Abstract
Advanced systemic mastocytosis (SM) is a rare myeloid neoplasm characterized by uncontrolled accumulation of neoplastic mast cells (MCs) in various organs with consecutive impairment of organ function, drug resistance, and a poor prognosis. Advanced SM may present as smoldering or slowly progressing neoplasm but may also present as rapidly progressing aggressive SM or even as MC leukemia. Approximately half of the patients have an associated hematologic non–MC-lineage disease (SM-AHNMD) or develop an AHNMD over time. Drug resistance may not only result from the KIT mutant D816V that is found in most patients, but also from KIT-independent pro-oncogenic signaling pathways that play a role in disease evolution. In patients with slow progression, advanced SM can often be kept under control for months with interferon-α or 2CdA. By contrast, in rapidly progressing aggressive SM and MC leukemia, even polychemotherapy and hematopoietic stem cell transplantation may fail, which points to the need to develop new drugs and treatment concepts for these patients. In SM-AHNMD, separate treatment plans should be established for the SM component and the AHNMD component of the disease, with recognition that the AHNMD often has to be managed and treated as a secondary and thus a high-risk neoplasm.
Introduction
Systemic mastocytosis (SM) is a clonal hematologic disease involving precommitted and mast cell (MC) committed hematopoietic progenitor cells.1-3 The disease is characterized by abnormal growth and accumulation of MCs in one or more visceral organs.1-5 The bone marrow (BM) is affected in almost all cases.4,5 In more than 80% of all patients, the KIT mutation D816V is detectable in the BM. The mutant is found in all categories of SM.6-10 Nevertheless, clinical features and courses in SM vary greatly and range from completely indolent with normal life expectancy to highly aggressive with short survival times.1-3,11-13 Thus, KIT D816V may be an important “transforming” oncoprotein in SM, but with regard to progression to advanced disease including MC leukemia (MCL), other as yet undefined pro-oncogenic molecules and signaling pathways may play a more decisive role.
The World Health Organization (WHO) classification has defined the following categories of SM: indolent SM (ISM), SM with an associated hematologic non–MC-lineage disease (SM-AHNMD), aggressive SM (ASM), and MCL.14-16 Smoldering SM (SSM), previously listed as a subcategory of ISM, is a new variant characterized by a high burden of MC, signs of dysplasia, and/or myeloproliferation in the BM (not fulfilling criteria of AHNMD), organomegaly, and multilineage involvement.14-17 Patients may stay in a smoldering state for decades. However, in some of these patients, transformation to SM-AHNMD, ASM, or MCL is seen.17 In ISM, disease progression to ASM may also occur, but it is an extremely rare event.
Patients with ISM and stable SSM are treated symptomatically with mediator-targeting drugs, including histamine receptor antagonists, but in general should not be treated with cytoreductive agents, interferon-α (IFN-α), KIT tyrosine kinase inhibitors, cladribine (2CdA), or other targeted (anticancer) drugs (Table 1).14,15,18 Bisphosphonates are prescribed in case of marked osteopenia (T score < −2) or frank osteoporosis (Table 1).18 In several patients with indolent SM, a coexisting allergy may be detected. In these patients, manifestation of allergic symptoms may be more severe (often life-threatening) than in patients without mastocytosis. When allergen-specific immunoglobulin E is detectable, specific immunotherapy may be considered depending on symptoms, patient characteristics, and type of allergen (Table 1).18
Commonly used basic supportive therapy and emergency therapy strategies for patients with SM
| Drug/therapy . | Recommended indication . | Schedule . |
|---|---|---|
| HR1 antagonists* | Mediator-related symptoms | Continuous orally |
| HR2 antagonists* | Gastrointestinal tract symptoms | Continuous orally |
| Proton pump inhibitors | Gastrointestinal tract symptoms | As needed orally |
| Cromolyn sodium or other MC stabilizers | Gastrointestinal tract symptoms | As needed orally |
| Glucocorticosteroids | Mediator-related symptoms and anaphylaxis not responsive to HR1/HR2 antagonists and MC-stabilizing agents | Short courses orally or intravenously |
| Bisphosphonates* | T score < −2, osteolyses | Continuous orally or intravenously |
| Allergen immunotherapy† | Allergy (IgE+)† | Lifelong for venom |
| Epinephrine pen | Anaphylactic shock | Emergency, subcutaneously |
| Drug/therapy . | Recommended indication . | Schedule . |
|---|---|---|
| HR1 antagonists* | Mediator-related symptoms | Continuous orally |
| HR2 antagonists* | Gastrointestinal tract symptoms | Continuous orally |
| Proton pump inhibitors | Gastrointestinal tract symptoms | As needed orally |
| Cromolyn sodium or other MC stabilizers | Gastrointestinal tract symptoms | As needed orally |
| Glucocorticosteroids | Mediator-related symptoms and anaphylaxis not responsive to HR1/HR2 antagonists and MC-stabilizing agents | Short courses orally or intravenously |
| Bisphosphonates* | T score < −2, osteolyses | Continuous orally or intravenously |
| Allergen immunotherapy† | Allergy (IgE+)† | Lifelong for venom |
| Epinephrine pen | Anaphylactic shock | Emergency, subcutaneously |
HR indicates histamine receptor; and IgE, immunoglobulin E.
These drugs are also prescribed as prophylactic therapy in patients with SM. Examples of commonly used HR1 and HR2 antagonists include: long-acting HR1 antagonists fexofenadine, cetirizine, or loratadine, which may be supplemented with short-acting drugs, such as diphenhydramine or hydroxyzine (the latter have sedating effects); HR2 antagonists commonly used include ranitidine, famotidine, or cimetidine.
Most commonly recommended for patients with hymenoptera venom allergy.
Mediator-associated problems occur quite frequently in ISM but are also seen in patients with advanced SM. However, in the latter group of patients, the predominant clinical problem is usually not related to mediator-associated symptoms but to the proliferation and often aggressive growth of MCs in diverse organs leading to organomegaly and organopathy, or even organ failure, also referred to as C-findings.19-21 In ASM and MCL, organ systems typically involved with C-findings are the BM (marked cytopenia), liver (hepatomegaly, ascites, increased liver enzymes), bones (osteolysis, pathologic fractures), and the gastrointestinal tract (malabsorption, weight loss; Table 2).4,19-21 An important aspect of ASM is that the disease can present as slowly progressing ASM (similar to SSM but with C-findings) or rapidly progressing ASM.17-19 The latter may behave like MCL and can progress to frank MCL within a short time.22 Another important aspect is that, during progression of ASM and MCL, the KIT mutant D816V may disappear,23 which is best explained by selection and transformation of more malignant subclones derived from a more primitive neoplastic stem cell compartment where KIT D816V is not expressed.
Typical symptoms and findings in various categories of SM
| Symptom/finding . | Typically found in . | |||
|---|---|---|---|---|
| ISM . | SSM . | ASM . | MCL . | |
| Mediator-related | ||||
| Hypotension and shock | + | + | +/− | +/− |
| Peptic ulcer disease | +/− | +/− | +/− | +/− |
| Headache and diarrhea | + | + | + | + |
| Organ-specific | ||||
| Maculopapular skin lesions | + | + | +/− | −/+ |
| Osteopenia/osteoporosis | +/− | +/− | −/+ | − |
| B-findings | ||||
| Hepatosplenomegaly | − | + | +/− | +/− |
| Lymphadenopathy | − | +/− | +/− | −/+ |
| Myeloproliferation or dysplasia | − | +/− | +/− | −/+ |
| C-findings | ||||
| Marked cytopenia | − | − | + | + |
| Osteolysis with or without pathologic fractures | − | − | + | + |
| Ascites and elevated liver enzymes | − | − | + | + |
| Malabsorption plus hypoalbuminemia | − | − | + | + |
| Symptom/finding . | Typically found in . | |||
|---|---|---|---|---|
| ISM . | SSM . | ASM . | MCL . | |
| Mediator-related | ||||
| Hypotension and shock | + | + | +/− | +/− |
| Peptic ulcer disease | +/− | +/− | +/− | +/− |
| Headache and diarrhea | + | + | + | + |
| Organ-specific | ||||
| Maculopapular skin lesions | + | + | +/− | −/+ |
| Osteopenia/osteoporosis | +/− | +/− | −/+ | − |
| B-findings | ||||
| Hepatosplenomegaly | − | + | +/− | +/− |
| Lymphadenopathy | − | +/− | +/− | −/+ |
| Myeloproliferation or dysplasia | − | +/− | +/− | −/+ |
| C-findings | ||||
| Marked cytopenia | − | − | + | + |
| Osteolysis with or without pathologic fractures | − | − | + | + |
| Ascites and elevated liver enzymes | − | − | + | + |
| Malabsorption plus hypoalbuminemia | − | − | + | + |
How we diagnose and classify patients with advanced SM
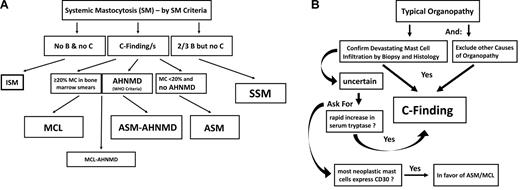
The diagnosis of SM is established using SM criteria provided by the WHO.14-16 At least one major and one minor or at least 3 minor SM criteria have to be fulfilled to establish the diagnosis of SM.14-16 After having diagnosed SM, the first 2 questions to be answered are: (1) whether the patient indeed has advanced SM leading to end-organ damage (C-findings) and (2) whether an addi-ional hematologic non–MC-lineage neoplasm is detectable (SM-AHNMD; Figure 1A). C-findings should lead to the assumption that the patient has ASM or MCL and thus will require a cytoreductive drug memory hook (eg, C = consider cytoreduction).14-16 It is important to note that SM-related organ damage only counts as a C-finding when the histology demonstrates a devastating MC infiltrate as a primary cause of organ damage, and other causes for organ damage have been excluded (Figure 1B). It is also important to know that one single C-finding is sufficient to diagnose advanced SM (requiring antineoplastic therapy). However, in most patients with ASM, multiple C-findings are recorded.14-16 In case the relationship between organopathy and SM is in question, serum tryptase levels should be reviewed: a rapid increase in serum tryptase levels supports the notion that the patient has ASM or MCL (Figure 1B). Another supportive marker is the phenotype of MC. Indeed, it has been described that CD30 is preferentially expressed in neoplastic MCs in ASM and MCL, and less abundantly in MCs in ISM.24-26 In other words, strong expression of CD30 in most neoplastic MC is in favor of the diagnosis ASM or MCL, and we expect that CD30 will serve as a potential immunohistochemical grading marker in SM in the future.
Diagnosis of advanced SM. (A) Diagnostic algorithm in advanced SM. After having established the diagnosis SM by SM criteria, the patient is examined for the presence of B-findings and C-findings. C-findings are indicative of severe organ damage caused by the SM-infiltrate and are typically found in ASM and MCL, but not in ISM or SSM. The smoldering variant is defined by the presence of at least 2 B-findings. To differentiate between ASM and MCL, the BM and peripheral blood smear have to be examined. When the percentage of MCs in the BM smear is more than or equal to 20%, the diagnosis is MCL. (B) Definition of C-finding. End-organ damage in SM is diagnosed in a stepwise manner. First, organopathy is judged as being typical for advanced SM (eg, huge osteolyses plus bone fractures, enlarged liver with ascites, SM-based severe cytopenia). In a second step, a histology of the affected organ is obtained to confirm that organ damage is indeed caused by a devastating mast MC infiltrate. Then, organ damage can be regarded as a C-finding and the final diagnosis is established: ASM or MCL with or without an AHNMD.
Diagnosis of advanced SM. (A) Diagnostic algorithm in advanced SM. After having established the diagnosis SM by SM criteria, the patient is examined for the presence of B-findings and C-findings. C-findings are indicative of severe organ damage caused by the SM-infiltrate and are typically found in ASM and MCL, but not in ISM or SSM. The smoldering variant is defined by the presence of at least 2 B-findings. To differentiate between ASM and MCL, the BM and peripheral blood smear have to be examined. When the percentage of MCs in the BM smear is more than or equal to 20%, the diagnosis is MCL. (B) Definition of C-finding. End-organ damage in SM is diagnosed in a stepwise manner. First, organopathy is judged as being typical for advanced SM (eg, huge osteolyses plus bone fractures, enlarged liver with ascites, SM-based severe cytopenia). In a second step, a histology of the affected organ is obtained to confirm that organ damage is indeed caused by a devastating mast MC infiltrate. Then, organ damage can be regarded as a C-finding and the final diagnosis is established: ASM or MCL with or without an AHNMD.
The final diagnosis in patients with advanced SM has to be based on the percentage of MCs in the BM smear and the presence or absence of an AHNMD (Figure 1A).14-16 In those with MCL, the percentage of MCs in the BM smear is more than or equal to 20%.14-16 Here the differential counts for MCs should be performed in areas sufficiently away from marrow spicules, and it is also important to note that this 20% threshold only counts in the smear but not in the BM histology. In other words, an infiltration grade of 50% in the histology can well be found in ISM but is not a sign or criterion for MCL or ASM.14-16 The other important checkpoint is AHNMD (Figure 1A). Here it is mandatory to apply further disease-related markers and to classify the AHNMD according to WHO criteria. Finally, both the SM and the AHNMD components of the disease are examined for expression of potential drug targets. Sometimes the AHNMD harbors KIT D816V, which is important as this mutant introduces resistance to various targeted drugs, including imatinib.27,28 In chronic myelomonocytic leukemia (CMML) associated with SM (SM-CMML), neoplastic monocytes usually display KIT D816V.29,30 In other AHNMDs, however, such as acute myeloid leukemia (AML), AHNMD cells (blast cells) may lack KIT D816V even when MC are KIT D816V+ cells.30,31
How we treat patients with SSM
SSM is defined by the presence of 2 or 3 B-findings, which include (1) a markedly elevated serum tryptase level (> 200 ng/mL), (2) splenomegaly or lymphadenopathy, and (3) BM dysplasia or myeloproliferation.14,15,17 Typically, KIT D816V is detectable in various myeloid lineages reflecting clonal multilineage involvement.17 In most patients with SSM, the course remains stable over years or even decades.17,32-34 For these patients, we recommend to wait and watch B-findings, including the serum tryptase level, which is a reliable marker of monitoring of (S)SM even when levels are highly elevated (sometimes exceeding 1000 ng/mL). SSM may be a “high-risk situation” for several reasons: first, progression to ASM or SM-AHNMD is sometimes found. In these patients, treatment follows the recommendation for newly diagnosed ASM or SM-AHNMD. In other patients, B-findings show progression, but no frank ASM or AHNMD develops.22,33,34 In these patients, it may be necessary to start treatment with a cytoreductive agent. In other patients, splenectomy may be required to reduce the MC burden.35 Although only a few pilot studies have been reported, it appears that one of the few effective agents for these patients is 2CdA.22,36-38 A difficult situation is the occurrence of life-threatening anaphylaxis in patients with otherwise stable SSM. If anaphylaxis is provoked by an (known) allergen, especially hymenoptera venom, immunotherapy should be considered with recognition of potential risks.39,40 Close monitoring is required for systemic reactions during immunotherapy in these patients. If no specific immunoglobulin E can be detected and anaphylaxis is repeatedly observed and life-threatening, and cannot be kept under control using conventional anti–mediator-type drugs, treatment with 2CdA or IFN-α may be justified. Indeed, 2CdA has been described to reduce the burden of KIT D816V+ MCs and thereby the frequency and intensity of anaphylactic reactions in these patients.22 Thus, as in essential thrombocythemia where patients receive cytoreductive agents to reduce the risk for life-threatening thromboembolism (but not to stop disease progression), patients with SSM may sometimes be considered as candidates for 2CdA or IFN-α therapy, to lower the risk of severe life-threatening anaphylaxis. Such treatment should preferentially be performed in controlled clinical trials. Unfortunately, only very few clinical trials have been initiated in SSM or ASM/MCL so far.
How we treat patients with ASM with slow progression
Many patients with ASM with slow progression can be kept under control for several months or even years by IFN-α or 2CdA.22,36-38,41-43 Therefore, we recommend these agents as first-line treatment in these patients. We usually start with prednisolone at 1 mg/kg body weight for 3 to 7 days and then start to add IFN-α (in hospitalized patients) at a dose of 3 million units 3 times a week. Thereafter, we try to reduce the dose of the steroid and to increase the dose of IFN-α to a maximum of 5 million units per day if possible. In patients with ascites or severe mediator-related symptoms, a small maintenance dose of prednisolone (≤ 5-10 mg/day) should be prescribed. Otherwise, prednisolone should be discontinued if possible. Monitoring of treatment responses is performed by repeated evaluation of C-findings, repeated BM investigations, and measurement of serum tryptase levels.43 In those patients who have an unsatisfactory or no response or a relapse, alternative therapy must be considered. We usually recommend to administer 2CdA (0.13 mg/kg per day intravenously over 5 days; 3-6 cycles) as second-line treatment in these patients or to enroll the patient in a clinical trial with a KIT D816V inhibitor, such as PKC412 (midostaurin). In those with rapid progression, more intensive treatment (as for ASM/MCL) is usually required. In patients with osteopathy (osteolysis, osteoporosis, or marked osteopenia: T score < −2) and those who receive long-term glucocorticosteroids, bisphosphonates are prescribed.18 It should be noted here that osteoporosis is a frequent finding in patients with indolent SM and does not qualify as a C-finding unless it is also associated with large osteolytic lesions and pathologic fractures.
In a few patients with slowly progressing ASM, no codon 816 mutation in KIT is detectable, even when BM cells are repeatedly tested.44,45 Some of these patients have other KIT mutations, and these mutants (as well as wild-type KIT) may sometimes respond to imatinib therapy.44,45 By contrast, in patients in whom KIT D816V is detectable, imatinib should not be used as the mutation introduces resistance. Detection of the KIT D816V mutant should ideally be performed in bone marrow aspirates or lesional tissue rather than peripheral blood as false-negative findings are common when (only) blood samples are tested.
How we treat patients with rapidly progressing ASM and MCL
Rapid progression in ASM is defined by (1) rapid deterioration of end-organ function caused by the MC infiltrate and (2) rapid increase in serum tryptase levels.14-16,18-20 Several of these patients progress to MCL within a short time. Responses to IFN-α are usually not seen, and responses to 2CdA usually are short-lived and followed by a relapse.14-16,22,46 Nevertheless, 2CdA is often applied in a first attempt to control the disease. In some of these patients, the burden of MC can indeed be reduced by this drug, at least for a certain time, so that further treatment can be planned.22,46 If the patient is young and has a suitable donor, hematopoietic stem cell transplantation (SCT) is the only chance to induce a stable remission in advanced SM. Before SCT, debulking should be performed by polychemotherapy (polychemotherapy regimens containing high-dose cytosine arabinoside and a nucleoside such as fludarabine) or repeated cycles of 2CdA. With this approach, the physician will learn whether the disease is responsive and whether the patient is fit enough to undergo SCT. Patients who fail 2CdA and polychemotherapy are candidates for experimental drugs. If the disease is completely resistant to chemotherapy and all other drugs, or relapses, palliative cytoreduction (with hydroxyurea) is initiated.14-16,18 The recommendations for treatment of patients with MCL are essentially the same as that for treatment of patients with rapidly progressing ASM. However, in contrast to ASM, 2CdA should not be considered as monotherapy in these patients. Whether patients with MCL may indeed benefit from combination polychemotherapy plus 2CdA remains unknown.
How we treat patients with SM-AHNMD
In approximately 50% of all patients with advanced SM, an AHNMD is diagnosed at presentation or develops during the course of disease.14-16,47 In these patients, both the SM and the AHNMD components of the disease have to be classified according to WHO criteria.14-16 In most cases, the KIT mutation D816V is detectable. Sometimes the mutant is not only expressed in MC, but also in AHNMD cells, depending on the type of AHNMD. In SM-CMML, neoplastic monocytes usually display KIT D816V.29,30 By contrast, in SM-AML, AML blasts often lack KIT D816V. Almost all types of hematopoietic neoplasms can develop as AHNMD in SM.14-16,47 However, whereas myeloid neoplasms are more commonly detected, B-cell neoplasms are less frequent (estimated frequency: 10% of all AHNMD), and a concomitant T-cell neoplasm represents an exceptional rarity, a distribution that is reminiscent of lineage-involvement in the blast phase of CML. The SM component in SM-AHNMD often presents as ASM and less frequently as MCL. A rather frequent constellation is ASM-CMML.14-16,47,48 From a practical viewpoint, it seems straightforward to treat the SM component of the disease as if no AHNMD was detected and the AHNMD component as if no SM was diagnosed,14-18 with one important exception: indeed, any type of AHNMD must be regarded as having developed secondary to SM, which may be of prognostic importance. Likewise, in SM-AML, AML should be treated as other patients with secondary (not de novo) and thus high-risk AML. Indeed, for most of these patients, high-dose chemotherapy and SCT are recommended.46-48 With regard to AML, it is also important to note that many patients with SM-AML are (mis)diagnosed as KIT D816V+ AML because the SM component has just been overlooked or the proliferation of AML blasts overwhelms the slower-growing coexisting SM component (occult SM).31 In some of these patients, SM can be more readily recognized when blast cells cleared after cytoreductive therapy for AML. Today, we recommend that bone marrow cells are examined for the presence of KIT D816V in all patients with AML and CMML. In a few patients with SM-AHNMD, the JAK2 mutant V617F is detected in AHNMD cells.49 In these patients, the AHNMD is often classified as primary myelofibrosis.49 Other patients have SM-PV or SM-MDS.46,47 Coexistence of SM with Ph+ CML is an extremely rare condition.
A special situation is SM with associated chronic eosinophilic leukemia (SM-CEL).50 These patients often have mildly or markedly elevated tryptase levels and spindle-shaped MCs expressing CD25. Neoplastic eosinophils often outnumber MCs in these patients, so that only CEL is diagnosed. In other patients, no SM is detectable, even after a thorough investigation. In most cases, the FIP1L1/PDFRA fusion gene product, but no KIT mutation, is detectable, even when SM is diagnosed. However, this does not mean that FIP1L1/PDGFRA is a molecular marker of SM.50 In rare cases, both mutants (FIP1L1/PDGFRA and KIT D816V) are detectable (P.V., unpublished observation, June 2010). In most patients, SM is classified as ISM, and only the CEL component requires antineoplastic therapy. As in CEL without SM, patients with SM-CEL start with imatinib (100 mg daily), a drug to which they are exquisitely responsive.21
Novel drugs and novel treatment approaches for ASM and MCL
Several novel drugs and approaches have been proposed for the treatment of patients with ASM and MCL (Table 3). Some of these concepts are based on the inhibition of KIT D816V in neoplastic cells. Examples for drugs blocking the tyrosine kinase activity of KIT D816V are dasatinib and PKC412 (midostaurin).21,23,45 Both drugs can also suppress histamine secretion from MCs. The disadvantage of dasatinib is its short half-life and its side effect profile. Indeed, dasatinib has yielded largely disappointing results in clinical trials in SM performed thus far.51,52 PKC412 is currently tested in clinical trials in patients with ASM and MCL. However, although KIT D816V+ subclones may respond, long-lasting effects have not been described, and it may happen that the patient has an early relapse with a KIT D186V− subclone.23 Other drugs block critical KIT-downstream signaling molecules, such as phosphatidylinositol 3 kinase or mammalian target of rapamycin (Table 3). However, in ASM and MCL, additional KIT-independent signaling molecules and pathways may play a more important, or even decisive, role in growth of neoplastic cells. Therefore, more recent treatment concepts focus on various signaling pathways and combinations of drugs targeting KIT D816V and other critical targets in SM.
Antiproliferative drugs used to treat patients with advanced systemic mastocytosis
| Drug . | Effects seen in SSM and slowly progressing ASM . | Effects seen in ASM or MCL with rapid progression . |
|---|---|---|
| Interferon-α | Responses including MR in a group of patients | Usually no responses seen |
| 2CdA | Responses frequently seen; MR in a group of patients | Transient responses in a few patients |
| PKC412 | Studies ongoing | Studies ongoing and transient responses in some cases23 |
| Dasatinib | Studies ongoing | Studies ongoing |
| Polychemotherapy | — | May induce remission or debulking before SCT |
| Experimental drugs | ||
| mTOR blockers | Studies ongoing | Studies ongoing |
| PI3 kinase blockers | Studies planned | Studies planned |
| Drug . | Effects seen in SSM and slowly progressing ASM . | Effects seen in ASM or MCL with rapid progression . |
|---|---|---|
| Interferon-α | Responses including MR in a group of patients | Usually no responses seen |
| 2CdA | Responses frequently seen; MR in a group of patients | Transient responses in a few patients |
| PKC412 | Studies ongoing | Studies ongoing and transient responses in some cases23 |
| Dasatinib | Studies ongoing | Studies ongoing |
| Polychemotherapy | — | May induce remission or debulking before SCT |
| Experimental drugs | ||
| mTOR blockers | Studies ongoing | Studies ongoing |
| PI3 kinase blockers | Studies planned | Studies planned |
All antiproliferative drugs used are experimental in nature.
MR indicates major clinical response; —, not applicable; mTOR, mammalian target of rapamycin; and PI3, phosphatidylinositol 3.
Response evaluation
Treatment responses in ASM and MCL should be evaluated by using criteria proposed by the consensus group.11,18 The following response categories are defined: a major response (disappearance of C-findings), a partial response (partial resolution of C-findings), and no response.11,18 MR can further be divided into a complete remission (complete disappearance of MC infiltrates and return of serum tryptase levels to < 20 ng/mL), incomplete remission (inomplete decrease in MC infiltrates and tryptase), and a pure clinial response (no decrease in MC infiltrates and tryptase levels). Partial response is further divided into a good partial response (> 50% regression of C-findings) and minor response. Finally, NR is divided into stable disease and progressive disease.11,18 Thus, responses are currently adapted to C-findings, which seems justified as most drugs are not inducing complete remissions. An important aspect in this evaluation is that C-findings may sometimes remain, although tryptase levels markedly decrease and MC infiltration is markedly reduced. In these patients, it may turn out that the clinical finding can no longer be judged as C-finding as the definition is no longer fulfilled (invasive MC infiltrate disappeared). Therefore, the evaluation of responses using C-finding criteria requires knowledge and some experience in the field, and exact application of all criteria. Another important aspect in response evaluation is that mediator-related symptoms may also respond to some of the cytoreductive (targeted) drugs applied in ASM and MCL. Likewise, as mentioned, some of these agents, like PKC412 or dasatinib, may block immunoglobulin E-dependent histamine secretion from MCs; and mediator-related symptoms may improve with 2CdA therapy. Therefore, it is also important to grade mediator-related symptoms in these patients and to determine responses using available response criteria. Again, it is recommended that the response criteria provided by the consensus group are applied.18
Perspectives in therapy of advanced SM: the foreseeable future
Treatment of advanced SM remains one of the most challenging areas in clinical hematology. Whereas mediator-related symptoms can be controlled in most cases, MC expansion is usually resistant to most conventional antineoplastic drugs. In nontransplantable patients exhibiting KIT D816V, some good, albeit transient, responses to 2CdA, PKC412, and polychemotherapy regimens containing high-dose cytosine arabinoside have been described. These approaches will hopefully be combined with each other and with transplantation strategies in the future, with the hope to achieve stable long-term remissions. There is also hope that novel more potent agents will be developed that can block not only KIT D816V-dependent signaling pathways in neoplastic MC, but also KIT-independent pro-oncogenic pathways that appear to play a predominant role in disease progression in ASM and MCL. Finally, with better knowledge and increased awareness, therapy of ASM and MCL should improve through earlier and more accurate diagnosis and early treatment intervention.
Acknowledgments
This work was supported by the Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich (grants P21173-B13 and SFB-F01820) and the Medical University of Vienna (Mastocytosis Research Grant).
Authorship
Contribution: P.V., W.R.S., and C.A. wrote the paper and approved the final version of manuscript.
Conflict-of-interest disclosure: P.V. received research grants from Novartis and Bristol-Myers Squibb and is Novartis consultant in a PKC412 project. C.A. has a consultancy agreement with Novartis for a PKC412 trial. W.R.S. declares no competing financial interests.
Correspondence: Peter Valent, Department of Internal Medicine I, Division of Hematology & Hemostaseology, Medical University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria; e-mail: peter.valent@meduniwien.ac.at.