the adenoviral load exceeds a certain critical level, depending on the risk group the patient is in. All high-risk patients get treated when the load exceeds > 100 cp/mL (mainly based on the above-mentioned fast kinetics in these highly vulnerable group of patients), all intermediate-risk patients get treated when load exceeds > 1000 cp/mL (a cutoff used in various guidelines for other viral reactivations; eg, EBV, CMV). Patients in the low-risk group are further divided into 2 groups on the basis of their immune status (number of CD3+ cells). Low-risk patients with both detectable numbers of T cells (CD3+ > 25/μL) present at the time of AdV detection and an adequate immune response (> 300/μL CD3+ T cells within weeks of AdV detection) only get treated if viral load exceeds > 10 000 cp/mL. A load > 10 000 cp/mL is regarded as failure of the wait-and-see policy and should result in initiation of treatment. In case of a suboptimal immune status, (absence of T cells, CD3+ < 25/μL, or an inadequate increase of CD3+ cells within 2 weeks' time) the low-risk patient gets treated similar to the intermediate group, when the load exceeds 1000 cp/mL.
Probenecid.
Treatment with probenecid (2 g, administered 3 hours before cidofovir infusion) and hyperhydration are started as supportive care to limit cidofovir nephrotoxicity.
Cidofovir.
Treatment with cidofovir 1 mg/kg 3 times a week (or 5 mg weekly as an alternative) is started when there are signs of AdV disease irrespective of the load.
Evaluation after the first week and the second week of cidofovir treatment.
When load increases by a log after 2 weeks of treatment, disease symptoms develop during preemptive treatment, or disease progresses during treatment for AdV disease, immunotherapy with AdV-specific CD4+/CD8+ CTLs is indicated for patients in centers where this is an available therapeutic option.
Taper therapy.
Adenoviral load detection.
From detection, adenoviral load detection by PCR is performed at least weekly for optimal timing of preemptive treatment and careful monitoring of the response to treatment.
Monitor evidence of disease.
An ophthalmologist should be consulted for screening of keratoconjunctivitis. In case of diarrhea perform gastroduodenal and colonoscopy with biopsies.
Discontinue therapy.
Discontinuation of therapy only when adenoviral load has been < 400 ccp/mL for 2 consecutive weeks and CD3+ T cells are > 300 μL.
Summary
Although HSCT has become much safer over the past decade, its main limitation remains transplantation-related mortality (eg, because of viral reactivations/disease) and relapse (in malignancies). The immune-suppressed period during the first weeks to months after HSCT is the most important determining factor. The ideal treatment strategy would aim to enhance the desired (antiviral and antitumor) immunity during the first weeks while preventing the undesired alloreactive immune response (aGVHD). HSCT is made safer with the use of uniform treatment guidelines, for which the validation has preferably been investigated in trials.
AdV infection and disease pose an important problem with considerable mortality in immunocompromised patients. Reactivation within the recipient and horizontal transmission are most common routes of acquisition of the virus. AdV infections are much more of a problem in pediatric HSCT than adult HSCT, possibly because of the reservoir of AdV in the general pediatric population who shed the virus for extended periods of time from lymphoreticular tissue before complete clearance can be secured. Antivirals are commercially available of which cidofovir is most effective as preemptive therapy. Because of toxicity and limited effectiveness, cidofovir is still not ideal, but, when used as a preemptive therapy, it is a reasonably safe option to buy time until immune recovery. Besides drug therapy, CTL immunotherapy is promising and is probably more effective. Unfortunately, this option is not available to all centers. However, we believe various issues currently preventing this treatment from introduction into general HSCT care will be addressed in the coming years. The treatment guideline presented in this article is based on the evidence currently available and may be a practical guideline to treat or prevent AdV disease. In the guideline, there is a steering role for stringent monitoring of adenoviremia by qPCR, immune reconstitution, preemptive treatment with cidofovir, and administration of AdV-specific CTLs for patients who do not adequately respond to cidofovir in centers for which CTL therapy is an option. Obvious limitations of current available therapies have been a motivation for development of new (immuno-)therapies that may make HSCT safer in the near future.
Authorship
Contribution: C.A.L., A.M.L., and J.J.B. equally contributed to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaap Jan Boelens, University Medical Center Utrecht, Department of Pediatrics, Blood and Marrow Transplant Program, Rm KC 03.063.0, Postbus 85090, 3508AB Utrecht, The Netherlands; e-mail: j.j.boelens@umcutrecht.nl.
References
National Institutes of Health