Abstract
Diagnostic errors in distinguishing between malignant and reactive processes can cause serious clinical consequences. We report 10 cases of unrecognized self-limited natural killer–cell proliferation in the stomach, designated as lymphomatoid gastropathy (LyGa). This study included 5 men and 5 women (age, 46-75 years) without any gastric symptoms. Gastroscopy showed elevated lesion(s) (diameter, ∼ 1 cm). Histologically, medium-sized to large atypical cells diffusely infiltrated the lamina propria and, occasionally, the glandular epithelium. The cells were CD2+/−, sCD3−, cCD3+, CD4−, CD5−, CD7+, CD8−, CD16−, CD20−, CD45+, CD56+, CD117−, CD158a−, CD161−, T cell–restricted intracellular antigen-1+, granzyme B+, perforin+, Epstein-Barr early RNA−, T-cell receptor αβ−, and T-cell receptor γδ−. Analysis of the 16 specimens biopsied from 10 patients led to a diagnosis of lymphoma or suspected lymphoma in 11 specimens, gastritis for 1 specimen, adenocarcinoma for 1 specimen, and LyGa or suspected LyGa for 3 specimens. Most lesions underwent self-regression. Three cases relapsed, but none of the patients died. According to conventional histopathologic criteria, LyGa is probably diagnosed as lymphoma, especially as extranodal natural killer/T-cell lymphoma, nasal type. However, LyGa is recognized as a pseudomalignant process because of its clinical characteristics. The concept of LyGa should be well recognized.
Introduction
The World Health Organization classification of tumors of hematopoietic and lymphoid tissues lists > 60 types of lymphomas.1 Several reactive or borderline lesions related to these overt lymphomas are well known. Some benign lymphoproliferative disorders, including infectious mononucleosis, drug-induced lymphadenitis especially related to anticonvulsants, and histiocytic/subacute necrotizing lymphadenitis (Kikuchi-Fujimoto disease),2,3 are occasionally misdiagnosed as malignancy because these lesions histopathologically mimic lymphoma.4 They are basically self-limited and require no cytoreductive therapies. Lymphomatoid papulosis, lymphomatoid granulomatosis, and methotrexate-associated lymphoproliferative disorder5 are listed as borderline lesions with uncertain malignant potential according to the World Health Organization. These disorders may also be diagnosed as overt lymphoma. Moreover, even if they are properly diagnosed, selection of a treatment strategy is then a matter of discussion because some of these cases undergo spontaneous regression. Therefore, conservative therapies are primarily favored in such cases, and these lesions should be treated as lymphoma only if they are clinically malignant. In any case, at the time these lesions are evaluated with biopsy specimens, the possibility of being benign should be well considered, and overtreatment must be carefully avoided.
Here, we report 10 cases of a pseudomalignant disorder caused by an unrecognized atypical natural killer (NK)–cell proliferation in the stomach; we have designated this disorder as lymphomatoid gastropathy (LyGa). According to conventional histopathologic criteria, such lesions are diagnosed as lymphoma, especially as extranodal NK/T-cell lymphoma, nasal type. However, considering its clinical characteristics, LyGa is recognized as a pseudomalignant process because it spontaneously regresses without any treatment.
Methods
Patients
During the 11-year period between 1998 and 2009, there were 10 cases of CD56-positive atypical lymphoid cell proliferation in the stomach (patients 1-3 presented at the Cancer Institute and patients 4-10 were referred to K.T. for consultation). The clinical records and pathology materials of the cases were reviewed.
Immunophenotyping and Epstein-Barr virus detection
Immunohistochemical examination was performed with Autostainer (Dako); dextran-polymer method (EnVision+; Dako); and antibodies against CD2, CD3, CD4, CD5, CD7, CD8, CD20, CD30, CD45, CD56, CD68 (KP1 or PGM1), T cell–restricted intracellular antigen-1 (TIA1), granzyme B, anaplastic lymphoma kinase, myeloperoxidase, Ki67, and T-cell receptor βF1 (TCRβF1). For flow cytometry, the following antibodies were used: CD2, CD3, CD7, CD56, TCRαβ, TCRγδ, TCRVa24, CD158a, and CD161. The presence of Epstein-Barr virus (EBV) was assessed by in situ hybridization for Epstein-Barr early RNA (EBER).
Polymerase chain reaction analysis for TCRγ gene rearrangement
DNA was extracted from the paraffin sections with the use of Recover All Total Nucleic Acid Isolation according to the manufacturer's instructions (Ambion). A seminested protocol involving 2 rounds of polymerase chain reaction (PCR) was used for the amplification of the rearranged TCRγ gene with the use of the primers TVγ, 5′-AGGGTTGTGTTGGAATCAGG-3′; TJγ-out, 5′-CGTCGACAACAAGTGTTGTTCCAC-3′; and TJγ-in, 5′-GGATCCACTGCCAAAGAGTTTCTT-3′. The 5′ end of TJγ-I was labeled by cyanine 5 for fragment analysis. In all the experiments, monoclonal (Jurkat cells) and polyclonal (placental tissue from a healthy person) controls were run in parallel with the samples. The PCR products were analyzed with CEQ8000 (Beckman Coulter Inc). DNA from each sample was amplified ≥ 6 times.
Results
Clinical history
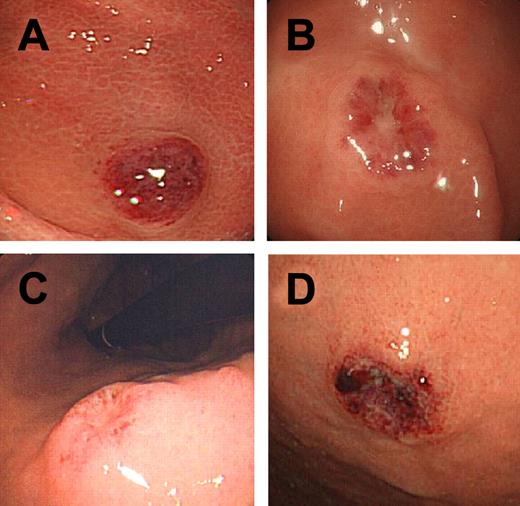
Of the 10 patients in this study, 5 were men and 5 were women. The age of these patients ranged from 46 to 75 years Three patients had a history of gastric cancer, of whom 1 had previously undergone endoscopic mucosal resection 2 times (case 1) and the other 2 had previously undergone partial gastrectomy (cases 3 and 8). At the time of the study, 3 patients had diabetes mellitus (cases 1, 2, and 9) and 4 had hypertension (cases 2, 7, 9, and 10). Blood cell counts and chemistry, including lactic dehydrogenase levels, were within the normal limits in all patients. There were no gastric symptoms at the time of gastroscopy. The 3 patients with history of gastric cancer underwent gastroscopy during a follow-up study for gastric cancer, and the procedure was performed on the other patients as a secondary checkup because gastric x-ray screening for cancer in these patients showed the presence of abnormal shadows. Gastroscopy showed ulcerative or elevated lesion(s) ∼ 1 cm in diameter in the stomach (Figure 1A-D). The pathologists of the institutions where the biopsies of the patients with LyGa were first performed diagnosed the patients' conditions as lymphoma or suspected lymphoma (cases 1, 2, 5-8, and 10), gastritis with histiocytic infiltration (case 3), and poorly differentiated adenocarcinoma (case 4). In case 3, the specimen was biopsied again 11 months later, and the patient's condition was then diagnosed as NK/T-cell lymphoma. Cases 5 and 9 were suspected of having lymphoma, and the pathologist consulted with one of the authors (K.T.), leading to the diagnosis of LyGa. In case 5, another biopsy was performed 3 weeks after the first biopsy for flow cytometry.
Gross appearance of LyGa. Cases 3 (A), 3 (B), 4 (C), and 10 (D) are shown.
An extensive workup, including ultrasonography (cases 1-4 and 9), computed tomographic (cases 1-4 and 6-9), and 2-[fluorine-18]fluoro-2-deoxy-d-glucose positron emission tomographic scans (cases 2, 4, 6, and 8); colonoscopy (cases 2, 4-6, and 9); and bone marrow biopsy (cases 1-4 and 7-9), was performed. The results showed no evidence of lymphoma in sites other than the stomach. Multiple serologic studies for celiac disease showed no evidence of high titers of anti–gliadin immunoglobulin A and immunoglobulin G antibodies in cases 2 and 4. Gastroscopy and biopsy were performed 1-4 months after the biopsies, which showed no evidence of lymphoma (cases 1, 2, 5, 7 and 10). Cases 4 and 6 underwent partial gastrectomy 1 month after the initial biopsy diagnosis, resulting in no evidence of carcinoma or lymphoma. All the patients were carefully watched and followed up without chemotherapy. Except in the case of patients 3, 8, and 9, none of the other patients had any recurrences. In case 3, the patient developed 3 lesions; on follow-up examination 11 months later, the lesions had regressed, and a new lesion was detected. The new lesion also regressed in 1 month from the second biopsy. In case 8, the patient developed another lesion 7 months after self-regression of the first lesion; this new lesion also regressed in 3 months without any treatment. In case 9, the first lesion could not be detected 4 months from the first biopsy; however, 2 new lesions were detected. After another 4 months, these 2 lesions could also not be detected, and 2 new lesions were identified. The consequence of the 2 lesions last detected is unknown because the patient refused further gastroscopic examination.
Morphology
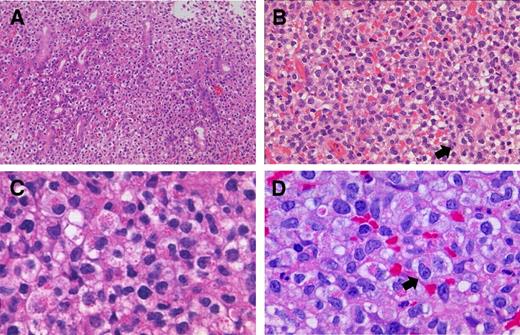
Grossly, the lesions were flat elevations with or without a shallow depression and were approximately 1 cm in diameter (Figure 1A-D). The atypical cells diffusely infiltrated the lamina propria and occasionally into the glandular epithelium (Figure 2A), simulating the lymphoepithelial lesion seen in extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue lymphoma, which was designated as lymphoepithelial-like lesion by NK cells (Figure 2B). In some cases, necrosis was present, but there were no angiocentric or angiodestructive growth patterns or apoptotic bodies. Mitotic figures were occasionally present. The atypical cells were medium to large with moderate to abundant clear or slightly eosinophilic cytoplasm. The nuclei were generally round to oval, but some were irregular and indented, with fine chromatin and a few inconspicuous nucleoli. These cytomorphologic features somewhat give a histiocyte-like impression. Interestingly, specimens for all the patients contained a variable proportion of cells (20%-90%) with eosinophilic granules in the cytoplasm (Figure 2C-D). In some cases, atypical cells with a prominent nucleolus were observed (Figure 2D). Small reactive lymphocyte aggregates and neutrophils may be occasionally found. Nine of the patients had Helicobacter pylori infection.
Histopathology of LyGa. The pattern of infiltration is diffuse (A; case 1; 20× objective). Atypical NK cells occasionally infiltrate the glandular epithelium (arrow), showing lymphoepithelial-like lesions by NK cells (B; case 10; 40× objective). Some atypical cells harbor large eosinophilic granules in the cytoplasm (C; case 3; 100× objective). In some cases, the nucleoli are prominent (arrow; D; case 5; 100× objective). Figures were taken with a microscope (BX51; Olympus) and a digital camera (KY-F75; Victor). Microsoft PowerPoint 2007 was used for image processing. Numeric apertures: 20×/0.40 (A), 40×/0.75 (B), 40×/0.95 (C), 60×/0.90 (D).
Histopathology of LyGa. The pattern of infiltration is diffuse (A; case 1; 20× objective). Atypical NK cells occasionally infiltrate the glandular epithelium (arrow), showing lymphoepithelial-like lesions by NK cells (B; case 10; 40× objective). Some atypical cells harbor large eosinophilic granules in the cytoplasm (C; case 3; 100× objective). In some cases, the nucleoli are prominent (arrow; D; case 5; 100× objective). Figures were taken with a microscope (BX51; Olympus) and a digital camera (KY-F75; Victor). Microsoft PowerPoint 2007 was used for image processing. Numeric apertures: 20×/0.40 (A), 40×/0.75 (B), 40×/0.95 (C), 60×/0.90 (D).
Immunophenotype and EBER in situ hybridization
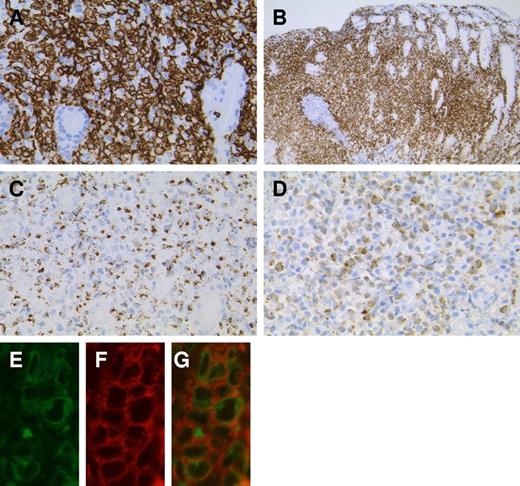
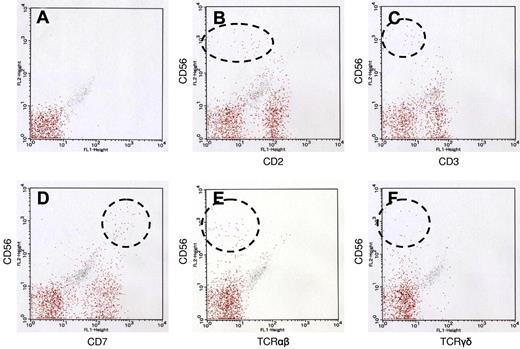
The atypical cells were strongly positive for CD7, CD56, and cytotoxic molecule-associated proteins (TIA1, granzyme B, and perforin; Figure 3A-C). CD2 and CD45 were variably positive. CD3ϵ was positive in the cytoplasm, but a membrane-staining pattern was not observed (Figure 3D). Anaplastic large cell lymphoma-associated markers (CD30 and anaplastic lymphoma kinase) were negative. Other common lineage markers, including B-cell (CD20), T-cell (CD4, CD5, and CD8), and myelomonocytic (CD68 and myeloperoxidase) markers, were all negative. EBER in situ hybridization was negative. The results of immunohistochemistry for individual cases are listed in Table 1. For case 5, flow cytometric analysis was performed with the second specimen, which was obtained from a biopsy performed 3 weeks after the first biopsy (Figure 4). Grossly, although the lesion was regressing, it remained present. The atypical cells of this case expressed CD7 and CD56 (both aberrantly bright) and CD2 (negative or dim). Other T or NK cell–related markers were negative (CD3, CD16, TCRαβ, TCRγδ, TCRVa24, CD158a, and CD161).
Immunophenotype of LyGa by immunohistochemistry. The atypical cells are positive for CD7 (A; case 5), CD56 (B; case 3), granzyme B (C; case 4), and cytoplasmic CD3ϵ (D; case 2). To confirm the cytoplasmic localization of CD3ϵ, fluorescein double immunohistochemistry for CD3ϵ (E) and CD56 (F) was performed (case 10). In the merged figure (G), the cytoplasmic localization of CD3ϵ is clearly shown, indicating that the atypical cells are of NK lineage. Figures were taken with a microscope (BX51; Olympus) and a digital camera (KY-F75; Victor). Microsoft PowerPoint 2007 was used for image processing. Numeric apertures: 40× (A,C,D), 10× (B), 60× (E-G).
Immunophenotype of LyGa by immunohistochemistry. The atypical cells are positive for CD7 (A; case 5), CD56 (B; case 3), granzyme B (C; case 4), and cytoplasmic CD3ϵ (D; case 2). To confirm the cytoplasmic localization of CD3ϵ, fluorescein double immunohistochemistry for CD3ϵ (E) and CD56 (F) was performed (case 10). In the merged figure (G), the cytoplasmic localization of CD3ϵ is clearly shown, indicating that the atypical cells are of NK lineage. Figures were taken with a microscope (BX51; Olympus) and a digital camera (KY-F75; Victor). Microsoft PowerPoint 2007 was used for image processing. Numeric apertures: 40× (A,C,D), 10× (B), 60× (E-G).
Patient characteristics and immunologic markers
| . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Case 8 . | Case 9 . | Case 10 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 52 | 58 | 51 | 50 | 55 | 46 | 65 | 56 | 59 | 75 |
| Sex | Male | Male | Male | Female | Male | Male | Female | Female | Female | Female |
| Past history | Two individual early gastric cancers at the ages of 48 and 51 y | NP | Advanced gastric cancer at the age of 47 y | NP | NP | NP | NP | Advanced gastric cancer at the age of 52 | NP | NP |
| H pylori | − | + | + | + | + | + | + | + | + | + |
| Original pathologic diagnosis | NK/T-cell lymphoma | NK/T-cell lymphoma | Gastritis with histiocytosis. NK/T-cell lymphoma* | Adenocarcinoma | Lymphoma, s/o LyGa* | NK/T-cell lymphoma; NK/T-cell lymphoma* | T-cell lymphoma | T-cell lymphoma; NK/T-cell lymphoma* | Lymphoma, s/o LyGa, s/o LyGa* | T-cell lymphoma |
| Follow-up examinations, days from the initial biopsy | 45, 73, 276, 577, 1165 | 55, 239, 442, 675, 896, 1121, 1497 | 336,† 365, 484, 701, 1065, 1429, 1793 | 41,‡ 167, 1360 | 13,† 132 | 50,† 56‡ | 38, 81, 137, 207, 361, 515, 742, 1029, 1281, 1515 | 98, 154, 236,† 256, 333, 452, 585, 790 | 113,† 232† | 30, 59, 143, 232, 354 |
| Patient status | Well at 145 mo | Well at 50 mo | Well at 60 mo | Well at 46 mo | Well at 33 mo | Well at 60 mo | Well at 56 mo | Well at 29 mo | Well at 18 mo | Well at 12 mo |
| Treatment | Observation | Observation | Observation | Subtotal gastrectomy | Observation | Total gastrectomy | Observation | Observation | Observation | Observation |
| CD2 | − | +w | +w | +w | − | + | +w | − | + | + |
| CD3 | + | + | + | + | + | + | + | + | + | + |
| CD4 | ND | − | − | − | − | − | − | − | − | − |
| CD5 | − | − | − | − | − | − | − | − | − | − |
| CD7 | + | + | + | + | + | + | + | + | + | + |
| CD8 | ND | − | − | ND | − | − | − | − | − | − |
| CD20 | − | − | − | − | − | − | − | − | − | − |
| CD56 | + | + | + | + | + | + | + | + | + | + |
| Cytotoxic molecules | TIA1+ | TIA1+, granzyme B+, perforin+ | TIA1+, granzyme B+, perforin+ | TIA1+, granzyme B+, perforin+ | Granzyme B+ | TIA1+, Perforin+ | Perforin+ | TIA1+ | Granzyme B+ | Granzyme B+ |
| EBER | − | − | − | − | − | − | − | − | − | − |
| Other markers | CD16−, betaF1− | CD16−, CD30−, CD45+w, CD57−, CD68−, CD123−, betaF1−, ALK−, MPO−, MIB1 index 10% | CD16−, CD30−, CD45+w, CD57−, CD68−, CD123−, betaF1−, ALK−, TdT−, MIB1 index 30% | CD16−, CD45+, CD68−, CD123−, betaF1−, CD43+ | TCRαβ−, TCRγδ−, CD161−, TCRVα24−, CD16−, CD158a− | CD10−, CD21−, BCL2+, CD45RO+, CD68−, MIB1 index 20% | MIB1 index 20%, CD21− |
| . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Case 8 . | Case 9 . | Case 10 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 52 | 58 | 51 | 50 | 55 | 46 | 65 | 56 | 59 | 75 |
| Sex | Male | Male | Male | Female | Male | Male | Female | Female | Female | Female |
| Past history | Two individual early gastric cancers at the ages of 48 and 51 y | NP | Advanced gastric cancer at the age of 47 y | NP | NP | NP | NP | Advanced gastric cancer at the age of 52 | NP | NP |
| H pylori | − | + | + | + | + | + | + | + | + | + |
| Original pathologic diagnosis | NK/T-cell lymphoma | NK/T-cell lymphoma | Gastritis with histiocytosis. NK/T-cell lymphoma* | Adenocarcinoma | Lymphoma, s/o LyGa* | NK/T-cell lymphoma; NK/T-cell lymphoma* | T-cell lymphoma | T-cell lymphoma; NK/T-cell lymphoma* | Lymphoma, s/o LyGa, s/o LyGa* | T-cell lymphoma |
| Follow-up examinations, days from the initial biopsy | 45, 73, 276, 577, 1165 | 55, 239, 442, 675, 896, 1121, 1497 | 336,† 365, 484, 701, 1065, 1429, 1793 | 41,‡ 167, 1360 | 13,† 132 | 50,† 56‡ | 38, 81, 137, 207, 361, 515, 742, 1029, 1281, 1515 | 98, 154, 236,† 256, 333, 452, 585, 790 | 113,† 232† | 30, 59, 143, 232, 354 |
| Patient status | Well at 145 mo | Well at 50 mo | Well at 60 mo | Well at 46 mo | Well at 33 mo | Well at 60 mo | Well at 56 mo | Well at 29 mo | Well at 18 mo | Well at 12 mo |
| Treatment | Observation | Observation | Observation | Subtotal gastrectomy | Observation | Total gastrectomy | Observation | Observation | Observation | Observation |
| CD2 | − | +w | +w | +w | − | + | +w | − | + | + |
| CD3 | + | + | + | + | + | + | + | + | + | + |
| CD4 | ND | − | − | − | − | − | − | − | − | − |
| CD5 | − | − | − | − | − | − | − | − | − | − |
| CD7 | + | + | + | + | + | + | + | + | + | + |
| CD8 | ND | − | − | ND | − | − | − | − | − | − |
| CD20 | − | − | − | − | − | − | − | − | − | − |
| CD56 | + | + | + | + | + | + | + | + | + | + |
| Cytotoxic molecules | TIA1+ | TIA1+, granzyme B+, perforin+ | TIA1+, granzyme B+, perforin+ | TIA1+, granzyme B+, perforin+ | Granzyme B+ | TIA1+, Perforin+ | Perforin+ | TIA1+ | Granzyme B+ | Granzyme B+ |
| EBER | − | − | − | − | − | − | − | − | − | − |
| Other markers | CD16−, betaF1− | CD16−, CD30−, CD45+w, CD57−, CD68−, CD123−, betaF1−, ALK−, MPO−, MIB1 index 10% | CD16−, CD30−, CD45+w, CD57−, CD68−, CD123−, betaF1−, ALK−, TdT−, MIB1 index 30% | CD16−, CD45+, CD68−, CD123−, betaF1−, CD43+ | TCRαβ−, TCRγδ−, CD161−, TCRVα24−, CD16−, CD158a− | CD10−, CD21−, BCL2+, CD45RO+, CD68−, MIB1 index 20% | MIB1 index 20%, CD21− |
NP indicates nothing in particular; s/o, suspected of; ND, not done; and +w, weakly positive.
In case 3, 5, 6, 8 and 9, multiple biopsies showed the presence of LyGa.
LyGa is present on follow-up examination.
In follow-up examinations, days of gastrectomy or gastroscopy with or without biopsy from the initial biopsy are described.
Immunophenotype of LyGa by flow cytometry. Flow cytometry was performed for case 5. The atypical cells were CD56bright, CD2dim (B), CD3− (C), CD7bright (D), TCRαβ− (E), and TCRγδ− (F). (A) Negative control.
Immunophenotype of LyGa by flow cytometry. Flow cytometry was performed for case 5. The atypical cells were CD56bright, CD2dim (B), CD3− (C), CD7bright (D), TCRαβ− (E), and TCRγδ− (F). (A) Negative control.
PCR analysis for TCRγ gene rearrangement
PCR analysis for TCRγ gene rearrangement was performed 6 times per case for cases 1-4 and 8. No reproducible rearranged bands were observed (data not shown).
Discussion
Here, we report 10 cases of self-limited lymphoma-like lesions in the stomach, which we designated as LyGa. These cases were almost identical to each other in morphology and immunophenotype of atypical cells. Gross examination showed that the lesions were ulcers or flat elevations with a shallow depression, measuring approximately 1 cm in diameter. Microscopic observation showed that they were composed of sheets of large peculiar cells that showed indented nuclei and clear cytoplasm with eosinophilic granules. Immunohistochemical analysis of the atypical cells of LyGa showed that they were CD2− or variably CD2+, CD3+ (cytoplasmic), CD4−, CD5−, CD7+, CD8−, CD16−, CD20−, CD45+, CD56+, CD117− and positive for cytotoxic molecule-related proteins (TIA1+, granzyme B+, and perforin+). This immunophenotype is highly suggestive of extranodal NK/T-cell lymphoma of the nasal type, which usually arises in extranodal sites, especially in the nasal cavity.1,6,7
Extranodal NK/T-cell lymphoma of the nasal type is rarely seen in Western countries and is more common in Asia and in Central and South American countries.1,6,7 It accounts for ∼ 2%,8 6%,9 8%,10 and 5%11 of all newly diagnosed lymphoma cases in Japan, Hong Kong, Korea, and Taiwan, respectively. Histologically, the lymphoma often has an angiocentric and angiodestructive infiltrate of atypical lymphocytes of various sizes leading to extensive necrosis.1 The immunophenotype of neoplastic cells usually indicates that they are of NK-cell lineage (surface CD3−, cytoplasmic CD3+, CD5−, and CD56+) but are occasionally of T-cell lineage by definition.1 In previous studies, neoplastic cells in almost all the cases were found to be infected by EBV.12,13 In localized diseases, the survival rate has recently improved with a combination of upfront radiotherapy and chemotherapy, whereas almost all patients with extensive disease die within a year after diagnosis.14-16
Of the 16 biopsied specimens in this study, 11 were diagnosed with lymphoma or suspected lymphoma. Fortunately, however, LyGa has several characteristic features that are not consistent with extranodal NK/T-cell lymphoma. First, the stomach is not a common site of origin in the case of NK/T-cell lymphoma. To the best of our knowledge, there are 10 reported cases of extranodal NK/T-cell lymphoma involving the stomach, and the lesions were not limited to the stomach in any of these cases.17-21 Second, although some of the cases of LyGa showed necrosis, but angiocentric or angiodestructive growth patterns, and prominent apoptotic bodies, which are common features of extranodal NK/T-cell lymphoma,1 were not observed. Third, LyGa may show epithelial invasion, that is, lymphoepithelial-like lesion by NK cells. Fourth, the cytomorphology of LyGa is atypical for extranodal NK/T-cell lymphoma. Although the cytologic spectrum of extranodal NK/T-cell lymphoma is broad,1 to the best of our knowledge, large eosinophilic cytoplasmic granules seen in the atypical cells of LyGa have never been observed in the histopathology section of extranodal NK/T-cell lymphoma although finer granules can often be seen in Giemsa-stained cytologic preparations. Finally, EBER in situ hybridization, which is almost always positive in NK/T-cell lymphoma of the nasal type,1,12,13 is consistently negative in LyGa. In addition, a differential diagnosis of CD56+ T-cell neoplasm with extensive loss of T-cell markers may be considered. In particular, the immunophenotype of LyGa overlaps the immunophenotype observed in some cases of enteropathy-associated T-cell lymphoma (type II).22 However, the negative PCR results for the TCRγ gene rearrangement (performed in cases 1-4 and 8; data not shown) were inconsistent with results obtained for T-cell lymphomas.
Vega et al23 reported a similar case of atypical NK-cell proliferation probably related to gluten sensitivity mimicking NK-cell lymphoma. In that study, the 32-year-old male patient was positive for anti–gliadin antibody and had persistent multiple lesions in the stomach, small bowel, and large bowel for 3 years.23 Two of our 10 patients were tested and found to be negative for anti–gliadin antibodies. Actually, gluten intolerance and celiac disease are extremely rare in Japan. However, the immunophenotype and morphology of the atypical cells of our patients were similar to those observed in the case of the 32-year-old man reported by Vega et al.23 In addition, our cases shared a significant clinical feature with the case reported by Vega et al,23 that is, “self regression.” The lesions of the 32-year-old man persisted for 3 years until he was placed on a gluten- and lactose-free diet, whereas the lesions of our patients did not seem to persist for such an extended period of time. Furthermore, none of our patients were found to have intestinal lesions. These differences might be due to the different stimulants, if any, although we were unable to identify any stimulant(s) in our cases.
Two types of gastric malignant neoplasms, namely, adenocarcinoma and mucosa-associated lymphoid tissue lymphoma, are related to H pylori infection. Nine of the 10 cases were positive for H pylori infection, and 3 of the patients had a history of gastric adenocarcinoma. Normal NK cells were present in both H pylori–infected and uninfected gastric mucosa at approximately 6% and 15% of the infiltrating lymphocytes, respectively.24 Several of our patients received H pylori eradication therapy, and their LyGa was observed to regress. There may be a pathogenetic relationship between H pylori and LyGa. However, ∼ 82% of the Japanese population is infected with H pylori.25 Moreover, even patients who did not undergo eradication therapy exhibited regression of LyGa. In terms of the relation of LyGa with adenocarcinoma, LyGa is more likely to be found in persons who have frequently undergone gastroscopy because LyGa shows no gastric symptoms. Therefore, although these concomitant occurrences appear coincidental, further studies are required for a better understanding of LyGa and its relationship with adenocarcinoma.
Whether LyGa is monoclonal proliferation or not remains a matter of debate. Unlike B or T cells, NK cells do not undergo any specific gene rearrangement, rendering it difficult to determine whether the proliferation of EBV-free NK cells is monoclonal or not. Vega et al23 indicated that the NK-cell proliferation in their study appeared polyclonal because of the heterogeneous expression of the immunoglobulin-like receptors CD158a, CD158b, and CD158e; nevertheless, they could not exclude the possibility of a low-grade neoplasm. Siu et al26 reported that the p73 gene was methylated in 94% of the NK-cell malignancies and that other methylated genes included hMLH1 (63%), p16 (63%), p15 (48%), and RAR β (47%). We analyzed the methylation status of several genes, including p16, p73, DAPK, MGMT, CDH1, and hMLH1, in 2 heterochronically biopsied specimens from case 3 to obtain evidence of monoclonality. No aberrant methylation, however, was found in the examined genes (data not shown). These results reconfirmed that LyGa is different from extranodal NK/T-cell lymphoma, but the results did not serve as evidence for the monoclonality of LyGa. Further investigation with a larger sample size is required to clarify this distinction. Cytogenetic analyses and studies involving the identification of genetic loss/gain (eg, studies involving single nucleotide polymorphism microarray analysis) or point mutations (eg, studies involving next-generation genome sequencing) may be helpful to clarify the biologic natures of LyGa, especially whether LyGa is monoclonal proliferation or not. Procurement of fresh materials for these studies is impeded by spontaneous regression of lesions after the index biopsy; the biopsy specimen is usually fixed in formalin and embedded in paraffin for routine pathologic diagnosis.
LyGa should be regarded as a distinctive clinicopathologic entity and be observed without treatment. However, if not well recognized, LyGa is probably to be histopathologically misdiagnosed as lymphoma. For example, Kikuchi-Fujimoto disease, a self-limiting disorder of unknown cause, is still often mistakenly diagnosed as lymphoma,4 although > 30 years have passed since it was first described in 1972. If LyGa is misdiagnosed as NK/T-cell lymphoma, it might be treated with radical therapeutic procedures, including chemotherapy, radiotherapy, gastrectomy, and stem cell transplantation. In fact, 2 patients of the present series underwent gastrectomy. The remaining 8 patients did not receive any treatment because the staging procedures followed by the initial diagnosis showed that the lesions regressed spontaneously. For 1 patient, however, the first biopsy specimen diagnosed as lymphoma was suspected to have been mistakenly identified to the patient. Fortunately, LyGa shows highly conserved and characteristic features in terms of clinical presentation, morphology, and immunophenotype (immunohistochemistry for CD3, CD5, CD7, CD56, and cytotoxic molecule(s) and EBER in situ hybridization are required to diagnose LyGa). Therefore, as long as LyGa is recognized as a distinct disease concept, there is no scope of misdiagnosis as malignancy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Hiroshi Takahashi, Toshio Kumasaka, Yukiko Itoh, Satoko Hatano, Keiko Yoshimura, Kazuya Kobori, and Takanori Kuwabara and the members of Ganken Ariake Lymphoma Study Group (GALSG) for their advice.
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Authorship
Contribution: K.T. and K.O. conceived the study, collected and analyzed the data, and drafted the paper; M.Y., Y.T., K. Marutsuka, M.N., N.F., T.Y., H.N., F.A., K. Hoshi, K. Matsue, and K. Hatake contributed patient materials and analyzed the data; and S.I. and K.N. performed special studies and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kengo Takeuchi, Pathology Project for Molecular Targets, Cancer Institute, Japanese Foundation for Cancer Research, 3-8-31 Ariake, Koto, Tokyo 135-8550, Japan; e-mail: kentakeuchi-tky@umin.net.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal