Abstract
Resistance to currently available therapies is a major impediment to the successful treatment of hematological malignancies. Here, we used a model of therapy-resistant B-cell nonHodgkin lymphoma (B-NHL) developed in our laboratory along with primary B-NHL cells to study basic mechanisms of bortezomib activity. In resistant cells and a subset of primary B-NHLs, bortezomib treatment led to stabilization of Bak and subsequent Bak-dependent activation of apoptosis. In contrast to sensitive cells that die strictly by apoptosis, bortezomib was capable of killing resistant cells through activation of apoptosis or caspase-independent mechanism(s) when caspases were pharmacologically inhibited. Our data demonstrate that bortezomib is capable of killing B-NHL cells via multiple mechanisms, regardless of their basal apoptotic potential, and contributes to growing evidence that proteasome inhibitors can act via modulation of B-cell lymphoma 2 (Bcl-2) family proteins. The capacity of bortezomib to act independently of the intrinsic apoptotic threshold of a given B-NHL cell suggests that bortezomib-based therapies could potentially overcome resistance and result in relevant clinical activity in a relapsed/refractory setting.
Introduction
NonHodgkin lymphoma (NHL) is a heterogeneous group of neoplasms with distinct natural histories, clinical features, responsiveness to therapy, and prognosis. Rituximab, a chimeric anti-CD20 monoclonal antibody, has changed the treatment paradigm for patients with B-cell nonHodgkin lymphoma (B-NHL). The integration of rituximab into several standard chemotherapy regimens has been shown to be superior to systemic chemotherapy alone in several randomized phase III clinical trials in various subtypes of lymphoma.1 The addition of rituximab (R) to standard doses of cyclophosphamide, doxorubicin, vincristine, and prednisone or fludarabine based-regimens has resulted in improved treatment outcomes in diffuse large B-cell and indolent B-cell lymphomas2-5 in recent years.
Despite the improvement in the outcome of NHL patients treated with R-chemotherapy, a significant number of patients with diffuse large B-cell lymphoma and the majority of patients with indolent B-cell lymphomas relapse after treatment. The mechanisms by which lymphoma cells acquire resistance to rituximab and/or chemotherapy agents are multifactorial and can be intrinsic to the cancer cell or host.6 Using a rituximab-resistance preclinical model characterized by our group, we previously demonstrated the existence of shared, cancer cell–intrinsic pathways of resistance to rituximab and conventional chemotherapy.7,8 Recent data in patients with diffuse large B-cell lymphoma undergoing salvage chemotherapy suggest that lymphomas that are resistant or relapse after upfront R-cyclophosphamide, doxorubicin, vincristine, and prednisone are indeed more resistant to subsequent treatment and further support the findings of our preclinical model.9,10
In 2 separate analyses, the level of expression of pro- versus antiapoptotic members of the B-cell lymphoma 2 (Bcl-2) family proteins proved prognostic for B-NHL patients treated with rituximab or chemotherapy.11,12 In our preclinical model of rituximab/chemotherapy resistance. we demonstrated that deregulation of the expression of pro- and antiapoptotic proteins is associated with acquired resistance to rituximab.8 Similar deregulation of Bcl-2 family proteins was reported in independently derived rituximab/chemotherapy-resistant cells,13 further validating the importance of the Bcl-2 family of proteins in therapy-resistant B-NHL.
Mounting evidence suggests that many Bcl-2 family proteins are targeted for proteasomal degradation in malignant cells.14 Inhibition of proteasomes may, therefore, lead to the selective induction and/or activation of Bcl-2 family proteins resulting in modulation of the apoptotic potential of malignant cells. Bortezomib (PS-341, Velcade) is a peptide boronic acid inhibitor of the 26S proteasome that binds to and inhibits the chymotrypsin-like catalytic domain of the 20S proteasome core.15 Hematological malignancies of the B-lineage appear especially sensitive to the antitumor activity of bortezomib, potentially due to their constitutive production of large amounts of immunoglobulin and enhanced sensitivity to a terminal unfolded protein response.16,17 In accord with this observation, bortezomib demonstrated clinical activity against and was approved by the United States Food and Drug Administration to treat relapsed or refractory multiple myeloma.18 Subsequently, bortezomib was Food and Drug Administration–approved for the treatment of relapsed/refractory mantle cell lymphoma19 and has shown activity against several other types of B-NHL in phase II trials.20-22
The mechanisms by which bortezomib induces cell death has yet to be fully elucidated. Bortezomib was thought to work by inhibiting nuclear factor-κB (NF-κB) activity via stabilization of IκB.23 In a B-NHL model system similar to the one used here, Jazirehi et al13 demonstrated that bortezomib could sensitize resistant B-NHL cells to chemotherapy by inhibiting NF-κB activity. In multiple cell types, including B-NHL, multiple myeloma, and solid tumor cells, proteasome inhibitors are capable of killing malignant cells via induction of the proapoptotic Bcl-2 family proteins Noxa or Bik.24-27 While each proposed mechanism of bortezomib action is likely valid, the only global inference that can be taken from the study of bortezomib's mechanism-of-action is that it can work via several distinct pathways that are dictated by the intrinsic nature of the tumor cells exposed to this agent.
In the current study, we used a previously described cell line model of rituximab/chemotherapy resistance7 to explore the therapeutic potential of bortezomib in therapy-resistant B-NHL. Data indicate that in our cell line model, and in a subset of primary B-NHL patient-derived tumor cell samples, bortezomib can induce apoptosis via stabilization of Bak. Surprisingly, death of therapy-resistant cells did not depend on Bak or caspase-dependent apoptosis. Knock-down of Bak or pharmacological inhibition of caspases was unable to inhibit their bortezomib-induced death, suggesting the activation of alternative cell death pathways (ie, necrosis or cell cycle arrest). The data presented here demonstrate the complexity of response to bortezomib treatment by demonstrating that within a cell population (ie, our resistant cells), bortezomib is capable of inducing apoptosis via Bak stabilization and/or caspase-independent cell death. Several caspase-independent mechanisms of cell death have been described and are currently being investigated as potential mediators of antitumor effects observed after exposure of B-cell lymphomas to bortezomib. Our data suggest that bortezomib may be useful in treating B-NHL patients who have failed previous therapy regimens because it can promote death through a variety of mechanisms distinct from other antineoplastic agents.
Methods
Cell Lines
Therapy-sensitive and -resistant cell lines were described previously.7 SUDHL-6 cells were obtained from Dr Shawn Murphy (University of Rochester, Rochester, NY) and multiple myeloma cells were obtained from Dr Kelvin Lee (Roswell Park Cancer Institute, Buffalo, NY).
Antibodies and reagents
Antibodies raised against Bak and Actin were obtained from Sigma Chemicals, Bik from Santa Cruz Biotechnology, Moesin (antibody-1) from Lab Vision, poly(adenosine diphosphate-ribose) polymerase (PARP)–1 from BD Pharmigen, and Noxa from Calbiochem. Propidium iodide and 3-methyladenine were obtained from Sigma Chemicals, zVAD-fmk and Q-VD-OPh from MBL International, and DiIC1 (1,1′-dimethyl-3,3,3′,3′-tetramethylindodicarbocyanine)5 from Invitrogen.
Patient samples
Primary malignant B-cells were isolated by magnetic-activated cell sorting (negative selection; Miltenyi Biotec) from pretreatment biopsy tissue obtained from B-NHL patients treated at Roswell Park Cancer Institute under an institutional review board–approved protocol (N = 18). Cells were seeded into 384-well plates at a density of 0.5 × 106/mL and treated in triplicate with a several doses of a panel of therapeutic agents under investigation in our laboratory including rituximab (10 μg/mL), cisplatinum (CDDP; 0.1μM, 1μM, and 10μM), adriamycin (0.1μM, 1μM, and 10μM) and bortezomib (1nM, 5nM, and 10nM) with or without the pan-caspase inhibitors Q-VD-OPh (2.5μM; MBL International). Twenty-four and 48 hours after treatment, viability was determined using CellTiter-Glo (Promega). Viability was determined using the following formula: viability = relative light unit from treated cells/relative light unit from control cell × 100%.
Western blotting
Western blots were performed as previously described.8
Proteasome activity
After overnight exposure to bortezomib, proteasome activity was determined as previously described.28
Cell death and apoptosis
Cells were incubated at a concentration of 0.5 × 106 cells/mL in complete media containing bortezomib or CDDP with or without chemical inhibitors (see figure legends for specific doses). At 24-, 48-, and 72-hour time points, aliquots were removed and stained with Alexa Fluor488-conjugated annexin V (Invitrogen), 50nM DiIC1,5 and 5 μg/mL propidium iodide in annexin-binding buffer (10mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]), 150mM potassium chloride, 1mM magnesium chloride, 1.3mM calcium chloride, 1 mg/mL glucose, 0.5% bovine serum albumin, pH 7.4). In addition, caspase-3/7 activity in equal numbers of treated versus untreated cells was detected in triplicate or quadruplicate wells using the luminometric Caspse-Glo 3/7 assay (Promega) following the manufacturer's protocols. Statistical differences were compared using unpaired Student t tests.
Transient expression of BAK1, BIK, or PMAIP1 (Noxa) transgenes
Expression constructs for Bak, Bik, and Noxa were created on the pIRES2-EGFP (Invitrogen) backbone using a previously described strategy.8 The coding regions of BAK1 or BIK were polymerase chain reaction (PCR) amplified from Raji cell cDNA, while PMAIP1 was PCR amplified from a Noxa expression construct kindly provided by Dr David Huang (Walter and Eliza Hall Institute, Melbourne, Australia). FLAG-tagged Bak constructs were generated by overlap-extension PCR. Resulting plasmids (2 μg) were transfected into resistant cells using an Amaxa Nucleofector following the manufacturer's protocol for Raji cells (Amaxa). Both mock transfected and empty pIRES2-EGFP vector transfected cells were used as negative controls.
Immunoprecipitation
Lysates were prepared from 2 × 107 cells treated with 25nM bortezomib (PS-341) or vehicle control (dimethyl sulfoxide [DMSO]) for 24 hours. Protein concentration was determined by bicinchoninic acid and normalized between samples. Lysates were then precleared using protein G sepharose beads. Anti-FLAG M2 monoclonal or anti-Bak polyclonal antibody was added and allowed to incubate overnight at 4°C with gentle agitation. Protein G sepharose beads were used to remove antibody and bound protein from solution, washed 4 times, and boiled in sodium dodecyl sulfate loading buffer. Beads were then removed by centrifugation, and eluted protein was separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels followed by Western blot analysis.
siRNA-mediated knockdown of Bak, Bik or Noxa
Knock-down of Bak, Bik, and Noxa was achieved using ON-TARGETplus SMARTpool siRNA (Dharmacon) designed to specifically target BAK1, BIK, or PMAIP1, respectively. Nontargeting SMARTpool siRNA (Dharmacon) was used as a negative control. Two hours following transfection of 2 μg of siRNA using an Amaxa Nucleofector, cells were incubated with bortezomib (20nM) for an additional 72 hours.
Results
Bortezomib induces delayed apoptosis of B-NHL cell lines that are resistant to rituximab and standard chemotherapy
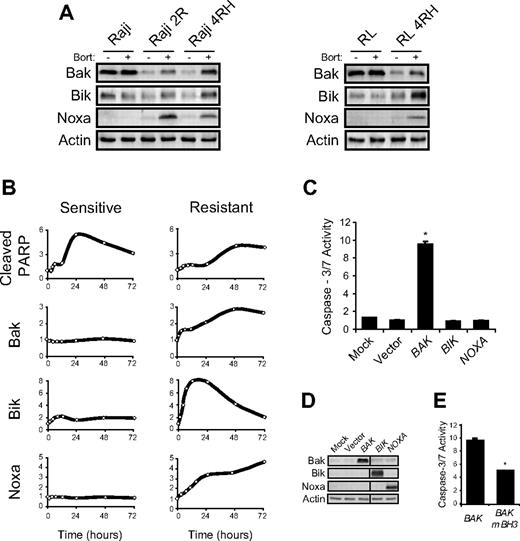
We previously described a cell line model of therapy-resistant B-NHL in which apoptosis was not induced by standard chemotherapeutic agents8 or rituximab.7 This model has become the basis for investigation of novel agents that may be used to treat therapy-resistant B-NHL. Bortezomib was found to induce the death of resistant cell lines Raji 2R, Raji 4RH, and RL 4RH, along with sensitive, parental cell lines Raji and RL (Figure 1A). compared with sensitive Raji or RL cells treated with the same dose (25nM) for the same duration (72 hours), bortezomib induced the death of a similar (P < .05) percentage of therapy-resistant cells. Parallel treatment of these cells with cisplatinum (100μM) was used to confirm the inability of standard chemotherapeutic agents to kill resistant cells (Figure 1A). To further understand the mechanism underlying bortezomib-induced cell death of sensitive and resistant cells, we examined the ability of either bortezomib or CDDP to induce the activation of caspase-3/7, a hallmark of apoptosis. We found that while CDDP was only capable of inducing caspase-3/7 activity in sensitive cells, bortezomib induced caspase-3/7 activity in sensitive and resistant cells, albeit to a much greater extent in sensitive cells (Figure 1B). These data suggested that bortezomib may induce apoptosis in cells that are resistant to apoptotic cell death. During the initial characterization of bortezomib-induced cell death in sensitive versus resistant cells, we found that sensitive cells died more rapidly over the first 48 hours of incubation (data not shown). This delay in cell death correlated with a delay in bortezomib-induced PARP cleavage (Figure 1C). The delay in apoptosis induction of resistant cells was further confirmed by flow cytometric analysis, which indicated that there was an approximately 24-hour delay in phosphatidylserine exposure, DNA fragmentation, and cell death of bortezomib-treated resistant cells compared with bortezomib-treated sensitive cells (Figure 1D).
Therapy-resistant B-NHL cell lines undergo delayed apoptosis when treated with bortezomib. (A) After a 72-hour incubation, bortezomib (25nM) induced the death of an approximately equal (P > .05) percentage of therapy-sensitive (Raji, RL) and therapy-resistant (Raji 2R, Raji 4RH, RL 4RH) cells. Data shown represent the average percent of propidium iodide–positive (PI+) cells ± SD from at least 5 independent experiments. Asterisks (*) indicate a significant increase compared with killing by cisplatinum (CDDP; 100μM). (B) Forty-eight hours following incubation with bortezomib (25nM), increased caspase-3/7 activity was detected in sensitive and resistant cells. Data shown are from 1 representative experiment repeated 3 times. Bars represent the average caspase-3/7 activity from quadruplicate wells ± SD. Asterisks (*) indicate a significant (P < .01) increase in caspase-3/7 activity compared with vehicle-treated cells. Double asterisks (**) indicate a significant increase in caspase-3/7 activity compared with vehicle-treated cells and compared with the same treatment in resistant cells. (C) A kinetic analysis of PARP cleavage indicated that sensitive cells undergo apoptosis more rapidly than resistant cells. PARP cleavage was observed following 24 hours of incubation of sensitive cells (Raji shown here) with 25nM bortezomib but not until 48 hours following incubation of resistant cells (Raji 4RH shown here) with 25nM bortezomib. Western blots shown are from a representative experiment that was repeated twice. (D) Analysis of apoptotic markers by flow cytometry confirmed that bortezomib (25nM) induced apoptosis with delayed kinetics in resistant cells (Raji 2R shown here) compared with sensitive cells (Raji shown here). Data shown represent the average ± SD of at least 3 independent experiments. Asterisks (*) indicate a significant (P < .05) difference between sensitive and resistant cells at a given time point. Other sensitive and resistant cell lines exhibited similar kinetic responses to bortezomib treatment (data not shown).
Therapy-resistant B-NHL cell lines undergo delayed apoptosis when treated with bortezomib. (A) After a 72-hour incubation, bortezomib (25nM) induced the death of an approximately equal (P > .05) percentage of therapy-sensitive (Raji, RL) and therapy-resistant (Raji 2R, Raji 4RH, RL 4RH) cells. Data shown represent the average percent of propidium iodide–positive (PI+) cells ± SD from at least 5 independent experiments. Asterisks (*) indicate a significant increase compared with killing by cisplatinum (CDDP; 100μM). (B) Forty-eight hours following incubation with bortezomib (25nM), increased caspase-3/7 activity was detected in sensitive and resistant cells. Data shown are from 1 representative experiment repeated 3 times. Bars represent the average caspase-3/7 activity from quadruplicate wells ± SD. Asterisks (*) indicate a significant (P < .01) increase in caspase-3/7 activity compared with vehicle-treated cells. Double asterisks (**) indicate a significant increase in caspase-3/7 activity compared with vehicle-treated cells and compared with the same treatment in resistant cells. (C) A kinetic analysis of PARP cleavage indicated that sensitive cells undergo apoptosis more rapidly than resistant cells. PARP cleavage was observed following 24 hours of incubation of sensitive cells (Raji shown here) with 25nM bortezomib but not until 48 hours following incubation of resistant cells (Raji 4RH shown here) with 25nM bortezomib. Western blots shown are from a representative experiment that was repeated twice. (D) Analysis of apoptotic markers by flow cytometry confirmed that bortezomib (25nM) induced apoptosis with delayed kinetics in resistant cells (Raji 2R shown here) compared with sensitive cells (Raji shown here). Data shown represent the average ± SD of at least 3 independent experiments. Asterisks (*) indicate a significant (P < .05) difference between sensitive and resistant cells at a given time point. Other sensitive and resistant cell lines exhibited similar kinetic responses to bortezomib treatment (data not shown).
Bortezomib treatment of resistant cells led to increased expression of proapoptotic Bak, Bik, and Noxa
Several members of the Bcl-2 family of apoptosis regulatory proteins are known to be altered by treatment with proteasome inhibitors.14 We previously showed that the lack of chemotherapy-induced apoptosis in our resistant cells was primarily due to mutation or posttranscriptional down-regulation of the proapoptotic Bcl-2 family proteins Bax and Bak, respectively.8 We therefore hypothesized that bortezomib may overcome the block in apoptosis in resistant cells by altering the ratio of Bcl-2 family proteins. In vitro exposure of sensitive and resistant B-NHL cells to bortezomib led to no visible change in several members of the Bcl-2 family of proteins, including Bcl-2, Bcl-xL, Bax, Bim, and Puma (data not shown). Induced myeloid leukemia cell differentiation protein (Mcl-1) in bortezomib-treated sensitive and resistant cells was found to be stabilized and cleaved in a caspase-dependent manner (data not shown) as was previously described.29,30 Most interestingly, however, 3 proapoptotic Bcl-2 family proteins (Bak, Bik, and Noxa) were induced in resistant cells following bortezomib exposure, while their expression remained constant in bortezomib-treated sensitive cells (Figure 2A). Moreover, quantitative analysis of the kinetics of Bak, Bik, and Noxa induction compared with PARP cleavage demonstrated clear induction of all 3 proapoptotic proteins before PARP cleavage in resistant cells (Figure 2B). These data suggest that increased expression of Bak and/or Bik and/or Noxa precede and may be necessary for induction of apoptosis in bortezomib-treated resistant cells.
Bak, Bik, and Noxa protein levels increase following bortezomib treatment of resistant cells. (A) Western blot analysis of whole cell lysates collected 24 hours following bortezomib (25nM) treatment of sensitive (Raji, RL) or resistant (Raji 2R, Raji 4RH, RL 4RH) cells demonstrated a striking increase in the expression of Bak, Bik, and Noxa in resistant but not sensitive, cells. Blots shown are from a representative experiment repeated more than 3 times. (B) Kinetic analysis of Bak, Bik, and Noxa expression following bortezomib treatment showed that their induction occurred before caspase activation (indicated by PARP cleavage) in resistant cells (Raji 2R shown here). Induction of Bak, Bik, and Noxa was not seen following bortezomib treatment of sensitive cells (Raji shown here), yet PARP cleavage occurred more rapidly than in resistant cells. Data shown are from a representative experiment repeated twice. Other bortezomib-treated sensitive and resistant cell lines displayed similar kinetics with respect to PARP cleavage along with Bak, Bik, and Noxa expression (data not shown). (C) Resistant cells (Raji 2R shown here) expressing a Bak transgene rapidly undergo apoptosis as indicated by caspase-3/7 activity. Eighteen hours following transfection of pIRES2-EGFP containing either a BAK1, BIK, or PMAIP1 (Noxa) transgene, or empty vector as a control, resistant cells were assayed for caspase-3/7 activity. Data shown are from a representative experiment repeated at least 3 times. Bars represent average caspase-3/7 activity from triplicate wells ± SD. Asterisks (*) denote a significant (P < .01) increase in caspase-3/7 activity compared with vector transfected cells. (D) Western blot confirmed expression of Bak, Bik, and Noxa transgenes. (E) Mutation of the BH3 domain of Bak abrogates its ability to induce apoptosis as indicated by caspase-3/7 activity. Data shown are from a representative experiment repeated twice. Bars represent average caspase-3/7 activity from triplicate wells ± SD. Asterisks (*) denote a significant (P < .01) decrease in caspase-3/7 activity compared with wild-type Bak transfected cells.
Bak, Bik, and Noxa protein levels increase following bortezomib treatment of resistant cells. (A) Western blot analysis of whole cell lysates collected 24 hours following bortezomib (25nM) treatment of sensitive (Raji, RL) or resistant (Raji 2R, Raji 4RH, RL 4RH) cells demonstrated a striking increase in the expression of Bak, Bik, and Noxa in resistant but not sensitive, cells. Blots shown are from a representative experiment repeated more than 3 times. (B) Kinetic analysis of Bak, Bik, and Noxa expression following bortezomib treatment showed that their induction occurred before caspase activation (indicated by PARP cleavage) in resistant cells (Raji 2R shown here). Induction of Bak, Bik, and Noxa was not seen following bortezomib treatment of sensitive cells (Raji shown here), yet PARP cleavage occurred more rapidly than in resistant cells. Data shown are from a representative experiment repeated twice. Other bortezomib-treated sensitive and resistant cell lines displayed similar kinetics with respect to PARP cleavage along with Bak, Bik, and Noxa expression (data not shown). (C) Resistant cells (Raji 2R shown here) expressing a Bak transgene rapidly undergo apoptosis as indicated by caspase-3/7 activity. Eighteen hours following transfection of pIRES2-EGFP containing either a BAK1, BIK, or PMAIP1 (Noxa) transgene, or empty vector as a control, resistant cells were assayed for caspase-3/7 activity. Data shown are from a representative experiment repeated at least 3 times. Bars represent average caspase-3/7 activity from triplicate wells ± SD. Asterisks (*) denote a significant (P < .01) increase in caspase-3/7 activity compared with vector transfected cells. (D) Western blot confirmed expression of Bak, Bik, and Noxa transgenes. (E) Mutation of the BH3 domain of Bak abrogates its ability to induce apoptosis as indicated by caspase-3/7 activity. Data shown are from a representative experiment repeated twice. Bars represent average caspase-3/7 activity from triplicate wells ± SD. Asterisks (*) denote a significant (P < .01) decrease in caspase-3/7 activity compared with wild-type Bak transfected cells.
To further study the role of Bak, Bik, and Noxa in the induction of apoptosis of resistant cells, we transfected resistant cells with pIRES2-EGFP containing either a BAK1, BIK, or PMAIP1 (Noxa) transgene. We found that expression of Bak, but not Bik or Noxa, in resistant cells led to a significant increase in caspase-3/7 activity and subsequent cell death (Figure 2C and data not shown). Western blots and flow cytometry were performed to verify the specific expression of enhanced green fluorescent protein and each transgene in transfected resistant cells (Figure 2D and data not shown). To illustrate the specificity of Bak induction of caspase-3/7 activity and cell death, an inactive, mutant form of Bak31 was transfected into resistant cells and was found to induce less caspase-3/7 activity than wild-type Bak (Figure 2E). These data demonstrate that induction of Bak is sufficient to induce apoptosis in resistant cells and support a role for increased Bak expression in bortezomib-induced death of resistant cells.
Bak induction occurred in a subset of bortezomib-treated primary B-NHL patient samples
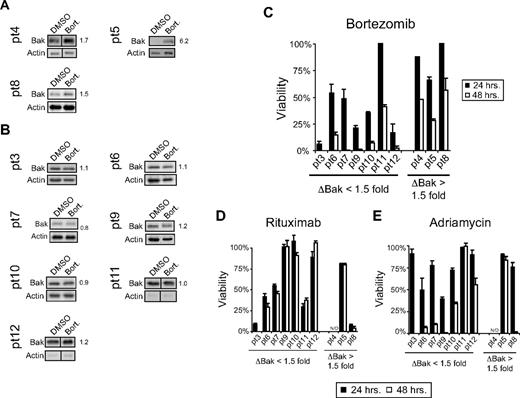
Data obtained from our B-NHL cell line model established 2 response patterns to in vitro bortezomib exposure: (1) sensitive cells die rapidly and do not show evidence of increased Bak expression following bortezomib exposure, and (2) resistant cells undergo slower cell death that is preceded by increased Bak, Bik, and Noxa expression following bortezomib exposure. When we examined several freshly isolated primary cultures of B-NHL cells from patients for their response to bortezomib, we found a similar pattern. Of the 10 primary isolates that yielded sufficient cells to perform cytotoxicity measurements and Western blots, 3 displayed a visible change in Bak expression following bortezomib exposure (Figure 3A), while the other 7 showed no evidence of increased Bak expression upon ex vivo bortezomib treatment (Figure 3B). Increased Bik expression was observed in the same 3 samples where increased Bak expression was seen while all 10 samples demonstrated increased Noxa expression upon ex vivo bortezomib exposure (data not shown). When the difference between Bak expression in vehicle-treated versus bortezomib-treated primary lymphoma cells was quantified, the 3 with visible Bak induction had greater than 1.5-fold changes in Bak (ΔBak ≥ 1.5), while the 7 with no visible changes in Bak expression had ΔBak values of < 1.5. We then separated the 10 samples into 2 groups based on their ΔBak values with 1.5 as our cutoff. When we compared the bortezomib-induced cytotoxicity between the 2 groups we found that, similar to our cell line model, primary cells in which Bak expression increased upon bortezomib exposure died slower over the 48-hour observation time than those with no change in Bak expression upon bortezomib exposure (Figure 3C). Sensitivity to rituximab-induced CMC (Figure 3D) or adriamycin treatment (Figure 3E) did not correlate with bortezomib sensitivity or changes in Bak expression following bortezomib. These data support findings from our cell line model and further suggest that Bak induction may precede and be necessary for bortezomib-induced cell death in those cells where Bak expression is controlled by the proteasome.
Increased Bak expression was observed in patient samples following bortezomib treatment. (A) A visible increase in Bak expression was observed in 3 of 10 primary B-NHL patient samples treated ex vivo with bortezomib (5-10nM). This visible increase in Bak expression correlated with a fold change in Bak of ≥ 1.5-fold. Individual fold change values were normalized to Actin and are indicated next to Bak Western blots. (B) Seven of 10 patient samples treated ex vivo with bortezomib had no visible change in Bak expression. (C) Following the general pattern established by sensitive and resistant cells, primary B-NHL cells that demonstrated no induction of Bak (ΔBak < 1.5-fold) following bortezomib treatment died more rapidly than those in which Bak increased (ΔBak ≥ 1.5-fold). B-NHL cells derived from patient 11 did not follow this general pattern. No pattern of sensitivity to rituximab (D) or adriamycin (E) was noted when the same 10 patient samples were segregated based on the ability of bortezomib to induce Bak expression.
Increased Bak expression was observed in patient samples following bortezomib treatment. (A) A visible increase in Bak expression was observed in 3 of 10 primary B-NHL patient samples treated ex vivo with bortezomib (5-10nM). This visible increase in Bak expression correlated with a fold change in Bak of ≥ 1.5-fold. Individual fold change values were normalized to Actin and are indicated next to Bak Western blots. (B) Seven of 10 patient samples treated ex vivo with bortezomib had no visible change in Bak expression. (C) Following the general pattern established by sensitive and resistant cells, primary B-NHL cells that demonstrated no induction of Bak (ΔBak < 1.5-fold) following bortezomib treatment died more rapidly than those in which Bak increased (ΔBak ≥ 1.5-fold). B-NHL cells derived from patient 11 did not follow this general pattern. No pattern of sensitivity to rituximab (D) or adriamycin (E) was noted when the same 10 patient samples were segregated based on the ability of bortezomib to induce Bak expression.
Proteasome inhibition by bortezomib stabilized expression of Bak in resistant cells
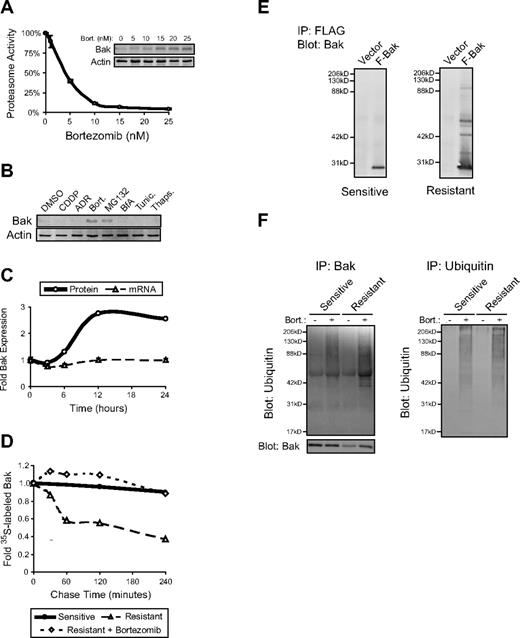
The first clear evidence that Bak was being regulated by proteasomes in resistant cells came from the observation that dose-dependent proteasome inhibition correlated with dose-dependent Bak induction in bortezomib-treated cells (Figure 4A). Treatment of cells with bortezomib is known to induce cell stress, particularly to the endoplasmic reticulum (ER),16 which could lead to induction of Bak. To address this possibility, we treated resistant cells with the ER-specific stress inducers brefeldin A, tunicamycin, or thapsigargin and compared Bak induction to proteasome inhibitor–treated cells. We found that Bak expression was induced by the proteasome inhibitors bortezomib, MG132, epoxomycin, and Ac-Leu-Leu-Nle (Figure 4B and data not shown). ER stressors and stresses induced by chemotherapeutic agents such as adriamycin and cisplatin did not increase Bak expression (Figure 4B). To determine whether the observed induction of Bak in bortezomib-treated resistant cells was dependent on transcription, we blocked transcription using actinomycin D and found that Bak protein expression still increased upon bortezomib exposure (data not shown). In support of the transcriptional independence of Bak induction, no change in BAK1 mRNA expression was detected by quantitative real-time PCR over the first 24 hours following bortezomib exposure of resistant cells. Bak protein expression examined in the same pool of cells increased approximately 2.5-fold over the same 24-hour time course, suggesting that the increase in Bak protein was independent of increased transcription of the BAK1 gene (Figure 4C). Because we found that proteasome inhibition in resistant cells led to increased Bak expression without increased BAK1 transcription, it seemed likely that Bak protein was stabilized by proteasome inhibition. We tested this idea using 35S-methionine pulse-chase experiments in untreated versus bortezomib-treated cells. We found that Bak stability was reduced in resistant cells compared with sensitive cells and that treatment of resistant cells with bortezomib increased Bak stability to a level comparable with untreated sensitive cells (Figure 4D). Overall, these data suggest that decreased steady-state Bak expression observed in resistant cells is due to increased proteasomal degradation of Bak and that inhibition of proteasomes leads to increased Bak stability and expression in resistant cells.
Bak is ubiquitinated and degraded by proteasomes in resistant B-NHL cell lines. (A) Dose-dependent inhibition of proteasome activity in resistant cells correlated with dose-dependent induction of Bak expression. Resistant cells (RL 4RH shown here) were incubated with 0-25nM bortezomib for 24 hours, then assayed for chymotrypsin-like activity of the 26S proteasome. Bak expression within the same cell population was determined by Western blot. Proteasome inhibition occurred at similar doses in other resistant and sensitive cell lines (data not shown). Bak induction correlated with proteasome inhibition in other resistant but not sensitive cell lines (data not shown). Data shown are from a representative experiment repeated 3 times. Points on the proteasome activity graph represent the average of triplicate wells ± SD (B) Increased Bak expression was observed in resistant cells treated with proteasome inhibitors and not other stress-inducing agents. Western blot analysis of whole cell lysates demonstrated increased Bak expression in resistant cells (Raji 2R shown here) treated for 48 hours with the proteasome inhibitors bortezomib (Bort.; 25nM) or MG132 (5μM) but not the standard chemotherapeutic agents cisplatinum (CDDP; 100μM) or adriamycin (ADR; 50μM), or the endoplasmic reticulum (ER) stress-inducers brefeldin A (BfA; 1 μg/mL), tunicamycin (Tunic.; 1 μg/mL), or thapsigargin (Thaps.; 1μM). (C) Incubation of resistant cells with bortezomib did not induce mRNA coding for Bak. Quantitative real-time PCR analysis of RNA extracted from resistant cells (Raji 4RH cells shown here) treated with bortezomib over a 24-hour time course showed no increased expression of BAK1, while Bak protein level within the same cell population increased approximately 2.5-fold. Data shown are from a representative experiment repeated twice. Real-time PCR data were analyzed using the ΔΔCt method and is expressed as fold change compared with time = 0 hours ± SD. (D) Bortezomib treatment of resistant cell lines stabilized expression of Bak protein. Bak stability was determined by standard 35S-methionine pulse-chase experiments. Bak was rapidly degraded in vehicle-treated (DMSO) resistant cells (open triangles) but not sensitive cells (closed circles). Incubation of resistant cells with bortezomib (50nM) stabilized the expression of Bak to a level comparable with untreated sensitive cells. Data shown are from a representative experiment repeated twice. (E) Exogenously expressed Bak appears ubiquitinated in resistant but not sensitive cells. FLAG-tagged Bak was immunoprecipitated using FLAG beads and subjected to Western blot for Bak expression. Higher molecular weight bands were detected in FLAG-IP/Bak Western blots from resistant (Raji 2R shown here) but not sensitive cells (Raji shown here). (F) Bortezomib treatment increases the ubiquitination of Bak in resistant cells. After 24 hours of bortezomib treatment (25nM), endogenous Bak or ubiquitin were immunoprecipitated from lysates of sensitive (RL shown here) or resistant (RL 4RH shown here) cells. Ubiquitin detected in the Bak IP from resistant cells increased to a greater degree following bortezomib treatment than did the ubiquitin detected in the Bak IP from sensitive cells while total ubiquitin levels increased similarly in sensitive and resistant cells treated with bortezomib.
Bak is ubiquitinated and degraded by proteasomes in resistant B-NHL cell lines. (A) Dose-dependent inhibition of proteasome activity in resistant cells correlated with dose-dependent induction of Bak expression. Resistant cells (RL 4RH shown here) were incubated with 0-25nM bortezomib for 24 hours, then assayed for chymotrypsin-like activity of the 26S proteasome. Bak expression within the same cell population was determined by Western blot. Proteasome inhibition occurred at similar doses in other resistant and sensitive cell lines (data not shown). Bak induction correlated with proteasome inhibition in other resistant but not sensitive cell lines (data not shown). Data shown are from a representative experiment repeated 3 times. Points on the proteasome activity graph represent the average of triplicate wells ± SD (B) Increased Bak expression was observed in resistant cells treated with proteasome inhibitors and not other stress-inducing agents. Western blot analysis of whole cell lysates demonstrated increased Bak expression in resistant cells (Raji 2R shown here) treated for 48 hours with the proteasome inhibitors bortezomib (Bort.; 25nM) or MG132 (5μM) but not the standard chemotherapeutic agents cisplatinum (CDDP; 100μM) or adriamycin (ADR; 50μM), or the endoplasmic reticulum (ER) stress-inducers brefeldin A (BfA; 1 μg/mL), tunicamycin (Tunic.; 1 μg/mL), or thapsigargin (Thaps.; 1μM). (C) Incubation of resistant cells with bortezomib did not induce mRNA coding for Bak. Quantitative real-time PCR analysis of RNA extracted from resistant cells (Raji 4RH cells shown here) treated with bortezomib over a 24-hour time course showed no increased expression of BAK1, while Bak protein level within the same cell population increased approximately 2.5-fold. Data shown are from a representative experiment repeated twice. Real-time PCR data were analyzed using the ΔΔCt method and is expressed as fold change compared with time = 0 hours ± SD. (D) Bortezomib treatment of resistant cell lines stabilized expression of Bak protein. Bak stability was determined by standard 35S-methionine pulse-chase experiments. Bak was rapidly degraded in vehicle-treated (DMSO) resistant cells (open triangles) but not sensitive cells (closed circles). Incubation of resistant cells with bortezomib (50nM) stabilized the expression of Bak to a level comparable with untreated sensitive cells. Data shown are from a representative experiment repeated twice. (E) Exogenously expressed Bak appears ubiquitinated in resistant but not sensitive cells. FLAG-tagged Bak was immunoprecipitated using FLAG beads and subjected to Western blot for Bak expression. Higher molecular weight bands were detected in FLAG-IP/Bak Western blots from resistant (Raji 2R shown here) but not sensitive cells (Raji shown here). (F) Bortezomib treatment increases the ubiquitination of Bak in resistant cells. After 24 hours of bortezomib treatment (25nM), endogenous Bak or ubiquitin were immunoprecipitated from lysates of sensitive (RL shown here) or resistant (RL 4RH shown here) cells. Ubiquitin detected in the Bak IP from resistant cells increased to a greater degree following bortezomib treatment than did the ubiquitin detected in the Bak IP from sensitive cells while total ubiquitin levels increased similarly in sensitive and resistant cells treated with bortezomib.
Bak is ubiquitinated in resistant, but not sensitive cell lines
The relative increase in proteasomal degradation of Bak observed in resistant cells could result from an increase in proteasome activity or selective targeting of Bak to the proteasome via increased ubiquitination. We previously demonstrated that resistant cells have a modest increase in total proteasome activity.7 However, this relatively small change in overall activity of proteasomes in resistant cells could not adequately explain the dramatic reduction of Bak protein expression. When FLAG-tagged Bak was immunoprecipitated from sensitive versus resistant cells and Bak Western blots were performed, we observed a distinct banding pattern in immunoprecipitates from resistant cells that was reminiscent of ubiquitination. In immunoprecipitates from sensitive cells, only a single band at the approximate molecular weight of Bak was observed (Figure 4E). We therefore examined the effect of bortezomib treatment on the ubiquitination of Bak in sensitive versus resistant cells. Immunoprecipitation of endogenous Bak followed by Western blot for Ubiquitin and Bak was carried out on lysates from sensitive and resistant cells treated with bortezomib. We observed a substantial increase in ubiquitinated Bak in bortezomib-treated resistant cells compared with bortezomib-treated sensitive cells and a banding pattern of ubiquitinated Bak similar to that observed in FLAG-Bak IPs (Figure 4F). To insure the increase in Bak ubiquitination in resistant cells was specific, immunoprecipitation of ubiquitin followed by Western blot for ubiquitin was preformed from the same lysates. As expected, bortezomib treatment increased the expression of ubiquitinated proteins in both sensitive and resistant cells to a similar extent (Figure 4F). These data suggest that Bak is specifically ubiquitinated and targeted for proteasomal degradation in resistant cells and helps to explain the reduced basal level of Bak observed in these cells.
Bak expression regulates bortezomib-induced apoptosis, but not cell death, of resistant cells
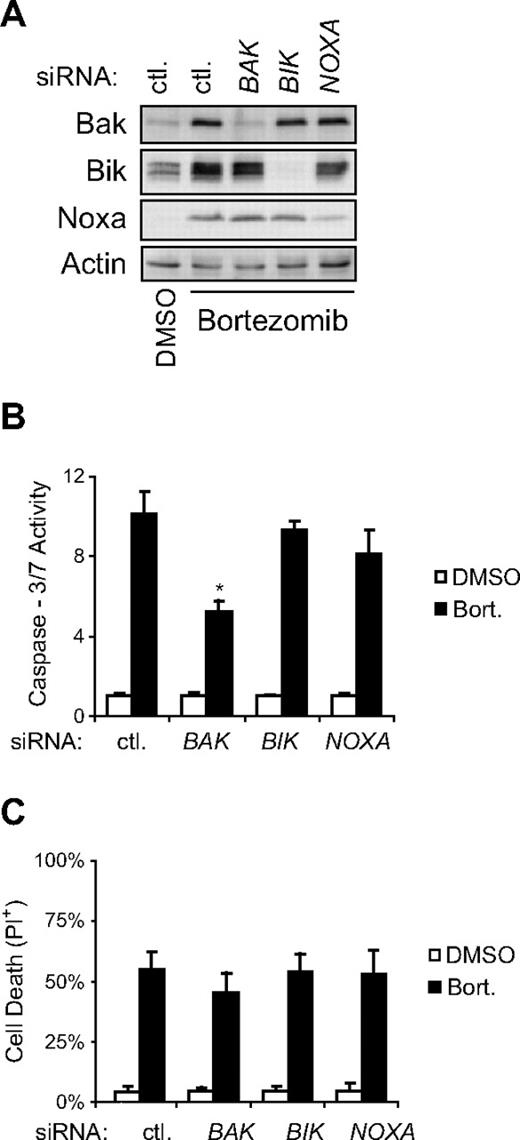
Inhibition of proteasome activity in resistant cells leads to increased expression of Bak, Bik, and Noxa and ultimately apoptotic cell death. To determine the contribution of Bak, Bik, or Noxa to bortezomib-induced apoptosis of resistant cells, siRNA specific for each were transfected 2 hours before incubation of cells with bortezomib, and their effect on bortezomib-induced apoptosis was determined. Western blot analysis of whole cell lysates from siRNA-transfected, bortezomib-treated resistant cells was used to confirm specific knock-down of each gene (Figure 5A). We found that siRNA-mediated inhibition of bortezomib-induced expression of Bak, but not Bik or Noxa, significantly (P < .01) reduced the ability of bortezomib to activate caspase-3/7 and induce DNA fragmentation (Figure 5B and data not shown). These data suggest that Bak is a critical mediator of bortezomib-induced apoptosis and that its stabilization in resistant cells allows for induction of apoptosis in cells that otherwise lack apoptotic potential. However, siRNA-mediated knockdown of Bak had no significant effect on the ability of bortezomib to kill resistant cells (Figure 5C). This suggests that bortezomib might be capable of activating additional Bak and caspase-independent death pathways in resistant cells.
Increased Bak expression is required for bortezomib-induced apoptosis, but not death, of resistant cells. (A) siRNA-mediated knock-down of Bak, Bik, or Noxa was confirmed in whole cell lysates collected 24 hours following bortezomib treatment. (B) Knock-down of Bak significantly inhibited the ability of bortezomib to induce apoptosis in resistant cells (Raji 4RH shown here). Resistant cells transfected with siRNA specific for BAK1, BIK, or PMAIP1 (Noxa), or non-targeting siRNA (ctl) as a control, were treated with bortezomib (20nM) for 24 hours and assayed for caspase-3/7 activity. Data shown are from a representative experiment repeated at least 3 times. Bars indicate average caspase-3/7 activity from triplicate wells ± SD. Asterisks (*) denote a significant decrease in caspase-3/7 activity compared with ctl siRNA transfected cells within the same treatment group. (C) Knock-down of Bak (or Bik or Noxa) does not alter the ability of bortezomib to kill resistant cells (Raji 4RH shown here). Cell death was determined by propidium iodide uptake in the same population of cells from B following 72 hours of incubation with bortezomib (20nM). Data shown are the average of at least 3 independent experiments ± SD. No significant difference (P > .05) in the percentage of propidium iodide–positive (PI+) cells was seen comparing BAK1, BIK, or PMAIP1 (Noxa) siRNA transfected to control siRNA transfected cells within the same treatment group.
Increased Bak expression is required for bortezomib-induced apoptosis, but not death, of resistant cells. (A) siRNA-mediated knock-down of Bak, Bik, or Noxa was confirmed in whole cell lysates collected 24 hours following bortezomib treatment. (B) Knock-down of Bak significantly inhibited the ability of bortezomib to induce apoptosis in resistant cells (Raji 4RH shown here). Resistant cells transfected with siRNA specific for BAK1, BIK, or PMAIP1 (Noxa), or non-targeting siRNA (ctl) as a control, were treated with bortezomib (20nM) for 24 hours and assayed for caspase-3/7 activity. Data shown are from a representative experiment repeated at least 3 times. Bars indicate average caspase-3/7 activity from triplicate wells ± SD. Asterisks (*) denote a significant decrease in caspase-3/7 activity compared with ctl siRNA transfected cells within the same treatment group. (C) Knock-down of Bak (or Bik or Noxa) does not alter the ability of bortezomib to kill resistant cells (Raji 4RH shown here). Cell death was determined by propidium iodide uptake in the same population of cells from B following 72 hours of incubation with bortezomib (20nM). Data shown are the average of at least 3 independent experiments ± SD. No significant difference (P > .05) in the percentage of propidium iodide–positive (PI+) cells was seen comparing BAK1, BIK, or PMAIP1 (Noxa) siRNA transfected to control siRNA transfected cells within the same treatment group.
Bortezomib kills resistant cells in the absence of caspase activation
To test the ability of bortezomib to kill cells in the absence of caspase activity, we incubated sensitive or resistant cells in bortezomib with or without the pan-caspase inhibitors zVAD-fmk or Q-VD-OPh. We found that in sensitive cells, caspase inhibition repressed bortezomib-induced cell death while in resistant cells caspase inhibition had no effect on or increased bortezomib-induced cell death (Figure 6A). Western blot was used to confirm that the doses of zVAD-fmk and Q-VD-OPh used inhibited caspase activity as measured by PARP cleavage (Figure 6B). These data suggest that in addition to triggering an apoptotic phenotype in resistant B-NHL cells, bortezomib is capable of killing them in a caspase-independent manner.
Caspase inhibition does not inhibit bortezomib-induced death of resistant cells and promotes phenotypic changes reminiscent of autophagy in bortezomib-treated cells. (A) Chemical inhibition of caspase activation inhibited bortezomib-induced cell death of sensitive but not resistant cells. After 72-hour incubations with bortezomib (25nM) alone or combined with the pan-caspase inhibitors zVAD-fmk (50μM) or Q-VD-OPh (5μM), sensitive (Raji, RL) and resistant (Raji 2R, Raji 4RH, RL 4RH) cells were assayed for cell death by propidium iodide uptake. Data shown are averages of at least 3 independent experiments ± SD. Asterisks (*) indicate significant (P < .05) inhibition of bortezomib-induced cell death. Double asterisks (**) indicate significant (P < .05) enhancement of bortezomib-induced cell death. (B) Western blot analysis of whole cell lysates from resistant cells (Raji 2R shown here) treated for 48 hours with bortezomib (25nM) with or without zVAD-fmk (50μM) or Q-VD-OPh (5μM) revealed that addition of pan-caspase inhibitors were sufficient to inhibit bortezomib-induced apoptosis, as demonstrated by inhibition of PARP cleavage.
Caspase inhibition does not inhibit bortezomib-induced death of resistant cells and promotes phenotypic changes reminiscent of autophagy in bortezomib-treated cells. (A) Chemical inhibition of caspase activation inhibited bortezomib-induced cell death of sensitive but not resistant cells. After 72-hour incubations with bortezomib (25nM) alone or combined with the pan-caspase inhibitors zVAD-fmk (50μM) or Q-VD-OPh (5μM), sensitive (Raji, RL) and resistant (Raji 2R, Raji 4RH, RL 4RH) cells were assayed for cell death by propidium iodide uptake. Data shown are averages of at least 3 independent experiments ± SD. Asterisks (*) indicate significant (P < .05) inhibition of bortezomib-induced cell death. Double asterisks (**) indicate significant (P < .05) enhancement of bortezomib-induced cell death. (B) Western blot analysis of whole cell lysates from resistant cells (Raji 2R shown here) treated for 48 hours with bortezomib (25nM) with or without zVAD-fmk (50μM) or Q-VD-OPh (5μM) revealed that addition of pan-caspase inhibitors were sufficient to inhibit bortezomib-induced apoptosis, as demonstrated by inhibition of PARP cleavage.
Bortezomib is capable of killing ex vivo treated primary B-NHL cells in a caspase-independent manner
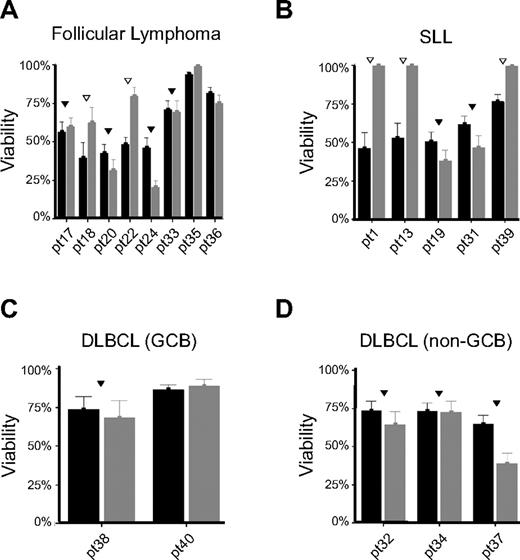
The ability of cell lines to activate “back-up” caspase-independent modes of cell death when apoptosis is inhibited has been extensively studied.32 In our cell line model, we have found that bortezomib is capable of inducing caspase-independent death (Figure 6A). To determine whether this phenomenon is specific to our B-NHL cells that were selected for therapy resistance, we tested 18 primary tumor specimens obtained from patients with follicular lymphoma (Figure 7A), small lymphocytic lymphoma (Figure 7B), and diffuse large B-cell lymphoma of germinal center (Figure 7C) and non–B-cell lymphoma of germinal center (Figure 7D) phenotypes to determine whether caspase-independent cell death occurred following bortezomib exposure. Bortezomib was capable of reducing the viability of ex vivo treated B-NHL cells by greater than 20% in 15 of 18 samples tested. Of these 15 patient samples that were sensitive to bortezomib, addition of the caspase inhibitor Q-VD-Oph significantly inhibited bortezomib-dependent loss of viability in 5 cases (Figure 7 open triangles). No specific pattern was observed regarding histology, subtype, or disease status (de novo vs relapsed/refractory). These data support our cell line findings and demonstrate that bortezomib is capable of killing B-NHL cells in a caspase-dependent or -independent manner.
Bortezomib induces caspase-dependent (▿) or -independent (▾) cell death in primary tumor cells isolated from patients with B-NHL. Tumor cells isolated from patients with (A) follicular lymphoma (n = 8), (B) small lymphocytic lymphoma (SLL, n = 5), (C) germinal center (GCB) diffuse large B-cell lymphoma (DLBCL, n = 2), and (D) non-GCB DLBCL (n = 3) were exposed in vitro to bortezomib (10μM) in the presence or absence of the pan-caspase inhibitor Q-VD-Oph (2.5μM). Viability was determined using the CellTiter Glo luminescent assay after 48 hours of incubation and expressed as percentage of luminescent signal compared with untreated controls. Samples in which caspase-dependent cell death was observed (n = 5) are denoted with an open upside-down triangle (▿) and those where caspase-independent cell death was observed (n = 10) are denoted with a filled upside-down triangle (▾) while those that were found resistant to bortezomib (> 80% viable) have no marks above them. Of interest, in some patient specimens, caspase inhibition further enhanced bortezomib activity (patients 24 and 37).
Bortezomib induces caspase-dependent (▿) or -independent (▾) cell death in primary tumor cells isolated from patients with B-NHL. Tumor cells isolated from patients with (A) follicular lymphoma (n = 8), (B) small lymphocytic lymphoma (SLL, n = 5), (C) germinal center (GCB) diffuse large B-cell lymphoma (DLBCL, n = 2), and (D) non-GCB DLBCL (n = 3) were exposed in vitro to bortezomib (10μM) in the presence or absence of the pan-caspase inhibitor Q-VD-Oph (2.5μM). Viability was determined using the CellTiter Glo luminescent assay after 48 hours of incubation and expressed as percentage of luminescent signal compared with untreated controls. Samples in which caspase-dependent cell death was observed (n = 5) are denoted with an open upside-down triangle (▿) and those where caspase-independent cell death was observed (n = 10) are denoted with a filled upside-down triangle (▾) while those that were found resistant to bortezomib (> 80% viable) have no marks above them. Of interest, in some patient specimens, caspase inhibition further enhanced bortezomib activity (patients 24 and 37).
Discussion
Proteasome inhibition by bortezomib can alter the expression of several pro- and antiapoptotic Bcl-2 family proteins in a variety of cell types and via distinct mechanisms.14 Bortezomib treatment of rituximab/chemotherapy resistant B-NHL cell lines developed by Dr Benjamin Bonavida's group led to down-regulation of Bcl-xL and sensitization to chemotherapy in an NF-κB–dependent manner.13 Here we report that bortezomib is capable of killing therapy resistant B-NHL cell lines in the absence of additional chemotherapeutic agents. Unpublished observations demonstrated that inhibition of NF-κB activity did not kill our therapy-resistant cell lines, even at high doses. Therefore, inhibition of NF-κB by bortezomib is not sufficient to kill therapy-resistant B-NHL cells, further suggesting that bortezomib must be acting on additional factors to promote cell death in our model of resistance.
Activation of BH3-only members of the Bcl-2 family of proteins is critical for initiation of the apoptotic cascade. BH3-only proteins are tightly regulated in healthy cells and can be activated transcriptionally or by posttranslational modification and/or stabilization. After bortezomib treatment, we found that 2 BH3-only proteins, Bik and Noxa, were induced in resistant, but not sensitive, cells. Previous studies in mantle cell lymphoma and multiple myeloma cell lines have demonstrated Noxa is constitutively degraded by proteasomes and is therefore induced by treatment with proteasome inhibitors.24,25 Similarly, Bik stabilization has been observed in cell lines derived from solid tumors treated with bortezomib.27,33 Recent evidence suggests that because of their limited binding specificities, expression of Noxa or Bik alone should not be sufficient to induce cell death in cells that express Bcl-xL and Mcl-1.34 Noxa should inhibit the binding of Mcl-1, but not Bcl-xL, to Bak while Bik should inhibit Bcl-xL, but not Mcl-1, binding to Bak and/or Bax. Simultaneous induction of Bik and Noxa in resistant cells (Figure 2) could potentially lead to apoptosis by blocking the inhibitory binding of both Mcl-1 and Bcl-xL to Bak, the only multidomain proapoptotic Bcl-2 family protein expressed in resistant cells. We were, however, unable to demonstrate a role for either Bik or Noxa in bortezomib-induced apoptosis (Figure 5). Additional experiments were conducted in which Bik and Noxa were simultaneously expressed or knocked down, and still no effect on apoptosis was observed (data not shown). These data suggest that while the expression of Bik and Noxa are clearly enhanced by proteasome inhibition, in the absence of adequate levels of Bak, this effect does not make a significant contribution to bortezomib-induced apoptosis in our resistant cells. Additional factors (perhaps other BH3-only proteins or stabilization of adequate levels of Bak) must be activated or induced in bortezomib-treated cells to promote Bak-dependent apoptosis.
Regardless of how bortezomib activates Bak- and/or Bax-dependent apoptosis, either Bax or Bak must be expressed in order for a cell to undergo apoptosis.35 In resistant cells, however, Bax is not expressed, and the expression of Bak is reduced. Reintroduction of either Bax or Bak led to apoptosis of resistant cells.8 Similarly, bortezomib treatment of resistant cells led to increased Bak expression and Bak-dependent apoptosis. Our data suggest that Bak is ubiquitinated and targeted for proteasomal degradation in resistant, but not sensitive, cells (Figure 4). Furthermore, we were able to observe Bak stabilization upon bortezomib treatment of additional B-NHL cell lines that were not selected for resistance (data not shown) and in isolated, primary B-NHL cells treated ex vivo with bortezomib (Figure 3). These observations suggest that increased proteasomal degradation of Bak may be a novel pathway that could contribute to therapy resistance in B-NHL.
The observation that bortezomib is capable of inducing apoptosis in cells that are otherwise resistant to apoptosis is important in our understanding of how these cells became therapy resistant. Identification of the molecular mechanisms underlying Bak degradation (eg, specific ubiquitin ligase[s]) could help guide the logical use of bortezomib to resensitize resistant B-NHL cells to apoptosis. However, identification of Bak as a major contributor to bortezomib-induced apoptosis of resistant cells did not complete our understanding of how bortezomib killed these cells. Bak induction, while necessary for caspase activation, was not required for bortezomib-induced death of resistant cells (Figure 5C), likely because bortezomib is capable of killing resistant cells in a caspase-independent manner (Figure 6A). Caspase-independent cell death is considered to be a secondary form of cell death that is only activated when apoptotic, caspase-dependent cell death is inhibited. Our data are consistent with this hypothesis yet further suggest that bortezomib may induce a form of caspase-independent death that is capable of killing some B-NHL cells (ie, our resistant cells) better than others (ie, our sensitive cells). To confirm and advance these data, we tested an additional 18 primary tumor cell specimens derived from patients with various subtypes of B-NHL and found that bortezomib was able to induce both caspase-dependent (n = 5) and -independent (n = 10) cell death. The caspase dependence of bortezomib-induced death did not correlate with histology or disease status (de novo vs relapsed/refractory). This could be explained by the heterogeneity within disease subtypes studied or could be a result of the limited number of samples currently available. Further study will be required to uncover correlations between the caspase dependence of cell death induced by bortezomib and clinical/molecular features of B-NHL.
Several caspase-independent, nonapoptotic modes of cell death have been described.36 Autophagy had been linked to proteasome activity,37 and its activation by proteasome inhibition made the most intuitive sense because the ubiquitin-proteasome system and autophagy are parallel protein degradation pathways that can compensate for one another. On the other hand, irreversible cell cycle arrest and/or necrosis had been observed following proteasome inhibition in preclinical cancer models38,39 Our data suggest that under normal circumstances apoptosis is the predominant mechanism by which bortezomib kills cells.
In addition, further studies to enhance our understanding of caspase-independent death pathways induced by bortezomib are ongoing in our laboratory. Caspase-independent cell death observed in resistant B-NHL cell lines and primary B-NHL cells may have clinical implications for the treatment of resistant B-NHL that have severe defects in their ability to undergo apoptosis. If caspase-independent pathway(s) are identified, bortezomib-based combination therapies could be designed to bypass the requirement for B-NHL cells to die by apoptosis and instead engage caspase-independent death pathways. Additional preclinical studies will be required to further understand bortezomib-induced cell death and to determine optimal treatment combinations that could benefit patients with resistant B-NHL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ping-Chaio Tsai, Elizabeth Repasky, PhD, Paul Wallace, PhD, and John Byrd, MD, for constructive criticism of this work.
This work was supported by National Institutes of Health R01 grant CA136907-01A1 awarded to Roswell Park Cancer Institute, Buffalo, NY; National Institutes of Health P01 grant CA103985-1 awarded to Garden State Cancer Center, Belleville, NJ; and the Eugene and Connie Corasanti Lymphoma Research Fund.
National Institutes of Health
Authorship
Contribution: S.H.O., J.B., A.B.-V., W.R., and C.M. performed experiments; J.G. provided surgical specimens; S.H.O., F.J.H.-I., and M.S.C. analyzed data and made the figures; and S.H.O., N.B., J.L.C., F.J.H.-I., and M.S.C. designed the research and wrote the paper.
Conflict-of-interest disclosure: M.S.C. has served on an advisory board and has received clinical research support from Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Myron S. Czuczman, MD, Chief, Lymphoma/Myeloma Section, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263; e-mail: myron.czuczman@roswellpark.org.