Abstract
Talin1 is a key integrin coactivator. We investigated the roles of this cytoskeletal adaptor and its target integrins in B-cell lymphogenesis, differentiation, migration, and function. Using CD19 Cre-mediated depletion of talin1 selectively in B cells, we found that talin1 was not required for B-cell generation in the bone marrow or for the entry of immature B cells to the white pulp of the spleen. Loss of talin1 also did not affect B-cell maturation into follicular B cells but compromised differentiation of marginal zone B cells. Nevertheless, serum IgM and IgG levels remained normal. Ex vivo analysis of talin1-deficient spleen B cells indicated a necessary role for talin1 in LFA-1 and VLA-4 activation stimulated by canonical agonists, but not in B-cell chemotaxis. Consequently, talin1 null B splenocytes could not enter lymph nodes nor return to the bone marrow. Talin1 deficiency in B cells was also impaired in the humoral response to a T cell-dependent antigen. Collectively, these results indicate that talin1 is not required for follicular B-cell maturation in the spleen or homeostatic humoral immunity but is critical for integrin-dependent B lymphocyte emigration to lymph nodes and optimal immunity against T-dependent antigens.
Introduction
Proper circulation of B lymphocytes between primary and secondary lymphoid organs is critical for their differentiation and function in the establishment of normal humoral protection.1-3 In adult mice, B-cell precursors rearrange their immunoglobulin (Ig) genes and differentiate into immature B cells in the bone marrow (BM).4,5 After negative selection, these cells egress from BM niches, enter the circulation, and home to the spleen where they reach final maturation or die.6 Immature spleen B cells can develop into either marginal zone (MZ) B cells or follicular B cells based on signaling through the B-cell receptor, Notch2, and the canonical nuclear factor-κB pathway.7 MZ B cells are a sessile population that reside at the border between the white and red pulps, and respond vigorously to blood-borne T cell-independent antigens.8 Follicular (FO) B cells are generated from immature B cells entering the white pulp3 and that differentiate into transitional 1 (T1) and transitional 2 (T2) cells based on their surface phenotype and functional characteristics.9,10 The T1 > T2 > FO pathway is considered the main B-cell differentiation pathway in the spleen and is critical for the ability of B cells to respond to blood-borne T-dependent antigens.11 A fraction of the mature follicular B lymphocytes leave the spleen and circulate in peripheral lymph nodes and mucosal-associated lymphoid tissues. Subsets of spleen and lymph node B cells can also home back to the BM, mainly after encountering antigen, and they differentiate into plasma or memory subsets.12
Similar to T-cell circulation through various lymphoid organs, the entry of B subsets into the BM, lymph nodes, and the spleen involves their crossing of different types of endothelial barriers.13 A key checkpoint in this emigration of both T and B cells is their ability to firmly attach to and protrude through the endothelial cell barrier lining these organs.14 Whereas leukocyte intravasation (egress) into blood15 is thought to take place independently of integrin-ligand interactions, B lymphocyte extravasation from the blood is thought to be mediated by distinct integrin-ligand interactions that generate shear-resistant adhesions.14,16 Nevertheless, once in the extravascular tissues, interstitial motility of B lymphocytes, like other leukocytes, is thought to take place independently of integrins via chemokine cues.17,18
Accumulating studies have established a key role for 3 B-cell integrins, VLA-4 (α4β1), α4β7, and LFA-1 (αLβ2) in the trafficking of mature B cells to lymph nodes, the BM,19 and peripheral sites of inflammation.20 Although LFA-1 has a key role in B-cell migration to peripheral lymph nodes and Peyer patches,21 it plays redundant roles in lymphocyte entry to the spleen and its white pulp.2,21 Similarly, although the roles of α4 integrins in B-cell trafficking to mucosal tissues and the BM are well established,22 these integrins, or their main endothelial ligands VCAM-1 and MAdCAM-1, have been suggested to be dispensable for B-cell entry to the spleen2,22 because of overlapping contributions of LFA-1 in this process.2 Overall, it remains unclear whether combined suppression of all 3 integrins would block B-cell entry to, and differentiation within, specific lymphoid organs and impair their specific humoral responses. Addressing these and other questions related to the contribution of integrins to B-cell proliferation, maturation, trafficking, and activation in vivo would require simultaneous knockdown of VLA-4, α4β7, and LFA-1 integrins, which is not experimentally feasible. We therefore took an alternative approach to simultaneously inhibit the adhesive functions of these key α4 and β2 integrins in B cells by deleting their expression of talin1.18
Talin1 is the predominant talin family member in B lymphocytes.23 It is a 270-kDa dimeric integrin tail and actin-binding focal adhesion protein that links integrins to the actin cytoskeleton24 and is critical for high integrin affinity to ligands.25 The critical role of talin1 in integrin function in platelets has been recently established in vivo.26,27 A recent study on ex vivo differentiated BM dendritic cells (DCs) indicated that depletion of talin1 in DCs resulted in complete loss of integrin function.18 We therefore adopted a similar approach to interfere with global integrin functions in B cells, by genetically ablating talin1 in all CD19-expressing B lineage cells using a CD19-driven Cre system.28 The maturation and trafficking of different subsets of B cells generated in these mice were carefully dissected both in naive mice and in mice exposed to a T-dependent antigen. Our results reveal major defects in integrin-dependent trafficking of talin1 null B cells to lymph nodes and the BM but largely conserved entry of talin1 null B cells to the spleen, with overall normal maturation of follicular subsets in the splenic white pulp. Nevertheless, mice deficient for talin1 in their B cells exhibit major defects in mounting efficient humoral responses to a peripherally introduced T-dependent antigen. Thus, talin1 and its major targets, α4 and β2 integrins, although dispensable both for B-cell development and their ability to mount systemic humoral responses, are required for B-cell entry to lymph node and the BM and for optimal humoral immunity to peripheral T-dependent antigens.
Methods
Reagents and antibodies, adoptive cell transfer, serum Ig determination, Western blot analysis, and immunohistochemistry protocols are all described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Mice and lymphocyte isolation
All animal procedures were approved the Institutional Animal Care and Use Committee at the Weizmann Institute of Science. Conditional talin1-deficient mice were generated by crossing CD19Cre mice hemizygous for talin1 with mice harboring a single talin1 allele flanked by loxP sites (Tln1−/fl) to achieve conditional deletion of Talin1 in B cells. The resultant CD19Cre/+Tln1−/fl mice, referred to as CD19Tln1−/− mice, were genotyped by polymerase chain reaction, as described.26 Cre-negative Tln1wt/fl mice (CD19+/+Tln1+/fl) were used throughout the study as the wild-type (WT) counterpart of CD19Tln1−/− mice. In some experiments, B cells from CD19Cre/+Tln1+/fl were used as controls and validated to display normal integrin activation properties (supplemental Figure 1). Murine lymphocytes were obtained from the various mice at 6 to 8 weeks of age, as previously described.29 B cells were purified from each mouse strain using CD45R (B220) beads (BD Biosciences). The purity of the B cells (96%-99%) was analyzed by fluorescence-activated cell sorter (FACS) after each experiment.
Flow cytometric assays
Isolated lymphocytes were incubated with primary antibodies (10 μg/mL) for 30 minutes at 4°C, washed, and incubated with secondary antibodies for 30 minutes at 4°C. Cells were washed and immediately taken for FACS analysis (BD Biosciences). For analysis of 5-bromo-2-deoxyuridine (BrdU) incorporation, mice were injected intraperitoneally with 10μM BrdU (Sigma-Aldrich). The percentage of BrdU-positive (proliferating) cells was determined by FACS analysis after 22 hours using the BrdU Flow Kit according to the manufacturer's instructions (BD Biosciences PharMingen).
Laminar flow adhesion and chemotaxis assays
Adhesion assays were performed on polystyrene plates either directly coated with the ligands mVCAM-Fc or mICAM-Fc, or with plates precoated with protein A before addition of ligand.30 Ligand densities were determined using radiolabeled monoclonal antibodies (mAbs), as described.30 Substrate-coated polystyrene plates were each assembled on the lower wall of a standard flow chamber (260-μm gap), as described.30 Bead isolated B lymphocytes were washed with H/H (HBSS containing 2 mg/mL bovine serum albumin [BSA] and 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4), resuspended in binding medium (H/H medium supplemented with 1mM CaCl2 and 1mM MgCl2), pretreated for 1 minute with agonists of interest, and perfused through the flow chamber at 37°C. Attachments were defined as “transient” if cells attached briefly (< 2 seconds) to the substrate, and as “arrests” if they remained stationary during at least 3 seconds of continuous flow.30 The murine brain-derived endothelial cell line BEnd.3 was plated at confluence on tissue culture dishes coated with fibronectin (20 μg/mL in phosphates buffered saline [PBS]), and cells were stimulated for 24 to 30 hours with tumor necrosis factor-α (500 U/mL). Where indicated, cells were suspended with function blocking anti-α4 (PS/2, Southern Biotechnology) or anti–LFA-1 (α-αL) mAb (I21/7, from Serotec) and perfused unwashed into the flow chamber. Chemotaxis assays toward CXCL12 and CXCL13 were performed in RPMI 1640 with 0.5% BSA using transwell culture inserts with 5-μm pore size (Corning Life Sciences) for 3 hours at 37°C in 5% CO2.
Immunization
Mice were immunized via the footpad with 25 μg of keyhole limpet hemocyanin conjugated to trinitrophenyl (KLH-TNP). After 2 and 4 weeks, immunization was repeated intraperitoneally, and both antigen and hapten specific antibodies were determined in the sera 2 weeks after the last immunization.
Statistical analysis
All data are reported as mean values plus or minus SD or range and were analyzed by a 2-tailed Student t test with equal sample variance. Differences between datasets were considered significant at P less than .05.
Results
B cell–specific deletion of talin1 impairs integrin activation by nonchemotactic inside-out agonists
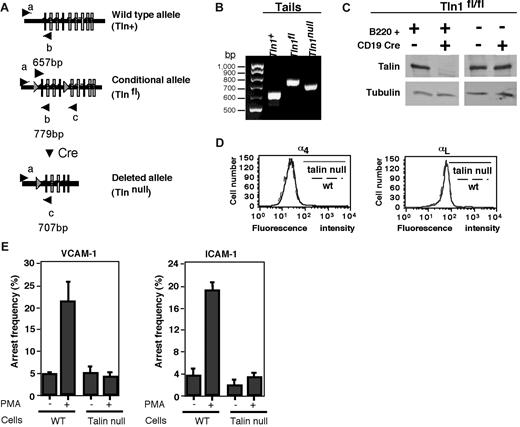
Disruption of the talin gene (Tln1) results in embryonic lethality.31 We therefore generated B cell–specific conditional talin1-deficient mice by crossing hemizygous mice harboring a single talin1 allele flanked by loxP sites (Tln1−/fl) with CD19Cre mice (CD19Cre/+).28 CD19 is transcribed exclusively in cells of the B lineage and is expressed at the earliest stages and throughout B-cell development and differentiation. Deletion of the floxed Tln1 allele was confirmed by polymerase chain reaction (Figure 1A-B), and nearly total loss of talin1 protein expression was validated in splenic B220+ CD19+ B cells derived from these mice (CD19Cre/+Tln1−/fl) herein referred to as CD19Tln1−/− mice, but not in their Cre-negative Tln1+/fl mice counterparts (CD19+/+Tln1+/fl herein “WT mice,” Figure 1C). Talin1 expression remained negligible up to 14 weeks after birth in all B220+ leukocytes but appeared normal in all B220− leukocytes (Figure 1C). Furthermore, BM from CD19Tln1−/− mice reconstituted into lethally irradiated WT recipients also gave rise to talin1-deficient B cells (not shown). Importantly, talin1 null B lymphocytes recovered from the spleens of CD19Tln1−/− mice were found to express normal levels of LFA-1 and of α4 integrins (Figure 1D).
Deletion of talin1 in B lineage cells using CD19 Cre targeting abrogates integrin activation. (A) Schematic representation of Tln1 WT (Tln+), loxP flanked (Tlnfl), and deleted (Tlnnull) alleles. The deletion of exons 1 to 4 takes place in B cells when Cre expression is driven by CD19. (B) Identification of Tln+ (657bp), Tlnfl(779bp), and Tlnnull (707bp) alleles by polymerase chain reaction. (C) Immunoblot for talin1 and tubulin in B220+ and B220− splenocytes derived from CD19Cre/+Tln1fl/fl mice. (D) FACS staining for expression of α4 and αL integrins in spleen-derived B cells from WT or CD19Cre/+Tln1−/fl (talin null) mice. For clarity, all isotype-matched negative controls, which yielded fluorescence intensity levels less than 10 arbitrary units, were omitted. (E) Frequency of arrest of WT and Tln1−/− B cells on VCAM-1 or on ICAM-1 triggered by 1-minute exposure to phorbol myristate acetate. Arrests were measured at a shear stress of 0.75 dyne/cm2 (VCAM-1) or 0.5 dyne/cm2 (ICAM-1). Results are the mean values ± range of 2 fields of view in 1 experiment representative of 4 independent experiments.
Deletion of talin1 in B lineage cells using CD19 Cre targeting abrogates integrin activation. (A) Schematic representation of Tln1 WT (Tln+), loxP flanked (Tlnfl), and deleted (Tlnnull) alleles. The deletion of exons 1 to 4 takes place in B cells when Cre expression is driven by CD19. (B) Identification of Tln+ (657bp), Tlnfl(779bp), and Tlnnull (707bp) alleles by polymerase chain reaction. (C) Immunoblot for talin1 and tubulin in B220+ and B220− splenocytes derived from CD19Cre/+Tln1fl/fl mice. (D) FACS staining for expression of α4 and αL integrins in spleen-derived B cells from WT or CD19Cre/+Tln1−/fl (talin null) mice. For clarity, all isotype-matched negative controls, which yielded fluorescence intensity levels less than 10 arbitrary units, were omitted. (E) Frequency of arrest of WT and Tln1−/− B cells on VCAM-1 or on ICAM-1 triggered by 1-minute exposure to phorbol myristate acetate. Arrests were measured at a shear stress of 0.75 dyne/cm2 (VCAM-1) or 0.5 dyne/cm2 (ICAM-1). Results are the mean values ± range of 2 fields of view in 1 experiment representative of 4 independent experiments.
Phorbol myristate acetate is a potent agonist of DAG-dependent PKCs, positive regulators of inside-out integrin activation critical for firm integrin adhesiveness in many hematopoietic cells triggered by nonchemotactic cytokine receptors and immunoreceptors.32 We therefore asked whether this potent regulator of integrins requires talin1 for its inside-out stimulatory activity. Strikingly, whereas a short exposure of WT B cells (either CD19+/+Tln1+/fl or CD19Cre/+Tln1+/fl) to phorbol myristate acetate triggered robust VLA-4 and LFA-1 adhesion to their respective ligands, VCAM-1 and ICAM-1, respectively, a similar exposure of talin1 null B cells failed to induce any detectable integrin activation (Figure 1E;supplemental Figure 1). B-cell receptor (BCR) ligation also failed to activate either VLA-4 or LFA-1 adhesiveness in talin1 null B cells (supplemental Figure 2). These data collectively suggest a critical role for talin1 in rapid inside-out integrin activation in B lymphocytes by nonchemotactic agonists.
Integrin activation by chemokines is abrogated in the absence of talin1 despite normal chemotactic responsiveness of talin-deficient B cells to chemokines
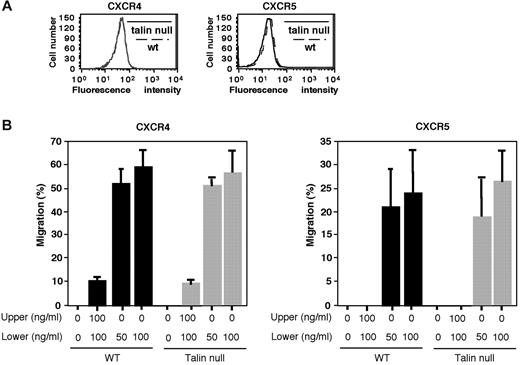
In light of the proposed involvement of LFA-1 and α4 integrins in B-cell trafficking, we next analyzed the migratory and adhesive properties of talin1-deficient and WT B220+ splenocytes ex vivo. We first compared the ability of talin1-deficient and WT B cells to migrate along gradients of 2 prominent B-cell chemokines, CXCL12 and CXCL13. Normal expression of the primary CXCL12 receptor, CXCR4, and the CXCL13 receptor, CXCR5, was confirmed on total B cells derived from CD19Tln1−/− spleens (Figure 2A). Chemotaxis toward different doses of CXCL12 or CXCL13 determined in transwell assays was comparable between talin1 null and WT B cells (Figure 2B). Thus, intrinsic signaling of CXCR4 and CXCR5 to the cytoskeletal machineries essential for B-cell motility and directionality does not require talin1.
B-cell chemotaxis does not require talin1. (A) FACS staining for CXCR4 (left) and CXCR5 (right) expression on CD19Tln1−/− mice (talin null) and WT spleen-derived B cells. (B) The B cells analyzed in panel A were compared for their ability to migrate toward different concentrations of CXCL12 (left) and CXCL13 (right). Results are the mean ± SD of triplicates and are representative of 2 independent experiments.
B-cell chemotaxis does not require talin1. (A) FACS staining for CXCR4 (left) and CXCR5 (right) expression on CD19Tln1−/− mice (talin null) and WT spleen-derived B cells. (B) The B cells analyzed in panel A were compared for their ability to migrate toward different concentrations of CXCL12 (left) and CXCL13 (right). Results are the mean ± SD of triplicates and are representative of 2 independent experiments.
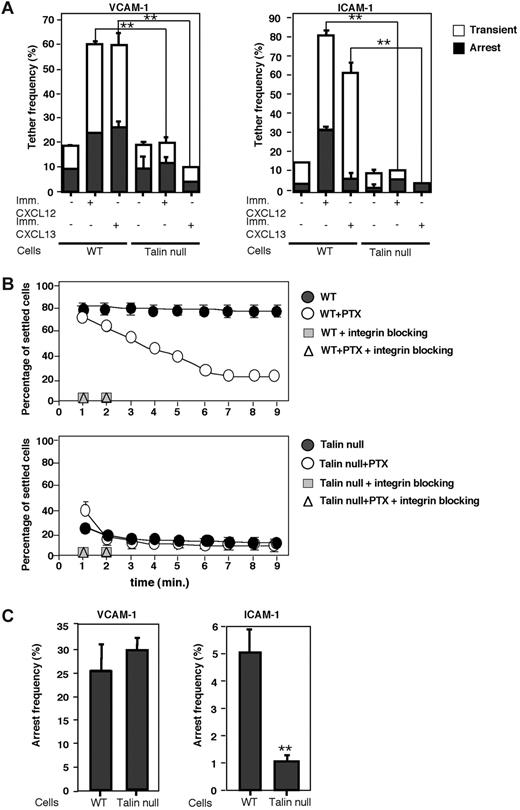
Because talin1 null B cells remained responsive to CXCL12 and CXCL13, we next assessed the ability of the 2 major B-cell integrins, VLA-4 and LFA-1, to undergo in situ activation by these chemokines. Neither CXCL12 nor CXCL13 could stimulate any VLA-4 or LFA-1 adhesiveness to their cognate endothelial ligands, VCAM-1 or ICAM-1, under shear flow in talin1 null spleen B cells (Figure 3A). In contrast, robust VLA-4 and LFA-1 activation by these chemokines was observed in talin1-expressing B cells derived from either CD19+/+Tln1+/fl or CD19Cre/+Tln1+/fl mice (Figure 3A; supplemental Figure 1). Talin1 null B lymphocytes also failed to develop any Gi protein-stimulated (pertussis toxin [PTX]-inhibited) integrin-dependent adhesion strengthening when settled on tumor necrosis factor-α-stimulated murine endothelial cells, which express high levels of both LFA-1 and VLA-4 ligands33 (Figure 3B). In contrast, WT B cells developed robust adhesion to identical endothelial cells, and blockage of their Gi-protein machineries by PTX pretreatment resulted in significant inhibition of adhesiveness (Figure 3B). Surprisingly, however, the ability of VLA-4 to spontaneously arrest spleen B cells on high- or low-density VCAM-1 in the absence of inside-out signals was not affected by loss of talin1, whereas spontaneous LFA-1-mediated arrests on high-density ICAM-1 developed by these B cells were greatly reduced (Figures 1E, 3C; supplemental Figure 1). Collectively, these results suggest an indispensable role for talin1 in physiologic settings where chemokine-triggered VLA-4- and LFA-1-mediated B lymphocyte adhesion to endothelial ligands can contribute to their extravasation across blood vessels.
Inside-out activation of VLA-4 and LFA-1 adhesiveness by rapid chemokine signals under shear flow is lost in talin1-deficient B cells. (A) Frequency of attachments (transient or arrests) of WT and CD19Tln1−/− spleen-derived B cells (talin null) to medium-density VCAM-1 or ICAM-1 triggered by either immobilized CXCL12 or CXCL13. Attachments were measured at a shear stress of 0.75 dyne/cm2 (on VCAM-1) or 0.5 dyne/cm2 (on ICAM-1). All adhesive interactions on VCAM-1 and ICAM-1 were blocked with either α4 or αL blocking mAbs, respectively. Results shown are the mean ± range in 2 fields of view from 4 independent experiments. **P < .05 for arrests of Tln1−/− versus WT cells. (B) WT and Tln1−/− B cells untreated or pretreated with PTX were settled for 2 minutes on tumor necrosis factor-α-stimulated bEnd.3 cells and then subjected to a shear stress of 2 dyne/cm2 for 10 minutes. The fractions of settled cells remaining adherent at the indicated time points are shown for each experimental group. Where indicated, intact or PTX treated lymphocytes were incubated with a cocktail of α4 and αL blocking mAbs. (C) Frequency of arrests of WT or Tln1−/− B cells on high-density VCAM-1 or ICAM-1 (500 sites/μm2) measured at a shear stress of 0.75 dyne/cm2 or 0.5 dyne/cm2, respectively, in 2 fields of view. Results are the mean values ± range in 2 fields of view from an experiment representative of 4 independent experiments. **P < .05.
Inside-out activation of VLA-4 and LFA-1 adhesiveness by rapid chemokine signals under shear flow is lost in talin1-deficient B cells. (A) Frequency of attachments (transient or arrests) of WT and CD19Tln1−/− spleen-derived B cells (talin null) to medium-density VCAM-1 or ICAM-1 triggered by either immobilized CXCL12 or CXCL13. Attachments were measured at a shear stress of 0.75 dyne/cm2 (on VCAM-1) or 0.5 dyne/cm2 (on ICAM-1). All adhesive interactions on VCAM-1 and ICAM-1 were blocked with either α4 or αL blocking mAbs, respectively. Results shown are the mean ± range in 2 fields of view from 4 independent experiments. **P < .05 for arrests of Tln1−/− versus WT cells. (B) WT and Tln1−/− B cells untreated or pretreated with PTX were settled for 2 minutes on tumor necrosis factor-α-stimulated bEnd.3 cells and then subjected to a shear stress of 2 dyne/cm2 for 10 minutes. The fractions of settled cells remaining adherent at the indicated time points are shown for each experimental group. Where indicated, intact or PTX treated lymphocytes were incubated with a cocktail of α4 and αL blocking mAbs. (C) Frequency of arrests of WT or Tln1−/− B cells on high-density VCAM-1 or ICAM-1 (500 sites/μm2) measured at a shear stress of 0.75 dyne/cm2 or 0.5 dyne/cm2, respectively, in 2 fields of view. Results are the mean values ± range in 2 fields of view from an experiment representative of 4 independent experiments. **P < .05.
Talin1 deficiency does not impair B lymphogenesis and maturation in the spleen
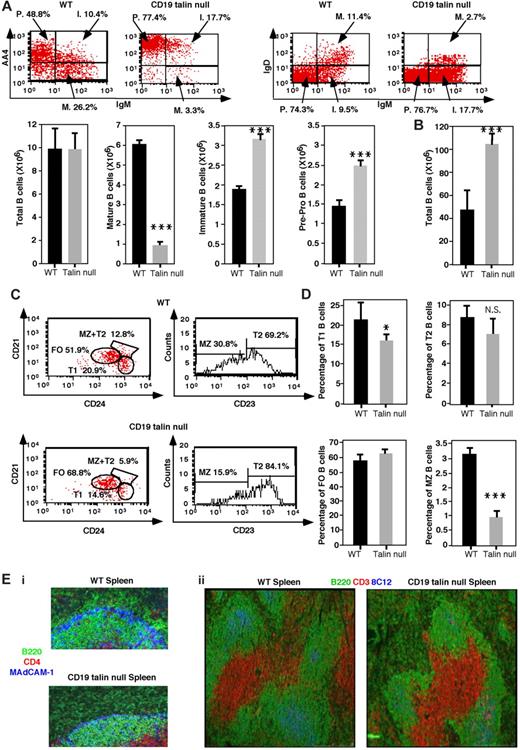
The lymphogenesis and differentiation of different B-cell subsets in CD19Tln1−/− mice were next analyzed in the BM and spleen, the major organs of B-cell generation and maturation in adult mice. Overall, the cellularity of B220+ cells was conserved in the BM of CD19Tln1−/− mice (Figure 4A). Nevertheless, a dramatically reduced percentage of mature B cells (M, IgM+AA4−) was detected in CD19Tln1−/− BM relative to WT BM (Figure 4A) as well as dramatically reduced levels of CD43lowIgMhighIgDhigh mature B cells (Figure 4A). Notably, this reduction was mirrored by an increased percentage of immature B cells (I, IgM+AA4+) and of pre and pro B cells (P, IgM−AA4+; Figure 4A) in the BM of CD19Tln1−/− mice.
Altered B-cell differentiation in CD19Tln1−/− mice. (A) Top left: FACS analysis of B220+ BM cells from WT or CD19Tln1−/− mice stained with anti-AA4 and anti-IgM mAbs, depicting pre-pro (P.), immature (I.), and mature (M.) B-cell subsets. Top right: FACS analysis of B220+ and CD43− BM cells from WT or CD19Tln1−/− (talin null) mice. These subsets were costained with anti-IgD and anti-IgM mAbs. Bottom panels: Absolute numbers of B220+ and of the indicated B subsets within equal volumes of BM suspensions from either WT or CD19Tln1−/− mice, determined by flow cytometry. The B220+ and CD43− subsets were also quantified by double staining of the AA4 and IgM markers. ***P < .001. n = 10. (B) Absolute numbers of total B220+ cells within spleen suspensions derived from either WT or CD19Tln1−/− mice (talin null), determined by flow cytometry. ***P < .001. n = 10. (C) FACS analysis of B220+ spleen cells isolated from WT or CD19Tln1−/− mice stained with anti-CD21, anti-CD24, and anti-CD23 mAbs, depicting FO, MZ, T2, and T1 B-cell subsets. (D) The fractions of spleen B220+ subsets determined by flow cytometry for WT versus CD19Tln1−/− mice (talin null). *P < .05. ***P < .001. N.S. indicates not significant. n = 10. (Ei) Spleen sections of WT or CD19Tln1−/− mice were stained with anti-B220 (green), anti-CD4 (red), and anti–MAdCAM-1 (light blue) antibodies. (Eii) Spleen sections of WT or CD19Tln1−/− mice were stained with anti-B220 (green), anti-CD3 (red), and anti-CR1 (8C12, light blue) antibodies.
Altered B-cell differentiation in CD19Tln1−/− mice. (A) Top left: FACS analysis of B220+ BM cells from WT or CD19Tln1−/− mice stained with anti-AA4 and anti-IgM mAbs, depicting pre-pro (P.), immature (I.), and mature (M.) B-cell subsets. Top right: FACS analysis of B220+ and CD43− BM cells from WT or CD19Tln1−/− (talin null) mice. These subsets were costained with anti-IgD and anti-IgM mAbs. Bottom panels: Absolute numbers of B220+ and of the indicated B subsets within equal volumes of BM suspensions from either WT or CD19Tln1−/− mice, determined by flow cytometry. The B220+ and CD43− subsets were also quantified by double staining of the AA4 and IgM markers. ***P < .001. n = 10. (B) Absolute numbers of total B220+ cells within spleen suspensions derived from either WT or CD19Tln1−/− mice (talin null), determined by flow cytometry. ***P < .001. n = 10. (C) FACS analysis of B220+ spleen cells isolated from WT or CD19Tln1−/− mice stained with anti-CD21, anti-CD24, and anti-CD23 mAbs, depicting FO, MZ, T2, and T1 B-cell subsets. (D) The fractions of spleen B220+ subsets determined by flow cytometry for WT versus CD19Tln1−/− mice (talin null). *P < .05. ***P < .001. N.S. indicates not significant. n = 10. (Ei) Spleen sections of WT or CD19Tln1−/− mice were stained with anti-B220 (green), anti-CD4 (red), and anti–MAdCAM-1 (light blue) antibodies. (Eii) Spleen sections of WT or CD19Tln1−/− mice were stained with anti-B220 (green), anti-CD3 (red), and anti-CR1 (8C12, light blue) antibodies.
As opposed to the conserved cellularity of total B220+ cells in the BM, a 2-fold increase in B220+ cell number was detected in the spleens of CD19Tln1−/− mice (Figure 4B). Interestingly, immature splenic B cells differentiated normally into T1 and T2 subsets as well as to FO B cells, as indicated by the fractions of T1 (CD21low CD24hi), T2 (CD21int/high CD24hi CD23hi), and FO (CD21int CD24int) subsets in CD19Tln1−/− mice (Figure 4C-D). Nevertheless, the fraction of MZ B cells (CD21int/high CD24hi CD23low) in the spleens of CD19Tln1−/− mice was significantly reduced relative to WT mice (Figure 4C-Ei).
As B cells require both intact VLA-4 and LFA-1 integrins to enter the spleen white pulp2 where they differentiate into T1, T2, and FO subsets, and for B-cell differentiation and retention in the MZ, these results collectively suggest that talin1 deficiency is permissive for the bulk of B-cell differentiation within the spleen white pulp. Indeed, both the cellularity and distribution of B cells remained largely intact in the white pulp of CD19Tln1−/− spleens (Figure 4Eii). Furthermore, the appearance of normal follicles and normal levels of FO B cells under steady-state conditions (Figure 4Eii) also suggested that talin1 is not required for immature B-cell entry and localization to, and differentiation within, the various white pulp niches, except for the marginal zone.
B-cell homing to lymph nodes and the BM is abrogated in the absence of talin1
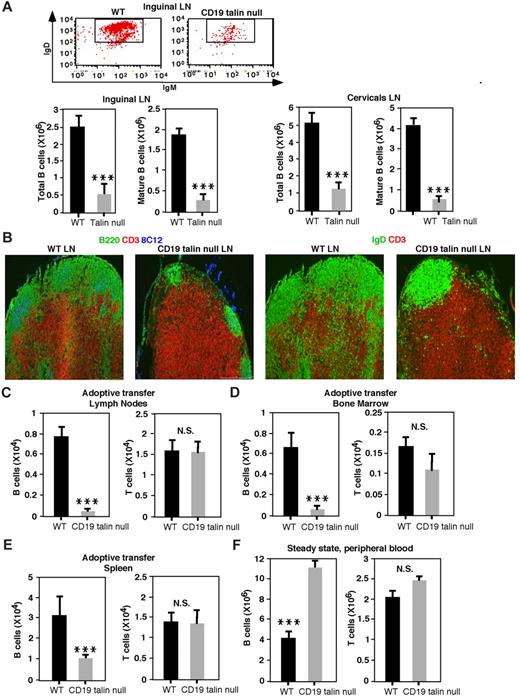
Mature B cells recirculate from the blood into peripheral lymph nodes. As this homing depends on proper chemokine signals to LFA-1 and VLA-4 integrins,34 we next wished to assess whether talin1 null B cells can enter these lymph nodes. Notably, at steady state, the numbers of IgD+ B cells recovered from inguinal and superficial cervical lymph nodes of CD19Tln1−/− mice were severely reduced (Figure 5A). Consequently, the size of B-cell follicles, visualized by either IgD or B220 staining, was also reduced in lymph nodes derived from naive CD19Tln1−/− mice (Figure 5B). To directly assess the ability of spleen-derived Tln1−/− B cells to migrate into lymph nodes, we next performed a competitive adoptive transfer experiment using WT and Tln1−/− splenic B cells labeled with distinct cell trackers and coinjected intravenously into WT recipient mice at a ratio of 1:1. The inguinal lymph nodes were excised and analyzed 24 hours after injection. Consistent with a key role for talin1 in B-cell entry to peripheral lymph nodes, Tln1−/− B cells failed to enter these organs (Figure 5C), whereas T cells derived from the same CD19Tln1−/− mice entered these lymph nodes with identical efficiency to their WT counterparts.
Mature Tln1−/− B cells cannot enter lymph nodes or home to BM. (A) Top: FACS analysis B220+ inguinal lymph node (LN) cells from WT or CD19 Tln1−/− mice stained with anti-IgD and anti-IgM mAbs. Bottom: Absolute numbers of total (B220+) and mature (IgD+) cells isolated from either inguinal (left) or cervical lymph nodes (right) of WT or CD19 Tln1−/− mice (talin null). ***P < .001 for the compared groups. n = 10. (B) Left images: Inguinal LN sections stained with anti-B220 (green), anti-CD3 (red), and anti-CR1 (8C12, light blue). Right images: Similar LN sections stained with anti-IgD (green) and anti-CD3 (red). (C) Competitive adoptive transfer of WT versus talin1-deficient B cells into WT recipient mice. The numbers of B220+ B cells from either WT or CD19Tln1−/− spleens, each labeled with a different fluorescent cell tracker (CMTMR or CFSE), recovered in both the inguinal and cervical lymph nodes of recipient mice 20 hours after intravenous. injection. The numbers of differentially dye labeled T cells recovered in these organs were determined by anti-CD3 staining and are shown for comparison. ***P < .001. n = 6. N.S. indicates no statistical significance. (D) The numbers of competitively transferred B220+ B cells or CD3+ T cells from either WT or CD19Tln1−/− spleens (CD19 talin null), labeled as in panel C and recovered in the BM of recipient WT mice, were determined after 20 hours by flow cytometry. The numbers of dye-labeled T cells recovered in these organs were determined by anti-CD3 staining and are shown for comparison. ***P < .001. n = 6. (E) Competitive adoptive transfer of WT versus talin1-deficient B cells into WT recipient mice. Dye-labeled splenocytes (15-25 × 106) from WT or CD19Tln1−/− spleens were coinjected into WT host mice. Dye-labeled B cells were determined in the spleen of the recipient mice after 20 hours by B220 staining. ***P < .001. n = 6. (F) Steady-state numbers of circulating WT and talin1-deficient B cells and WT T cells. Absolute cell numbers of either B220+ or CD3+ peripheral blood lymphocytes determined in equal blood volumes of either WT or CD19Tln1−/− mice (CD19 talin null). ***P < .001. n = 4.
Mature Tln1−/− B cells cannot enter lymph nodes or home to BM. (A) Top: FACS analysis B220+ inguinal lymph node (LN) cells from WT or CD19 Tln1−/− mice stained with anti-IgD and anti-IgM mAbs. Bottom: Absolute numbers of total (B220+) and mature (IgD+) cells isolated from either inguinal (left) or cervical lymph nodes (right) of WT or CD19 Tln1−/− mice (talin null). ***P < .001 for the compared groups. n = 10. (B) Left images: Inguinal LN sections stained with anti-B220 (green), anti-CD3 (red), and anti-CR1 (8C12, light blue). Right images: Similar LN sections stained with anti-IgD (green) and anti-CD3 (red). (C) Competitive adoptive transfer of WT versus talin1-deficient B cells into WT recipient mice. The numbers of B220+ B cells from either WT or CD19Tln1−/− spleens, each labeled with a different fluorescent cell tracker (CMTMR or CFSE), recovered in both the inguinal and cervical lymph nodes of recipient mice 20 hours after intravenous. injection. The numbers of differentially dye labeled T cells recovered in these organs were determined by anti-CD3 staining and are shown for comparison. ***P < .001. n = 6. N.S. indicates no statistical significance. (D) The numbers of competitively transferred B220+ B cells or CD3+ T cells from either WT or CD19Tln1−/− spleens (CD19 talin null), labeled as in panel C and recovered in the BM of recipient WT mice, were determined after 20 hours by flow cytometry. The numbers of dye-labeled T cells recovered in these organs were determined by anti-CD3 staining and are shown for comparison. ***P < .001. n = 6. (E) Competitive adoptive transfer of WT versus talin1-deficient B cells into WT recipient mice. Dye-labeled splenocytes (15-25 × 106) from WT or CD19Tln1−/− spleens were coinjected into WT host mice. Dye-labeled B cells were determined in the spleen of the recipient mice after 20 hours by B220 staining. ***P < .001. n = 6. (F) Steady-state numbers of circulating WT and talin1-deficient B cells and WT T cells. Absolute cell numbers of either B220+ or CD3+ peripheral blood lymphocytes determined in equal blood volumes of either WT or CD19Tln1−/− mice (CD19 talin null). ***P < .001. n = 4.
The dramatically reduced number of mature B cells in the BM of CD19Tln1−/− mice (Figure 4A) also suggested an indispensable role of talin1 in the ability of mature B lymphocytes to return to the BM, once released into the circulation from the spleen. To directly assess the ability of spleen-derived Tln1−/− B cells to enter the BM, we repeated transfer experiments similar to those described in Figure 5C. As was observed in lymph nodes, only a minute fraction of transferred talin1 null spleen-derived B lymphocytes could be recovered in the BM of WT recipients, whereas a large number of transferred WT B spleen cells were recovered from the same BM tissues (Figure 5D). The reduced number of mature B cells found at steady state in the BM of CD19 Tln1−/− mice (Figure 4A) is therefore the outcome of impaired homing of mature spleen B lymphocytes to the BM. Interestingly, despite the increased B cellularity in the spleen of CD19Tln1−/− mice (Figure 4B,E), the rate of entry of spleen-derived Tln1−/− B cells adoptively transferred to a WT recipient spleen was 60% lower than that of WT spleen B cells (Figure 5E). Consistent with their reduced entry to lymph nodes, the BM, and to a lesser extent, the spleen (Figure 5C-E), a nearly 3-fold increase in the number of circulating B cells was observed in CD19Tln1−/− mice at steady state, whereas the number of circulating T cells remained unchanged (Figure 5F). Collectively, these results suggest an indispensable role of talin1 in B-cell entry to lymph nodes and the BM and a partial requirement for this integrin adaptor in B-cell entry to the spleen.
Talin1 deficiency does not alter B-cell generation and survival in the BM and the spleen
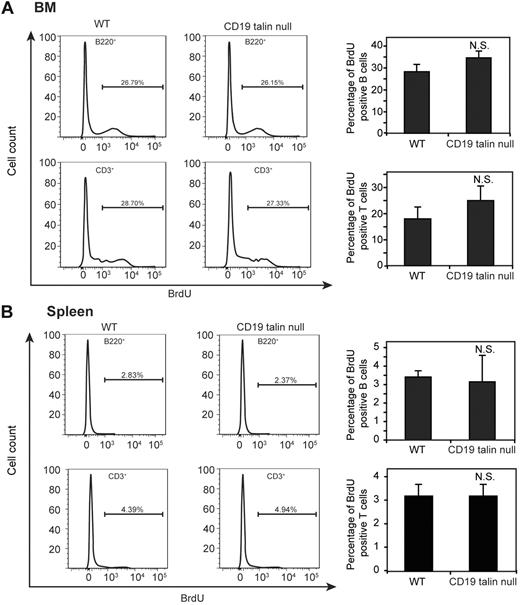
The elevated levels of circulating B cells in CD19Tln1−/− mice might result not only from their deficient entry to lymph nodes and return to the BM, but also from increased lymphogenesis. We therefore next compared BrdU incorporation by BM and spleen B and T cells in WT and CD19Tln1−/− mice. Notably, proliferation rates of both B cells (B220+) and T cells (CD3+) derived from either the BM (Figure 6A) or the spleen (Figure 6B) of CD19Tln1−/− mice were comparable. Furthermore, both talin1 null BM and spleen-derived B cells exhibited comparable degrees of spontaneous apoptosis (as determined by intracellular caspase 3 and 7 activities, data not shown). These findings collectively suggest that the 3-fold higher number of B lymphocytes circulating in the peripheral blood of CD19Tln1−/− mice (Figure 5F) and the 2-fold higher number of B cells populating the spleen (Figure 4B) did not result from abnormal rates of proliferation and survival of talin1 null B cells. Rather, the higher numbers of circulating talin1 null B cells reflect an inherent defect in the ability of these lymphocytes to extravasate blood vessels at all major lymphoid organs. Interestingly, spleens of CD19Tln1−/− mice were approximately 2-fold enlarged in volume and in content of both B and T lymphocytes, whereas peripheral lymph nodes of CD19Tln1−/− mice remained normal in size. Because the fraction and composition of DC subsets in CD19Tln1−/− spleens were comparable (data not shown), the increased numbers of B and T splenocytes in CD19Tln1−/− mice were probably the result of the dramatically elevated number of blood-circulating B lymphocytes that could not emigrate at the periphery, and therefore overpopulated this organ.
Normal Tln1−/− B-cell proliferation in the BM and spleen. BM cells (A) or splenocytes (B) from BrdU-treated WT or CD19 Tln1−/− mice were stained with anti-BrdU antibodies, and for either B220 or CD3, as indicated. Data on left are histograms depicting the percentage of BrdU-incorporated cells from those that stained positively for either B220 or CD3; each histogram shows 1 experiment representative of 6. Data on right are the mean ± SD of the BrdU-incorporated cells, which stained for B220 (B cells) or CD3 (T cells). n = 6. N.S. indicates not significant.
Normal Tln1−/− B-cell proliferation in the BM and spleen. BM cells (A) or splenocytes (B) from BrdU-treated WT or CD19 Tln1−/− mice were stained with anti-BrdU antibodies, and for either B220 or CD3, as indicated. Data on left are histograms depicting the percentage of BrdU-incorporated cells from those that stained positively for either B220 or CD3; each histogram shows 1 experiment representative of 6. Data on right are the mean ± SD of the BrdU-incorporated cells, which stained for B220 (B cells) or CD3 (T cells). n = 6. N.S. indicates not significant.
Talin1 null B cells generate reduced humoral responses to T-dependent antigens
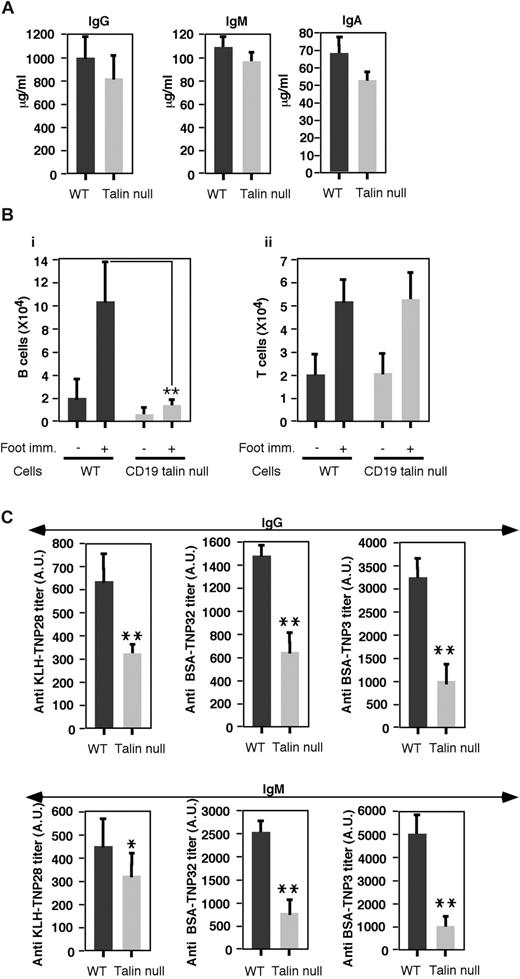
The hallmark of B-cell function is Ig production. Surprisingly, at steady state, the levels of total serum IgG and IgM of naive CD19Tln1−/− mice were only marginally reduced (Figure 7A), reflecting normal ability of FO B220+ talin1 null cells to encounter both T-dependent and -independent blood-borne antigens in the splenic white pulp. Conserved IgM levels could also result from normal numbers of B220+CD5+ cells found in the peritoneal cavity of CD19Tln1−/− mice (data not shown), a source of natural IgM production.35 In contrast, the level of serum IgA was only marginally reduced in a statistically insignificant manner (Figure 7A), suggesting conserved steady-state humoral responses of mucosal talin1 null B cells.
Defective humoral responses in CD19Tln1−/− mice. (A) IgG, IgM, and IgA serum levels in naive WT or CD19 Tln1−/− mice (talin null). Results are the mean ± SEM of 10 mice in each experimental group. The differences between the compared experimental groups were statistically insignificant. (B) Absolute numbers of B cells (i, B220+) or T cells (ii, CD3+) in the footpad draining peripheral lymph nodes of either WT or CD19Tln1−/− mice, either naive or 10 days after primary immunization with the T-dependent antigen, KLH-TNP (KLH-TNP28). **P < .002. n = 2. (C) Antigen- and hapten (TNP)–specific IgG and IgM titers, expressed as arbitrary units, after immunization with the T-dependent antigen, KLH-TNP (KLH-TNP28). CD19Tln1−/− (labeled as talin null) and WT mice were each immunized in the footpad. After 2 and 4 weeks, mice received additional antigenic boosts into their peritoneal cavity; and 2 weeks later, serum was analyzed for KLH, KLH-TNP, or BSA-TNP specific antibodies by enzyme-linked immunosorbent assay. Results are mean ± range and are expressed as arbitrary units. *P < .05. **P < .01. n = 2. Top panel: Serum IgG reactivity against KLH only, KLH-TNP28, or BSA conjugated with TNP at either low or high density (TNP32 and TNP3, respectively). Bottom panel: Serum IgM reactivity against KLH only, KLH-TNP28, or BSA carrying either high or low density of TNP (TNP32 and TNP3, respectively).
Defective humoral responses in CD19Tln1−/− mice. (A) IgG, IgM, and IgA serum levels in naive WT or CD19 Tln1−/− mice (talin null). Results are the mean ± SEM of 10 mice in each experimental group. The differences between the compared experimental groups were statistically insignificant. (B) Absolute numbers of B cells (i, B220+) or T cells (ii, CD3+) in the footpad draining peripheral lymph nodes of either WT or CD19Tln1−/− mice, either naive or 10 days after primary immunization with the T-dependent antigen, KLH-TNP (KLH-TNP28). **P < .002. n = 2. (C) Antigen- and hapten (TNP)–specific IgG and IgM titers, expressed as arbitrary units, after immunization with the T-dependent antigen, KLH-TNP (KLH-TNP28). CD19Tln1−/− (labeled as talin null) and WT mice were each immunized in the footpad. After 2 and 4 weeks, mice received additional antigenic boosts into their peritoneal cavity; and 2 weeks later, serum was analyzed for KLH, KLH-TNP, or BSA-TNP specific antibodies by enzyme-linked immunosorbent assay. Results are mean ± range and are expressed as arbitrary units. *P < .05. **P < .01. n = 2. Top panel: Serum IgG reactivity against KLH only, KLH-TNP28, or BSA conjugated with TNP at either low or high density (TNP32 and TNP3, respectively). Bottom panel: Serum IgM reactivity against KLH only, KLH-TNP28, or BSA carrying either high or low density of TNP (TNP32 and TNP3, respectively).
To directly address whether talin1 null B cells can develop a strong humoral response to an exogenously administered T-dependent antigen, we next compared the ability of CD19Tln1−/− mice to generate a secondary response to the prototypic T cell-dependent antigen, KLH-TNP. The antigen was introduced in complete Freund adjuvant into the footpad of CD19Tln1−/− mice to elicit T-dependent priming of mature B cells in the footpad draining peripheral lymph nodes. Indeed, the number of B cells accumulated in these draining lymph nodes 10 days after initial KLH-TNP immunization was reduced by approximately 65% in CD19Tln1−/− mice, whereas T cells accumulating in these lymph nodes remained similar in WT and CD19Tln1−/− mice (Figure 7B). As expected, this primary response did not recruit B lymphocytes to the spleen (supplemental Figure 3). After 2 and 4 weeks, KLH-TNP was repeatedly introduced into the abdominal cavity, and both antigen and hapten (ie, TNP) specific antibodies were determined in the sera from the mice 2 weeks after the last immunization. Notably, both antigen- and hapten-specific IgG and IgM levels were markedly reduced (Figure 7C middle and right bars), and the levels of high-affinity hapten-specific IgG or IgM Abs (ie, reactive to low-density hapten, Figure 7C) were reduced to a similar extent in immunized CD19Tln1−/− mice. Thus, the reduced ability of talin1 null B lymphocytes to enter tissue draining lymph nodes where they can participate in primary and secondary responses to a T-dependent antigen (Figure 7B) resulted in severely impaired humoral responses to this antigen.
Discussion
Firm adhesions mediated by the LFA-1 and α4 integrins are one of the key checkpoints in lymphocyte exit through blood vessels within lymphoid organs and sites of inflammation.14 To mediate adhesion, these integrins must undergo in situ activation by appropriate chemokine signals presented by blood vessel endothelial cells.36 In T cells, talin1 has been shown to play a critical role in both LFA-1 and VLA-4 activation by chemokine signals.37-39 The present study is the first demonstration that talin1 is also a critical factor in the ability of B-cell VLA-4 and LFA-1 to undergo activation by chemokine signals commonly found in the lymph node and the BM vasculature. Our in vivo results also suggest that talin1 is required for B lymphocyte entry to peripheral lymph nodes and the BM, trafficking processes mediated by LFA-1 and VLA-4, respectively.34 Talin1 is also critical for B-cell VLA-4 and LFA-1 activation by BCR signaling. Talin1 is dispensable, however, for B-cell chemotaxis, a process that does not involve integrin function. B-cell talin1 is also dispensable for the residual VLA-4 adhesiveness to VCAM-1 retained by nonstimulated B cells but is still critical for residual LFA-1 adhesiveness. In human T cells, this spontaneous VLA-4 adhesiveness is reduced by transient suppression of either talin1 or paxillin,40 a specific adaptor of the α4 subunit of VLA-441 critical for its spontaneous adhesiveness, particularly under shear forces.40 It is therefore possible that, in our conditional talin1 depletion B-cell model, prolonged depletion of talin1 allows paxillin to substitute for talin1 in conferring low spontaneous adhesiveness of VLA-4 but not of LFA-1.
Our in vivo analysis shows that, at steady state, both immature and mature talin-negative B cells cannot optimally exit the circulation and therefore accumulate in larger numbers in the blood circulation. Because we have ruled out a defect in proliferation of talin1 null B cells in the BM and spleen, as well as in their maturation in the spleen, the elevated cellularity of talin1 null B cells in the blood and the spleen appears to be a direct reflection of 2 major defects: (1) enhanced release of immature B cells from the BM sinusoids, possibly the result of abrogated VLA-4-dependent association with sinusoid VCAM-1 and endothelial chemokines; and (2) an inability of mature B cells that leave the spleen to emigrate from blood to lymph nodes or return to the BM.
Despite these 2 defects, talin1 null B cells enter almost normally into the spleen's white pulp and follicles where they seem to differentiate and mature into follicular B cells. B-cell migration through the marginal sinus into the white pulp has been postulated to involve integrin-mediated lymphocyte attachments to cells lining the marginal sinus that express high levels of the α4 integrin ligand, MAdCAM-1, as well as considerable ICAM-1 and VCAM-1.2 Because MAdCAM-1 is not involved in B-cell entry to white pulp, VLA-4 engagements of VCAM-1 and fibronectin associated with marginal sinus lining cells, together with LFA-1-ICAM-1 engagements, were suggested to cooperate in B-cell crossing of the marginal sinus into the white pulp.2 Because B cells also require proper activation by chemokine signals for their entry into the white pulp,42 the entry of these lymphocytes from the marginal sinus into the white pulp would be expected to involve combined activation of LFA-1 and VLA-4 by inside-out chemokine signals,2 a process that we confirmed ex vivo to require B-cell talin1. Because we found that talin1 is not required for B-cell entry into the white pulp in vivo, our results argue that talin1-deficient B cells do not need to optimally activate their LFA-1 and α4 integrins via in situ GPCR signals to cross the marginal sinus and enter the white pulp. It is possible that talin1-deficient B cells may use their residual VLA-4 adhesiveness to cross this sinus and enter the white pulp. Notably, adoptively transferred talin1-deficient spleen B cells entered host spleens at approximately 2-fold lower rates than their WT counterparts. Thus, talin1 null B cells do exhibit an inherently reduced ability to cross endothelial barriers within the spleen, probably because of their impaired LFA-1 adhesiveness. Nevertheless, combined VLA-4 and LFA-1 blocking of talin1-deficient B cells could not further reduce their overall 2-fold reduced entry to the spleen (data not shown). Taken together, talin1-deficient B cells clearly overcome their multiple adhesive defects and eventually cross the endothelial barrier of the marginal sinus into the white pulp. Talin1-deficient B cells may also enter white pulp follicles via the red pulp, using integrin-independent interactions.
In contrast to their overall intact ability to enter and differentiate within the white pulp and its follicles, the fraction of fully differentiated marginal zone B cells found in CD19 talin1 null mice was dramatically reduced. Thus, fully intact talin1-dependent LFA-1 and VLA-4 activities are necessary for the entrapment, full maturation and retention of these B cells in the marginal zone, processes shown by the Cyster's laboratory to be tightly regulated by VLA-4 and LFA-1.43 Interestingly, despite the marked reduction of MZ B cells in CD19 talin1 null mice, MZ macrophage distribution was fully conserved (data not shown). Thus, although differentiation of immature B cells into MZ B cells is talin1 and integrin-dependent, talin1 is dispensable for the entry of immature B cells into the spleen white pulp and for their maturation into follicular B cells. Talin1 also appears dispensable for the generation and retention of peritoneal CD5+CD220+ B cells (data not shown).
In contrast to their ability to enter the spleen, talin1-deficient B cells could not use any talin1 or integrin-independent route to attach and to cross endothelial cells within peripheral lymph nodes and the BM. One plausible explanation for this dichotomy is the apparently negligible shear forces exerted on B lymphocytes migrating through the spleen marginal sinuses compared with the high shear forces exerted on B lymphocytes attached to lymph node high endothelial venules or the BM sinusoids.44 Integrin adhesiveness is indeed positively regulated by shear forces16,45 as is selectin adhesiveness. Notably, lymphocyte attachment to lymph node high endothelial venules and the BM sinusoids involve either L-selectin- or E-selectin-mediated interactions,46 whereas lymphocyte entry to the spleen white pulp does not involve any of these selectin-mediated interactions.47 These results may therefore reflect a general specialization between lymphocyte attachment and crossing of the lymph node and the BM blood vessels on the one hand, and the spleen sinuses on the other hand.
Importantly, the inability of mature talin1 mutant B cells to enter lymph nodes was observed both in naive and immunized mice. We assessed the ability of talin1-deficient mature B lymphocytes to generate a humoral response to a T-dependent antigen, KLH-TNP, after its drainage into skin-associated peripheral lymph nodes and found that the entry of talin1-deficient B cells to lymph nodes draining the tissue where the antigen was initially introduced was negligible. Importantly, even when reexposed to a secondary and tertiary boost of this antigen, talin1 null B cells still failed to generate a significant humoral response to this antigen, evident from reduced generation of both low- and high-affinity IgM and IgG against this antigen. These results reflect both the reduced lymph node entry of B cells in talin1 null mice into the antigen collecting lymph nodes together with potential defects in plasma B-cell migration to the BM.
Another possibility to account for the reduced T-dependent humoral response of talin1-deficient B cells is a defect in integrin-facilitated antigen recognition by the BCR. In addition to regulating B lymphocyte interactions with endothelial ligands, α4 and LFA-1 integrins, and their talin1 coactivator can reduce the threshold of B-cell activation by the BCR48 because, on encounter of antigen, B cells use these integrins to prolong B-cell contacts with antigen-presenting VCAM-1-expressing, follicular dendritic cells.49 We also cannot exclude that the intrinsic ability of talin1-deficient B cells to differentiate into plasma cells within lymph node follicles is also defective. Notably, when tested ex vivo, talin1 null B cells completely failed to activate their integrins in response to rapid BCR signals. On the other hand, significant IgM and IgG production by nonimmunized mice seems to be independent on talin1 expression by B cells. It is not clear how B cells capable of producing these homeostatic antibodies can overcome their talin1 deficiency. Addressing these and related issues using additional integrin function deficient B-cell models may deepen our understanding of the enormous flexibility of antigen driven B-cell activation and antibody production within various lymphoid organs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr S. Schwarzbaum for editorial assistance and L. Bar-On for help with determination of spleen DC subsets.
This work was supported by the US-Israel Binational Science Foundation and the Israel Science Foundation. R.A. is an incumbent of the Linda Jacobs Chair in Immune and Stem Cell Research. The work in the laboratory of D.R.C. was supported by the Wellcome Trust.
This manuscript is dedicated to the loving memory of Dr Valentin Grabovsky, who passed away while the article was in press.
Wellcome Trust
Authorship
Contribution: E.M.-M. designed parts of the study, performed research, analyzed data, and assisted in manuscript preparation; V.G. performed experiments; S.W.F. designed parts of the study, performed research, and assisted in manuscript preparation; G.C. designed parts of the study; Y.G. assisted with FACS analysis of lymphoid organs; G.G. performed immunohistochemistry analysis; S.J.M. generated talin1 floxed mice; R.M. assisted with the in vivo experiments; D.M. designed parts of the study and performed experiments; R.E.M. designed parts of the study and performed research; D.R.C. provided talin1 floxed mice and provided input into manuscript preparation; I.S. designed parts of the study and assisted in manuscript preparation; and R.A. designed and supervised all aspects of the work and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronen Alon, Department of Immunology, Weizmann Institute of Science, 1 Herzl St, Rehovot, Israel 76100; e-mail: ronen.alon@weizmann.ac.il.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal