Abstract
The requirements for tonic T-cell receptor (TCR) signaling in CD8+ memory T-cell generation and homeostasis are poorly defined. The SRC homology 2 (SH2)-domain–containing leukocyte protein of 76 kDa (SLP-76) is critical for proximal TCR-generated signaling. We used temporally mediated deletion of SLP-76 to interrupt tonic and activating TCR signals after clearance of the lymphocytic choriomeningitis virus (LCMV). SLP-76–dependent signals are required during the contraction phase of the immune response for the normal generation of CD8 memory precursor cells. Conversely, LCMV-specific memory CD8 T cells generated in the presence of SLP-76 and then acutely deprived of TCR-mediated signals persist in vivo in normal numbers for more than 40 weeks. Tonic TCR signals are not required for the transition of the memory pool toward a central memory phenotype, but the absence of SLP-76 during memory homeostasis substantially alters the kinetics. Our data are consistent with a model in which tonic TCR signals are required at multiple stages of differentiation, but are dispensable for memory CD8 T-cell persistence.
Introduction
Protection against recurrent infections resulting from the same pathogen is a hallmark of adaptive immunity. After acute infection by an intracellular pathogen, naive CD8+ T cells expressing epitope-specific T-cell receptors (TCRs) are activated. The effector phase of the response is short, with a rapid expansion of antigen-specific T cells and pathogen clearance. The expanded effector cells undergo a contraction phase, while approximately 5% to 10% of antigen-specific cells are maintained to establish a memory pool and provide long-term protection from reinfection by the same pathogen.1-3
In the early stages of the effector response to acute lymphocytic choriomeningitis virus (LCMV) infection, activated CD8+ cells differentiate into 2 subsets with distinct fates. These populations can be phenotypically identified by cell-surface expression of killer cell lectin-like receptor G1 (KLRG-1) and the receptor for interleukin-7 (IL-7R).4 Short-lived effector cells (SLECs) express high levels of KLRG-1 and the transcription factors Blimp-1 and T-bet, and decreased levels of IL-7R.5-8 SLECs are dependent on signals from the environment, including TCR signals, inflammatory cytokines such as IL-12 and IFNγ, and common γ chain cytokine signaling from IL-2 and IL-15.4,9 Conversely, memory precursor (MP) cells express low levels of KLRG-1 and higher levels of IL-7Rα, CXCR3, and CD27.4,10,11 While these cells possess effector function, they also have the potential to further differentiate into long-lived memory T cells after the resolution of infection. The molecular nature of the proximal signals involved in the SLEC/MP cell-fate decision and those required for normal homeostasis of these populations have not been extensively studied.
Previous studies have shown that IL-15– and IL-7–generated signals are required for memory T-cell homeostasis.12-15 Depending on the experimental system and the characteristics used to define the memory population, TCR signals have been shown to be required or dispensable. For example, H-2Db-restricted, male-specific (H-Y TCR-transgenic) memory CD8+ T cells require expression of either H-2Db or H-2Dd for survival.16 The absence of all major histocompatibility complex (MHC) class I expression leads to the disappearance of the cells, suggesting that a tonic MHC-TCR signal is required. In addition, CD8+CD44hi cells do not persist in mice after gene deletion of the TCRα chain.17 In contrast, polyclonal CD8+ T-cell populations containing memory CD8+ T cells generated by viral infection persist indefinitely when transferred into MHC class I–deficient mice.18 CD44hi cells and TCR-transgenic memory T cells persist long term, even when the expression of the src family tyrosine kinase Lck or of TCR itself is substantially decreased by a bitransgenic tetracycline regulatory system.19-21
An obstacle to characterizing the requirements for memory population generation and persistence is the heterogeneity of definitions for memory CD8+ T cells or memory CD8+ T-cell populations. Memory T cells have been defined by a combination of their capacity to mount a recall response, their effector function, and their expression of cell-surface markers such as high levels of CD44.22 However, CD44 expression may be increased on effector T cells, on cells generated by proliferation in a lymphopenic environment or in response to environmental antigens, and on antigen-specific memory T cells. Therefore, the identification of a memory CD8+ T-cell population by an isolated elevation of CD44, as has been used in some studies of memory T-cell homeostasis, may be misleading.
In the present study, we investigated the role of TCR signaling in the activation, differentiation, and response to reinfection by combining 2 well-defined systems. Antigen-specific (AgSp) memory cell populations were generated using a well-described pathogen model, LCMV, and CD8+ memory T cells were tracked using TCR-specific binding to LCMV-specific MHC:peptide tetramers. We chose to manipulate the expression of the adaptor molecule SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76), because this adaptor is critical for the transduction of TCR-derived signals in Jurkat T cells,23 developing thymocytes,24-26 and peripheral T cells27 (and G. F. Wu, E.C., J.S.M., unpublished data, September 2010). Antigenic and tonic TCR-generated signals are abrogated through in vivo, temporally controlled conditional deletion of SLP-76 during either the contraction or maintenance phases of the immune response. Using this system, we show that SLP-76–dependent signals are required for normal AgSp memory differentiation during the contraction phase of the immune response. SLP-76 expression is critical for TCR-signal generation, inflammatory-cytokine production, and proliferation of AgSp memory CD8+ T cells in response to reinfection. Lastly, we demonstrate that SLP-76 is critical for naive T-cell maintenance, but is dispensable for CD8+ antigen-specific memory T-cell homeostasis.
Methods
Mice and tamoxifen treatment
Floxed SLP-76, the R26R-YFP reporter, SLP-76null, and UBC-CreT2 transgenic mice have been described previously.24,26,28,29 SLP-76F/FR26RYFP/YFP mice were intercrossed with SLP-76+/−CreT2+ to generate the SLP-76F/−R26RYFPCreT2+ (cKO) and SLP-76F/+R26RYFPCreT2+ (cHET) mice used in the studies. Tamoxifen in corn oil was administered for 5 consecutive days by oral gavage using a weight-based regimen (200 μg/g/d). All mice were maintained at the University of Pennsylvania, and protocols were approved by the institutional animal care and use committee.
Viral infection
Mice received 2 × 105 plaque-forming units (PFUs) of LCMV-Armstrong by intraperitoneal injection to initiate the acute infection. Recall responses were generated by inoculation with 2 × 106 PFU of LCMV clone 13 intravenously.
Antibodies and flow cytometry
Fluorochrome-conjugated antibodies used for staining were purchased from BioLegend, eBioscience, Invitrogen, BD Biosciences, Beckman Coulter, or Caltag Laboratories. H-2Db:GP33 tetramers conjugated to allophycocyanin were made and used as described previously.30 Lymphocytes from the peripheral blood were isolated on a density gradient using Histopaque (Sigma-Aldrich). Lymphocytes from spleen were isolated after hypotonic lysis with BioWhittaker ACK solution (Lonza). For intracellular cytokine analysis, cells were stimulated in the presence of brefeldin A with media alone, 2C11 (Bio X Cell), 5 ng/mL GP33 peptide (GenScript), or with 5 ng/mL phorbol myristate acetate (PMA) and 1 μg/mL ionomycin. After stimulation, cells were harvested and stained using a Cytofix/Cytoperm kit (Becton Dickinson) according to the manufacturer's instructions. All samples were acquired using a flow cytometer (LSRII; Becton Dickinson) and analyzed using FlowJo Version 8 (TreeStar).
Statistical analyses
Where indicated, P values were determined by a 2-tailed unpaired Student t test; P values < .05 were considered statistically significant. All graphs show averages of the mean ± SD. Linear regression modeling for CD62L expression was calculated in Prism software (GraphPad); statistical analysis of points indicating 50% CD8+ CD62L, and corresponding points along the x-axis (time).
Quantification of T lymphocytes from peripheral blood
Frequencies taken from the FlowJo analysis of fluorescence-activated cell sorting (FACS) data were calculated to generate relative numbers. The frequency of CD8+ T cells was multiplied by 106 to yield the relative number of CD8+ T cells per 1 million lymphocytes. Frequencies of the daughter gates (ie, YFP+, CD44hi, GP33+) were then multiplied by the CD8+ T-cell number to calculate the relative number of populations of interest per million lymphocytes. Relative numbers derived from all cKO mice or all cHET mice were averaged, and the mean ± SD was plotted.
Results
A system to generate SLP-76–deficient memory T cells
To determine whether TCR-generated signals mediated by SLP-76 are involved in AgSp memory generation and homeostasis, we used mice in which the SLP-76 gene can be deleted in a temporally controlled fashion by the administration of the estrogen analog tamoxifen. To generate conditional knockouts, we mated mice with a floxed allele of SLP-76 to mice transgenic for a tamoxifen-regulated Cre recombinase (CreT2) that is under the transcriptional control of the ubiquitin promoter.29 To identify cells that have undergone Cre-mediated deletion, mice were intercrossed with a Rosa26-based EYFP Cre-reporter.28 To improve deletion efficiency, we used experimental mice in which only one allele of SLP-76 needed to be deleted because the second allele was a germline-null mutation (SLP-76Flox/nullCreT2R26RYFP, termed cKO; Figure 1A). As controls, we used mice with one wild-type allele and one floxed allele (SLP-76Flox/WTCreT2R26RYFP, termed cHET). To control for Cre expression and drug treatment, all mice used in these studies expressed CreT2 and were treated with tamoxifen. Before tamoxifen administration, T-cell development and differentiation occurred normally (data not shown); after tamoxifen administration, SLP-76 was deleted and YFP was expressed. Using matched sets of cHET and cKO mice, we examined the effects of ablating TCR-generated signals during the generation or maintenance of AgSp memory CD8+ T cells.
Tamoxifen treatment 30 days after LCMV infection results in efficient deletion of SLP-76 in a normal memory T-cell population. (A) Schematic diagram of SLP-76 conditional deletion. cHET and cKO mice contain LoxP sites flanking exon 3 of the SLP-76 gene. The second SLP-76 allele in cHET mice is a wild-type allele and is a germline-null allele in cKO mice. All mice have one copy of the R26R-YFP Cre reporter. All mice express a transgene with the CreT2 cDNA under the transcriptional control of the ubiquitin promoter. CreT2 recombinase activity is induced by tamoxifen administration, resulting in excision of the lox-STOP-lox cassette in the reporter construct and the floxed SLP-76 alleles. (B) Intracellular staining of CD8+ T cells for SLP-76 in cHET and cKO mice. After tamoxifen treatment, splenocytes were permeabilized and stained for SLP-76. The dot plots show an overlay of CD8-gated cells from cKO (left panel) and cHET (right panel) mice. The numbers in the upper right quadrant are relative percentages for cKO cells in the gated areas. Results are representative of 4 experiments. (C) Schematic of LCMV infection and Cre-mediated deletion regimen. cKO and cHET mice were infected with LCMV-Armstrong on day 0, allowed to clear the virus, and contracted to a memory T-cell population before Cre-mediated deletion. More than 30 days later, tamoxifen was administered for 5 days with subsequent SLP-76 deletion and YFP expression. The solid line represents the relative abundance of LCMV-specific cells and the dashed line represents the relative abundance of YFP+ cells. (D) Representative FACS plots of cells from cHET and cKO mice after infection but before tamoxifen treatment. Mice were bled at day 8 and day 20 after infection, and the peripheral blood was assayed using polychromatic flow cytometry. Contour plots are gated on CD8+ T cells (left panels). Histogram overlays of CD8+CD44hiH-2Db:GP33+-gated cells from cHET mice (solid gray) and cKO mice (black line; right panels). Results are representative of 2 experiments.
Tamoxifen treatment 30 days after LCMV infection results in efficient deletion of SLP-76 in a normal memory T-cell population. (A) Schematic diagram of SLP-76 conditional deletion. cHET and cKO mice contain LoxP sites flanking exon 3 of the SLP-76 gene. The second SLP-76 allele in cHET mice is a wild-type allele and is a germline-null allele in cKO mice. All mice have one copy of the R26R-YFP Cre reporter. All mice express a transgene with the CreT2 cDNA under the transcriptional control of the ubiquitin promoter. CreT2 recombinase activity is induced by tamoxifen administration, resulting in excision of the lox-STOP-lox cassette in the reporter construct and the floxed SLP-76 alleles. (B) Intracellular staining of CD8+ T cells for SLP-76 in cHET and cKO mice. After tamoxifen treatment, splenocytes were permeabilized and stained for SLP-76. The dot plots show an overlay of CD8-gated cells from cKO (left panel) and cHET (right panel) mice. The numbers in the upper right quadrant are relative percentages for cKO cells in the gated areas. Results are representative of 4 experiments. (C) Schematic of LCMV infection and Cre-mediated deletion regimen. cKO and cHET mice were infected with LCMV-Armstrong on day 0, allowed to clear the virus, and contracted to a memory T-cell population before Cre-mediated deletion. More than 30 days later, tamoxifen was administered for 5 days with subsequent SLP-76 deletion and YFP expression. The solid line represents the relative abundance of LCMV-specific cells and the dashed line represents the relative abundance of YFP+ cells. (D) Representative FACS plots of cells from cHET and cKO mice after infection but before tamoxifen treatment. Mice were bled at day 8 and day 20 after infection, and the peripheral blood was assayed using polychromatic flow cytometry. Contour plots are gated on CD8+ T cells (left panels). Histogram overlays of CD8+CD44hiH-2Db:GP33+-gated cells from cHET mice (solid gray) and cKO mice (black line; right panels). Results are representative of 2 experiments.
After tamoxifen treatment, a population of CD8+ T cells began to express YFP. To verify that YFP-expressing T cells also lacked SLP-76 expression, splenocytes were isolated and analyzed for expression of YFP and SLP-76. Based on SLP-76 protein expression (Figure 1B left) and expression of CD69 in response to TCR cross-linking (data not shown), 90% to 99% of CD8+ T cells had undergone deletion at the SLP-76 locus, depending on the experiment. Deletion at the Rosa26 locus occurred in only 40% to 60% of the cells. In cKO T cells, the expression of YFP was highly correlated with deletion at the SLP-76 locus, as indicated by a lack of YFP+SLP-76+ cells. Conversely, the absence of YFP expression was not correlated with the deletion status at the SLP-76 locus. Treatment of cHET mice resulted in a similar deletion efficiency at the Rosa26 locus and deletion of the SLP-76Flox allele (Figure 1B right and data not shown). Although cHET T cells consistently expressed slightly reduced levels of SLP-76 relative to wild-type, TCR cross-linking–induced activation was similar to that of wild-type controls (data not shown). Evaluation of SLP-76–deficient versus –sufficient cells could therefore be achieved by comparing YFP+ cells from tamoxifen-treated cKO and cHET mice, respectively.
Memory CD8+ T cells lacking SLP-76 fail to induce effector cytokines in response to TCR-mediated stimuli
To investigate the requirement for SLP-76–dependent signals in AgSp CD8+ memory T cells, we first generated an immune repertoire enriched for LCMV-specific memory T cells by infection with LCMV-Armstrong in cHET and cKO mice before tamoxifen-induced deletion (Figure 1C). To confirm normal CD8+ memory T-cell generation in mice expressing the SLP-76 floxed allele and CreT2, before tamoxifen treatment we characterized the phenotype of CD8+ T cells reactive to the immunodominant epitope GP33 after infection with LCMV-Armstrong. Both experimental and control mice generated a population of cells that were specific to the immunodominant epitope and recognized by the H-2Db:GP33 tetramer (Figure 1D left). In both cKO and cHET mice, the H-2Db:GP33 tetramer-specific population expressed high levels of CD44. In addition, cell-surface expression of CD127 (IL-7Rα chain), CD122 (IL2R/IL15R β chain), CD25 (IL-2Rα chain), L-selectin (CD62L), KLRG-1, and the CXCR3 chemokine receptor were all comparable on CD44hi H-2Db:GP33 tetramer-positive cells from cKO and cHET mice (Figure 1D and data not shown).
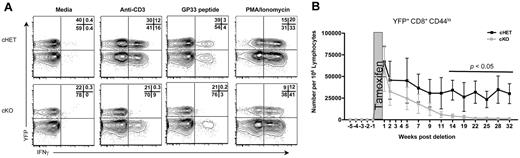
To assess SLP-76–dependent signaling in AgSp memory CD8+ T cells, cKO and cHET mice were treated with tamoxifen beginning more than 30 days after LCMV infection. Single-cell interferon-gamma (IFNγ) production was assessed as a functionally significant TCR-dependent readout. Four hours after stimulation, memory but not naive, CD8+ T cells were primed to express high levels of IFNγ.22,31 Ten days after SLP-76 deletion, splenocytes from cKO and cHET mice were stimulated in vitro for 4 hours with anti-CD3 or LCMV-specific peptide or were left unstimulated (Figure 2A). Cross-linking of the TCR or peptide stimulation both induced IFNγ production in cells from cHET mice. In contrast, cross-linking of the TCR by antibody or stimulation with peptide failed to induce substantial IFNγ production in memory CD8+ T cells expressing YFP (ie, those lacking SLP-76). Because the YFP− cells were a mixture of deleted and nondeleted cells, induction of IFNγ was detectable in the YFP− cKO but at a decreased frequency compared with YFP− cHET controls. Incubation with a combination of phorbol ester and calcium ionophore, a stimulus that bypasses proximal TCR-mediated signal transduction, resulted in a comparable induction of IFNγ in YFP+ CD8+ T cells from both cHET and cKO mice, indicating that despite the loss of SLP-76, the CD44hi cells maintained potentialeffector function in cKO mice. Thus, SLP-76 is required for cytokine production in response to TCR stimulation of memory CD8+ T cells.
SLP-76 is necessary for TCR-mediated signaling in memory CD8+ T cells. (A) Two weeks after tamoxifen treatment, whole-spleen suspensions from cHET and cKO mice were stimulated in vitro. All plots are gated on CD8+CD44hi cells. Numbers in the top right quadrant show the relative percentage of cells in each gate. Data are representative of 2 experiments. (B) Lymphocytes from cHET and cKO mice were isolated from peripheral blood longitudinally and analyzed by multicolor flow cytometry. The number of naive (CD8+CD44loYFP+) T cells in the peripheral blood was calculated longitudinally as described in “Quantification of T lymphocytes from peripheral blood.” The shaded area represents the time of tamoxifen administration. Each time point shows a mean and standard deviation derived from a total of 6 cHET and 8 cKO mice in 2 separate experiments.
SLP-76 is necessary for TCR-mediated signaling in memory CD8+ T cells. (A) Two weeks after tamoxifen treatment, whole-spleen suspensions from cHET and cKO mice were stimulated in vitro. All plots are gated on CD8+CD44hi cells. Numbers in the top right quadrant show the relative percentage of cells in each gate. Data are representative of 2 experiments. (B) Lymphocytes from cHET and cKO mice were isolated from peripheral blood longitudinally and analyzed by multicolor flow cytometry. The number of naive (CD8+CD44loYFP+) T cells in the peripheral blood was calculated longitudinally as described in “Quantification of T lymphocytes from peripheral blood.” The shaded area represents the time of tamoxifen administration. Each time point shows a mean and standard deviation derived from a total of 6 cHET and 8 cKO mice in 2 separate experiments.
SLP-76–dependent signals are required for the generation of tonic TCR signals
To address whether deletion of SLP-76 could affect tonic TCR signals generated by TCR:MHC interaction, we assessed the persistence of CD8+ naive T cells in the absence of SLP-76. Results using several different experimental approaches have shown that naive CD8+ T cells fail to persist in vivo when deprived of the TCR:MHC interaction.16,17,32,33 We hypothesized that if deletion of SLP-76 abrogated tonic TCR-generated signals, then there would be a gradual disappearance of naive SLP-76–deficient CD8+ T cells over time. To determine whether this is the case, CD8+CD44loYFP+ cells were quantitated longitudinally in peripheral blood after tamoxifen-induced deletion (Figure 2B). The number of YFP+ naive T cells decreased substantially during the first 11 weeks after deletion, and became almost undetectable by 20 weeks after deletion. Absolute numbers of CD8+CD44loYFP+ naive cells were similarly decreased in the spleen (data not shown). Numbers of total CD8+ and CD8+CD44loYFP− peripheral blood T cells in cKO mice were slightly lower, but not statistically different from those seen in cHET mice (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These data suggest that, similar to pre-TCR signaling in developing thymocytes,24,25 expression of the SLP-76 adaptor is required for tonic TCR-dependent signals in peripheral T cells.
SLP-76–dependent signals are not required for persistence of AgSp memory CD8+ T cells
Next, we assessed the persistence of CD8+ T cells in the absence of SLP-76 by serial measurement of total and antigen-specific CD8+ T-cell numbers after deletion of SLP-76. CD8+ T cells in the peripheral blood before and after tamoxifen administration were quantitated using multiparameter flow cytometry. LCMV-Armstrong inoculation resulted in an equivalent expansion of CD8+ cells and contraction to a memory population before tamoxifen administration (Figure 1D and supplemental Figure 1A). We hypothesized that the mice would not become lymphopenic, because the deletion was incomplete, and any potential decrease in lymphocyte numbers due to SLP-76 deficiency would be compensated for by maintenance and potential expansion of the YFP− SLP-76+ population. Consistent with this hypothesis, the numbers of CD8+YFP− and CD8+CD44hiYFP− cells in the peripheral blood of cKO and cHET mice were not statistically different (supplemental Figure 1C-D). Similarly, the numbers of total T and CD8+ T cells in the spleen were equivalent in cKO and cHET mice at all time points assessed up to 48 weeks after deletion (supplemental Figure 1E-F). Therefore, an environment without significant lymphopenia was maintained in the CD8+ T-cell compartments of cKO mice, allowing evaluation of CD8 memory T-cell homeostasis and function in a comparable in vivo setting.
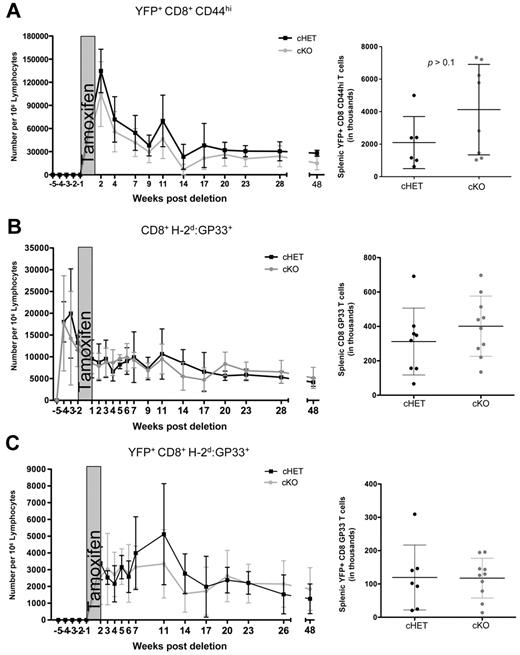
To evaluate the effect of SLP-76 deficiency, we gated on the YFP+ cells. CD8+CD44hiYFP+ T-cell numbers in the peripheral blood of cKO mice were modestly decreased compared with the cHET controls, but persisted for up to 48 weeks after deletion (Figure 3A). Similar to peripheral blood, the total numbers of CD8+CD44hi T cells in the spleen were maintained in the absence of SLP-76, and were not significantly different than in the cHET mice (Figure 3A right).
Maintenance of antigen-specific memory cells is not dependent on SLP-76. cHET and cKO mice were bled longitudinally, and peripheral blood lymphocytes were isolated and stained for multicolor flow cytometry. (A) SLP-76–deficient CD44hi cell number was determined longitudinally in peripheral blood samples (left) and spleens (right). (B) Total GP33-specific CD8+ T cells enumerated from peripheral blood (left) and spleen (right). (C) YFP+H-2Db:GP33-specific CD8+ T cells enumerated from peripheral blood (left) and from spleen (right). For the longitudinal studies, cells were gated on populations as indicated, and normalized to number of cells per 1 million lymphocytes, as described in “Quantification of T lymphocytes from peripheral blood.” Data points are shown as mean and standard deviations. Black represents cHET; and gray, cKO. Longitudinal bleeding data are compiled from 15 mice in 2 independent infections. Splenocyte numbers are compiled from mice at 6, 10, and 48 weeks after infection in a total of 4 independent infections.
Maintenance of antigen-specific memory cells is not dependent on SLP-76. cHET and cKO mice were bled longitudinally, and peripheral blood lymphocytes were isolated and stained for multicolor flow cytometry. (A) SLP-76–deficient CD44hi cell number was determined longitudinally in peripheral blood samples (left) and spleens (right). (B) Total GP33-specific CD8+ T cells enumerated from peripheral blood (left) and spleen (right). (C) YFP+H-2Db:GP33-specific CD8+ T cells enumerated from peripheral blood (left) and from spleen (right). For the longitudinal studies, cells were gated on populations as indicated, and normalized to number of cells per 1 million lymphocytes, as described in “Quantification of T lymphocytes from peripheral blood.” Data points are shown as mean and standard deviations. Black represents cHET; and gray, cKO. Longitudinal bleeding data are compiled from 15 mice in 2 independent infections. Splenocyte numbers are compiled from mice at 6, 10, and 48 weeks after infection in a total of 4 independent infections.
Because the CD8+CD44hi population is composed of cells generated through multiple processes (ie, infection, lymphopenia-induced proliferation, recent activation), using this measurement of cell numbers could be misleading.18,34,35 To specifically evaluate the persistence of AgSp memory CD8+ T cells, we used MHC:peptide tetramer staining to identify LCMV-specific cells within the CD44hi pool. Total CD8+H-2Db:GP33+ cell numbers in the peripheral blood and spleen were equivalent in the cHET and cKO mice for up to 48 weeks after deletion (Figure 3B), suggesting that the persistence of AgSp memory CD8+ T cells does not require SLP-76–mediated TCR signals.
Because CreT2-mediated deletion is less than 100% efficient, it is possible that a small proportion of AgSp memory CD8+ T cells retaining SLP-76 expression were expanding in response to homeostatic signals to maintain the H-2Db:GP33 compartment in the cKO mice. To address this possibility, we took advantage of the correlation between SLP-76 deletion and YFP expression. After tamoxifen treatment, AgSp YFP+ cells were clearly identifiable in the peripheral blood and were quantitated longitudinally (Figure 3C). All H-2Db:GP33+YFP+ tetramer-positive cells were CD44hi. Consistent with the total CD8+GP33+ cell numbers, YFP+ AgSp CD8+ memory T-cell numbers were similar between the cKO and cHET mice in both the peripheral blood and spleen. Thus, SLP-76 expression is not required for the maintenance of AgSp memory CD8+ T cells.
Memory T-cell populations persist through slow turnover, long-term survival, and resistance to apoptosis. Cell-intrinsic survival factors, such as the increased expression of the anti-apoptotic protein Bcl-2, support memory T-cell persistence.36,37 To further investigate the potential role of SLP-76 in mediating memory CD8+ T-cell homeostasis, we also examined whether SLP-76 deletion affected Bcl-2 expression in AgSp CD8+ memory T cells. We assayed for Bcl-2 expression by intracellular staining of peripheral blood lymphocytes. As expected based on their long-term persistence, equivalent levels of Bcl-2 protein were found comparing CD8+, CD8+H-2Db:GP33+, and CD8+ H-2Db:GP33+YFP+ populations from cKO and cHET mice (supplemental Figure 2).
TCR signals are not required to form a central memory population
We next sought to determine whether the loss of TCR signaling has a long-term impact on continuing differentiation of the AgSp CD8+ memory T-cell population. First, we assessed whether memory CD8+ T cells lacking SLP-76 maintained cell-surface and phenotypic characteristics of memory T cells 48 weeks after the deletion of SLP-76. The long-term absence of SLP-76–dependent signals did not alter the level of cell-surface TCR on AgSp persisting memory CD8+ T cells, as assessed by the relative intensity of tetramer staining (Figure 4A). YFP+ LCMV-specific cells from blood and secondary lymphoid organs were also assessed for expression of markers of T-cell memory. Expression of CD44, CXCR3, CD127, CD122, and CD25 were equivalent regardless of SLP-76 expression (Figure 4A bottom panels and data not shown).
Lack of persistent SLP-76–dependent TCR signals alters CD8+ Tcm differentiation. (A) Immunophenotyping of splenocytes from cHET and cKO mice 48 weeks after SLP-76 deletion. (Top) Contour plots gated on CD8+ T cells with a gate drawn around the CD44hiH-2Db:GP33+ population. (Bottom) Histograms of CD44hiH-2Db:GP33+-gated populations comparing cell-surface expression of CXCR3, CD122, CD127, and CD62L in cHET and cKO mice splenocytes. cHET cells are in solid gray; the black line represents cKO. (B) Representative histograms of CD62L expression on CD44hiH-2Db:GP33+–gated cells from cHET (top) and cKO (bottom) peripheral blood. The time after deletion is indicated in weeks. Relative percentages of cells within the indicated gated regions are shown. (C) Frequency of CD62LhiYFP+CD44hiH-2Db:GP33+ T cells in peripheral blood. Averages and standard deviations from of cHET (black) and cKO (gray) cohorts are shown from infection with LCMV-Armstrong up to 48 weeks after deletion of SLP-76. Timing of LCMV and tamoxifen are indicated below the x-axis. Dotted vertical lines indicate the point at which 50% of each population was CD62Lhi. Asterisks show time points with statistical differences (P < .05).
Lack of persistent SLP-76–dependent TCR signals alters CD8+ Tcm differentiation. (A) Immunophenotyping of splenocytes from cHET and cKO mice 48 weeks after SLP-76 deletion. (Top) Contour plots gated on CD8+ T cells with a gate drawn around the CD44hiH-2Db:GP33+ population. (Bottom) Histograms of CD44hiH-2Db:GP33+-gated populations comparing cell-surface expression of CXCR3, CD122, CD127, and CD62L in cHET and cKO mice splenocytes. cHET cells are in solid gray; the black line represents cKO. (B) Representative histograms of CD62L expression on CD44hiH-2Db:GP33+–gated cells from cHET (top) and cKO (bottom) peripheral blood. The time after deletion is indicated in weeks. Relative percentages of cells within the indicated gated regions are shown. (C) Frequency of CD62LhiYFP+CD44hiH-2Db:GP33+ T cells in peripheral blood. Averages and standard deviations from of cHET (black) and cKO (gray) cohorts are shown from infection with LCMV-Armstrong up to 48 weeks after deletion of SLP-76. Timing of LCMV and tamoxifen are indicated below the x-axis. Dotted vertical lines indicate the point at which 50% of each population was CD62Lhi. Asterisks show time points with statistical differences (P < .05).
All of the H-2Db:GP33+ cells at 48 weeks after deletion expressed high levels of CD62L (Figure 4A bottom panel), suggesting conversion to a central memory phenotype regardless of continued expression of SLP-76. To further investigate TCR-signaling dependence on the conversion from effector memory (Tem) to central memory (Tcm), we used CD62L expression to evaluate the kinetics of this shift in the peripheral blood. However, we found that the kinetics of change in the population from CD62Llo to CD62Lhi differed between AgSp memory CD8+ T cells that were SLP-76 sufficient or deficient. Loss of SLP-76 was correlated with a more rapid conversion from CD62Llo to CD62Lhi (Figure 4B). To quantitate the difference in kinetics more precisely, we determined the number of days needed for 50% of the cells to express high levels of CD62L (Figure 4C). Fifty percent of cHET memory CD8+ T cells converted by day 109 after infection, which was comparable with wild-type C57BL/6 mice.38 In contrast, deletion of SLP-76 30 days after infection resulted in a more rapid conversion (89 days after infection). This pattern of accelerated conversion to Tcm was also observed in the spleens of cHET and cKO mice at 10 weeks after deletion (98 days after infection). A higher frequency of splenic YFP+ H-2Db:GP33 cells was found to be CD62Lhi in cKO mice compared with cHET mice (supplemental Figure 4). These data suggest that continuous TCR-generated signals are important for regulating the kinetics of CD62L up-regulation as memory CD8+ T cells convert from Tem to Tcm.
SLP-76–deficient AgSp memory cells fail to transduce TCR signals but maintain memory potential long-term
To determine whether TCR signaling required SLP-76 in the persisting CD8+ LCMV-specific T cells, we assessed the ability of memory CD8+ T cells in cKO and cHET mice to proliferate in vivo in response to reinfection. Previously infected cHET and cKO mice that had undergone deletion of SLP-76 48 weeks earlier were challenged with LCMV clone 13, which presents the same CD8+ T-cell epitopes as LCMV-Armstrong but induces a chronic infection. T-cell expansion was assessed by serial bleeds at 3 and 5 days after infection, and splenocyte numbers were determined at day 5 after infection. CD8+ T cells had an equivalent increase in numbers after reinfection in the cKO and cHET mice, indicating either that CD8+ memory T cells do not require SLP-76 for a secondary response or that a sufficient number of nondeleted CD8+ memory T cells were still present in the cKO mice (supplemental Figure 5A). To differentiate these possibilities, the response of YFP+-gated CD8+ T cells was determined. No expansion in the CD8+YFP+, CD8+CD44hiYFP+, or CD8+H-2Db:GP33+YFP+ populations was seen at day 3 or 5 after reinfection of the cKO mice (Figure 5A-C left column). However, rechallenge with clone 13 elicited expansion of CD8+CD44hiYFP−- and CD8+H-2Db:GP33+YFP−-specific populations in both cHET and cKO mice, which accounted for the overall expansion of CD8 T cells observed (Figure 5A-C right column and supplemental Figure 5A). To assess the overall H-2Db:GP33-specific T-cell response to reinfection, tetramer-positive cell numbers were quantitated in the spleen and compared with preinfection controls generated from earlier time points (supplemental Figure 5B). GP33-specific T-cell numbers increased in both cHET and cKO mice; however, the increase was confined to the YFP-negative population in the cKO mice, while both YFP+ and YFP− expanded in the cHET mice. These results show that SLP-76 is required for the proliferative response to reinfection, and that antigen-specific T cells in cKO mice that maintain SLP-76 expression are still capable of responding.
Persisting SLP-76–deficient H-2Db:GP33+ CD8+ T cells from cKO mice are unable to respond to TCR signals. Forty-eight weeks after SLP-76 deletion, mice were rechallenged with LCMV clone 13 infection, and expansion was assessed quantitatively in peripheral blood at days 3 and 5 after infection. Data are gated on: (A) total CD8+, (B) CD8+CD44hi, and (C) CD8+H-2Db:GP33+ cells. Cells are further gated for YFP expression, with the YFP+ populations shown in the left column and YFP− populations shown in the right column. Each data point shows mean and standard deviation from 2 mice in each cohort. (D) Splenocytes from cHET and cKO mice were isolated at day 5 after rechallenge and stimulated in vitro for 4 hours. Samples were stained for immunophenotyping and intracellular cytokine expression. Histograms are gated on YFP+CD8+CD44hi cells. Numbers in the top right quadrant show the relative percentage of cells in each gate. Shaded histograms represent cHET mice; black line histograms represent cKO mice. Data are representative of stimulations from 2 cHET and 2 cKO mice.
Persisting SLP-76–deficient H-2Db:GP33+ CD8+ T cells from cKO mice are unable to respond to TCR signals. Forty-eight weeks after SLP-76 deletion, mice were rechallenged with LCMV clone 13 infection, and expansion was assessed quantitatively in peripheral blood at days 3 and 5 after infection. Data are gated on: (A) total CD8+, (B) CD8+CD44hi, and (C) CD8+H-2Db:GP33+ cells. Cells are further gated for YFP expression, with the YFP+ populations shown in the left column and YFP− populations shown in the right column. Each data point shows mean and standard deviation from 2 mice in each cohort. (D) Splenocytes from cHET and cKO mice were isolated at day 5 after rechallenge and stimulated in vitro for 4 hours. Samples were stained for immunophenotyping and intracellular cytokine expression. Histograms are gated on YFP+CD8+CD44hi cells. Numbers in the top right quadrant show the relative percentage of cells in each gate. Shaded histograms represent cHET mice; black line histograms represent cKO mice. Data are representative of stimulations from 2 cHET and 2 cKO mice.
To further assess the functional capacity of the persisting CD8+ memory T cells, we isolated splenocytes after 48 weeks of SLP-76 absence, followed by in vivo reinfection, and used them in single-cell assays of cytokine expression. Cells were either left unstimulated or were stimulated with PMA and ionomycin to bypass proximal TCR signaling, and then IFNγ production was assessed. In contrast to the inability to proliferate in vivo or to produce cytokine in response to peptide stimulation, YFP+CD44hiCD8+ T cells from both cHET and cKO produced IFNγ in response to PMA and ionomycin treatment (Figure 5D). Thus, persisting SLP-76–deficient CD8+ memory T cells have the capacity to produce effector cytokines rapidly, but the block in proximal TCR signals resulted in an inability to respond functionally to repeated viral infection.
Continuous SLP-76 expression is required for normal memory cell differentiation
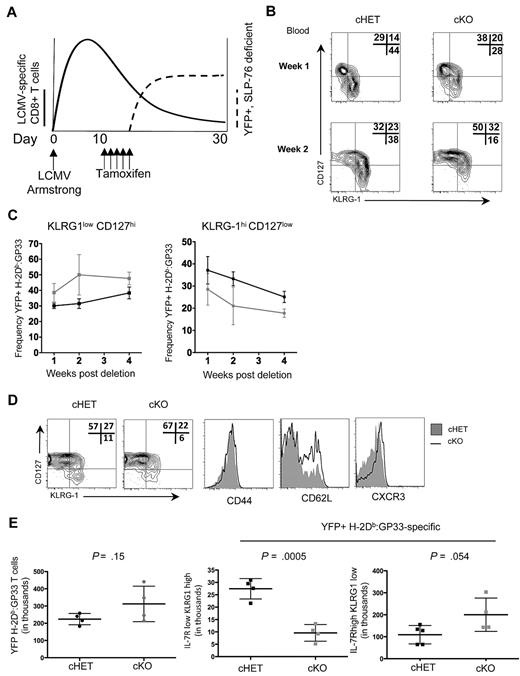
The accelerated kinetics of Tem to Tcm differentiation in persisting cKO H-2Db:GP33-specific T cells when SLP-76 was deleted in established memory T-cell populations led us to question whether differentiation earlier in memory generation was also dependent on SLP-76–mediated signals. The peak of CD8+ T-cell expansion in response to LCMV-Armstrong was approximately 8 to 10 days after exposure, at which point viral replication was undetectable in immunocompetent mice,30 but distinct populations of LCMV-specific KLRG1lowCD127hi MPs and KLRG1hiCD127low SLECs were detectable. To investigate whether alterations in TCR signaling after the peak of CD8+ T-cell expansion influences the kinetics and dynamics of memory T-cell generation, we modified the timing of deletion and administered tamoxifen beginning at day 10 after infection (Figure 6A).
SLP-76 is required for normal memory generation after acute LCMV infection. (A) Schematic of the experimental design. cHET and cKO mice were infected with LCMV-Armstrong on day 0, with subsequent expansion of CD8+ T cells (bold line). On days 11 to 15, mice were administered tamoxifen with subsequent deletion of SLP-76 and expression of YFP (dashed line). (B) Contour plots of CD8+H-2Db:GP33+YFP+ gated peripheral blood lymphocytes isolated at 1 and 2 weeks after deletion from cHET and cKO mice. Frequencies in each gate are indicated in the top right corner of each plot. Data are representative of 5 cHET and 5 cKO mice. (C) Longitudinal quantitation of CD8+H-2Db:GP33+YFP+ KLRG1lowCD127high (left) and KLRG1highCD127low (right) frequencies from cHET versus cKO peripheral blood lymphocytes. Mean and standard deviations are a composite from 2 independent experiments using a total of 5 cHET and 5 cKO mice. (D) Contour plots from splenocytes isolated from cHET and cKO mice 4 weeks after deletion. All plots are gated on CD8+H-2Db:GP33+YFP+. Shaded histograms are from cHET mice; solid black histograms are from cKO mice. Data are representative of 2 independent experiments. (E) Quantitation of CD8+H-2Db:GP33+YFP+ MP and SLEC populations in cHET and cKO mice spleens 4 weeks after deletion. Each point represents an individual mouse.
SLP-76 is required for normal memory generation after acute LCMV infection. (A) Schematic of the experimental design. cHET and cKO mice were infected with LCMV-Armstrong on day 0, with subsequent expansion of CD8+ T cells (bold line). On days 11 to 15, mice were administered tamoxifen with subsequent deletion of SLP-76 and expression of YFP (dashed line). (B) Contour plots of CD8+H-2Db:GP33+YFP+ gated peripheral blood lymphocytes isolated at 1 and 2 weeks after deletion from cHET and cKO mice. Frequencies in each gate are indicated in the top right corner of each plot. Data are representative of 5 cHET and 5 cKO mice. (C) Longitudinal quantitation of CD8+H-2Db:GP33+YFP+ KLRG1lowCD127high (left) and KLRG1highCD127low (right) frequencies from cHET versus cKO peripheral blood lymphocytes. Mean and standard deviations are a composite from 2 independent experiments using a total of 5 cHET and 5 cKO mice. (D) Contour plots from splenocytes isolated from cHET and cKO mice 4 weeks after deletion. All plots are gated on CD8+H-2Db:GP33+YFP+. Shaded histograms are from cHET mice; solid black histograms are from cKO mice. Data are representative of 2 independent experiments. (E) Quantitation of CD8+H-2Db:GP33+YFP+ MP and SLEC populations in cHET and cKO mice spleens 4 weeks after deletion. Each point represents an individual mouse.
To assess the differentiation kinetics of H-2Db:GP33+ CD8+ T cells into MP and SLEC populations, peripheral blood lymphocytes were isolated longitudinally and stained for effector and memory cell surface markers before and after tamoxifen-induced deletion. To ensure that cells lacking SLP-76 were evaluated, all data were gated on YFP+ cells. Before deletion of SLP-76 (ie, day 8 after infection), similar proportions of MP and SLEC populations were identified within the CD44hi and CD44hi H-2Db:GP33+ CD8+ T cells in both cHET and cKO mice (Figure 1D and data not shown). However, within 1 week after SLP-76 deletion, the relative ratio of MP to SLEC cells had shifted to favor memory precursor cells in cKO animals (Figure 6B top panels). By 2 weeks after deletion, the shift was more pronounced (Figure 6B bottom panels). Quantitation of H-2Db:GP33+ MP and SLEC CD8+ T-cell populations was assessed longitudinally up to 4 weeks after deletion in the peripheral blood (Figure 6C). At each time point evaluated, fewer peripheral blood SLEC CD8+ T cells were detectable in cKO relative to cHET mice, while cKO mice possessed a higher frequency of MP CD8+ T cells.
IL-7R expression is inversely correlated with ligand-induced TCR signals,39 making it possible that decreased levels of CD127 in cells from the cKO did not represent a true conversion to memory precursor cells. To verify the memory phenotype of these CD127hi T cells, we assessed the expression of 2 additional cell-surface markers, CXCR3 and CD62L. Within the H-2Db:GP33 YFP+ population, there were greater frequencies of CXCR3hi and CD127hiCD62Lhi CD8+ T cells in cKO mice compared with cHET mice (supplemental Figure 5A-B). Four weeks after deletion (6 weeks after infection), the phenotype and cellularity of splenic CD8+ T-cell populations were assessed (Figure 6D-E). All tetramer-specific T cells were CD44hi and, consistent with findings from the peripheral blood, YFP+ LCMV-specific CD8+ T cells within the spleen exhibited a shift toward MPs and away from SLECs, as evidenced by increased frequencies of CD127hi, CXCR3hi, and CD62Lhi cells. Quantitation of populations in the spleen demonstrated no difference in overall CD8+ YFP+ H-2Db:GP33+ cellularity, but there was a statistically significant decrease in the CD127loKLRG1hi population in the cKO compared with the cHET mice. A trend toward higher numbers of splenic MP cells in the cKO animals was not statistically significant 4 weeks after deletion. Thus, disruption of TCR signaling affects both early memory T-cell generation and the conversion of Tem to Tcm in normally generated CD8+ memory T cells.
Discussion
In this study, we combined temporally controlled genetic deletion of a key signaling molecule, a model of in vivo infection, and memory T-cell identification using tetramer-based reagents to address the requirement for tonic TCR signaling in AgSp memory T-cell generation and persistence. We showed that TCR signaling in memory T cells requires SLP-76, as evidenced by a striking lack of IFNγ production in vitro and a lack of proliferation in response to in vivo rechallenge. Both the generation of CD8+ MPs and the kinetics of Tem to Tcm conversion in existing CD8+ memory T-cell populations were altered after the deletion of SLP-76. Despite the requirement for SLP-76 in antigen-driven signals and CD8+ memory differentiation, its continued expression was not required for AgSp CD8+ memory T-cell persistence.
This study expands our understanding of the SLP-76 adaptor protein to include a role in memory CD8+ T-cell activation. In vivo infection followed by in vitro stimulation with LCMV-peptide allowed us to clearly define the memory cells used for this study. While SLP-76 has been shown previously to be critical for ligand-independent24,25 and ligand-dependent26 TCR signals in thymocytes, it is possible that it was not required for signaling in previously activated T cells. The expression of the related adaptor cytokine-dependent hemopoietic cell linker (CLNK) in previously activated but not naive T cells,40 and the decreased levels of SLP-76 in memory CD4+ T cells, would have offered a potential mechanism by which SLP-76 was not required to mediate TCR signals. While there may be additional pathways that rely on CLNK, TCR-mediated IFNγ production and proliferation are clearly dependent on SLP-76.
Our system evaluates the effect of genetic ablation of a key signaling molecule in a defined polyclonal memory T-cell population in the absence of lymphopenia. Previous studies using conditional gene expression and adoptive transfer techniques have demonstrated that the persistence of memory CD8+ T cells requires16,17 or does not require TCR-generated signals.18-21 Studies supporting TCR signal independence of CD8+ memory T cells have been criticized for their lack of complete signal abrogation and recipient host lymphopenia. Our results using a system in which deletion effectively eliminates both tonic and antigen-induced TCR signals, and in which the competitive environment between SLP-76–sufficient and SLP-76–deficient cells prevents significant lymphopenia (supplemental Figure 1), further supports a model in which TCR signals are not required for memory cell persistence. Because the compartment is full throughout the life of the animal, this model also allowed us to examine the effect of losing TCR signaling, not only on memory CD8+ T cell persistence, but also on Tem to Tcm differentiation.
We found that SLP-76 signaling plays a role in the normal generation and differentiation of CD8+ memory T cells. The investigation of memory T-cell lineage differentiation into Tcm and Tem populations has been addressed in multiple studies.5,41-43 T-cell competition, duration, and strength of initial stimulation and persistent TCR:MHC interaction have both been shown to affect the kinetics of the shift from Tem to Tcm.38,44,45 Similar to our findings, the shift from Tem to Tcm was previously shown to be accelerated after LCMV infection of mice harboring mutations at critical tyrosine residues of SLP-76,46 which may be explained either by alterations in the initial priming or alterations in signal strength continuing well after the formation of the Tem compartment. Our results, however, clearly show that the early shift seen with the complete deletion of SLP-76 during the memory phase cannot be explained by differences in precursor frequency or signal strength of initial activation, because these events occur before deletion. One potential mechanism to explain our findings is that, similar to CD8+ memory T cells generated in response to chronic infection,47 terminally differentiated Tem cells require continuous TCR signaling for survival. Loss of SLP-76 leads to an early loss of the Tem population, with Tcm cells expanding to maintain total numbers of AgSp CD8+ memory T cells.
Signals resulting from inflammatory cytokines affect the generation and maintenance of CD8 memory T cells.48 In addition to TCR signal disruption, we have shown that the deletion of SLP-76 also results in the inability of CD8+ cells to produce cytokines. It is possible that conditional deletion of SLP-76 after LCMV infection results in an altered inflammatory environment in vivo, leading to altered memory differentiation. However, because the cells in the YFP− fraction are still capable of producing cytokines, we believe that the inflammatory environment is not profoundly altered in a way that would significantly affect Tem survival. An alternative potential mechanism is that Tem populations may convert to Tcm in a linear manner, with the loss of tonic signaling increasing the rate of conversion. Thus, while we cannot rule out the possibility that precursor frequency and signal strength at activation can play a role, we favor a model in which cell-intrinsic, continuous TCR signals have an independent role after the memory compartment is formed.
In addition to the differentiation of CD8+ Tcm populations, we used timed deletion during the contraction phase of the immune response to assess the requirement of SLP-76 signaling in memory cell development. We observed a significant increase in both the relative percentage and the absolute numbers of MP cells. This increase was accompanied by a concomitant decrease of SLEC cells in both the peripheral blood and spleen. The removal of SLP-76–dependent TCR signaling accelerated the disappearance of SLEC cells, suggesting either that tonic TCR signaling is required or that another SLP-76–dependent signaling pathway contributes to the homeostasis of this effector population. Conversely, SLP-76 ablation during the contraction phase results in a greater number of MP cells, and consequently does not affect the overall number of AgSp CD8+ T cells during contraction. Our experiments cannot differentiate between a model in which, in the absence of TCR signals, SLEC convert to a memory precursor phenotype versus one in which SLEC and MP populations may share a requirement for a SLP-76–independent factor.
The terminal differentiation of KLRG1hi cells is associated with elevated levels of the T-box transcription factor T-bet,4,49,50 while memory precursor cells express lower levels. Conversely, eomesodermin (Eomes) has been shown to execute memory differentiation programs.6 Interestingly, modulation of signals through the mammalian target of rapamycin (mTOR) pathway alters the balance of T-bet to Eomes; inhibition of mTOR signaling favors Eomes expression over T-bet.49 Decreased mTOR activity during the first 30 days of LCMV infection also results in a relative skewing toward memory precursor cells,51 similar to our findings when SLP-76 was deleted immediately after viral clearance. Thus, a possible mechanism to explain our findings is an alteration of a SLP-76→mTOR→Tbet signaling pathway. In addition, Tbet expression levels may be decreased through a phosphorylation event involving the ITK kinase52 (also John T. Chang and Steven L. Reiner, unpublished data), implicating an SLP-76→ITK→Tbet pathway. It remains to be determined whether the primary defect of SLP-76 deletion can be overridden by rescuing Akt or ITK activity, and whether this would result in normal differentiation from effector cells to memory cells.
We used a novel system combining temporally mediated ablation of SLP-76 and in vivo infection to address the requirements for SLP-76 in memory CD8+ T-cell TCR signaling and its role in homeostasis. SLP-76 is required for transducing both tonic and antigenic signals generated by the TCR in CD8+ cells. Lack of SLP-76 leads to an increase in memory precursor cells and a more rapid shift to central memory, but does not affect the homeostasis of the AgSp memory compartment. This suggests that appropriately timed, transient targeting of SLP-76 may improve immune memory generation in response to new pathogens and vaccines while not affecting preexisting memory populations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors extend their gratitude to Jiyeon Kim and Martha Jordan for critical review of this manuscript, Eleni Argyropoulou for mouse husbandry, and Drs Frank Costantini and Eric Brown for mouse strains.
This work was partially funded by the National Institutes of Health (R01 AI085160). J.S.M. received additional support from a John Merrill Grant of the American Society of Nephrology and American Society of Transplantation. E.J.W. was funded in part by the National Institutes of Health (R01 AI71309).
National Institutes of Health
Authorship
Contribution: K.R.W. designed and performed experiments, analyzed and interpreted the data, and wrote the manuscript; E.C. performed experiments and analyzed data; M.S. performed experiments; H.S. offered technical assistance and discussion; E.J.W. designed experiments, interpreted the data, and generated critical reagents; and J.S.M. designed the study, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan S. Maltzman, University of Pennsylvania School of Medicine, Department of Medicine, 754 Biomedical Research Bldg II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: maltz@mail.med.upenn.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal