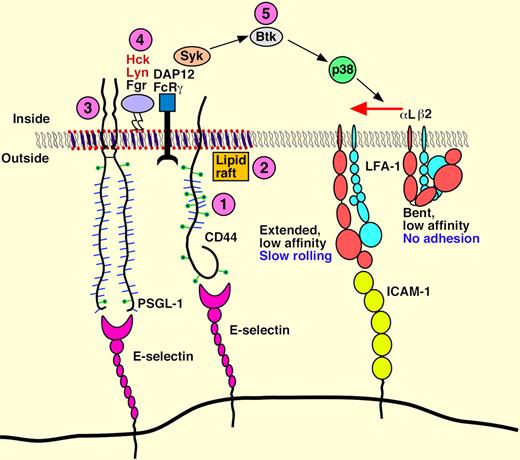
In this issue of Blood, Yago and colleagues identify CD44 and new components to an intracellular kinase signaling cascade in murine neutrophils that activates LFA-1 integrins, and thus enables E-selectin–mediated slow rolling.
Functional E-selectin ligands identified on murine neutrophils include E-selectin ligand-1 (ESL-1), PSGL-1, and CD44. So far, PSGL-1 is the only ligand reported to support neutrophil slow rolling over inflamed venules.1,2 In this issue of Blood, Yago et al3 identify CD44 and new components to an existing intracellular kinase signaling cascade in neutrophils that activates LFA-1 integrins to enable E-selectin–mediated slow rolling. These studies report that E-selectin engagement of either CD44 or PSGL-1 in bone marrow leukocytes (> 90% neutrophils) triggered a sequential tyrosine kinase cascade consisting of (1) Src family kinases Hck, Lyn and Fgr, (2) Syk, (3) Btk, and (4) p38, and led to LFA-1 engagement of ICAM-1 (see figure) for slow rolling in vivo and in vitro. The importance of these pathways to neutrophil recruitment was corroborated by neutrophil “competitive” adoptive transfer studies where cells treated with inhibitors and/or cells from knockout animals exhibited defective slow rolling on TNF-α–treated venules in the cremaster muscle or diminished recruitment in a model of thioglycollate-induced peritonitis in wild-type mice. Furthermore, they corroborated many of their findings with human neutrophils, which also exhibit slower rolling on E-selectin plus ICAM-1 than on E-selectin alone via SFK, Syk, and p38 and lipid raft–mediated LFA-1 activation under flow conditions in vitro.
E-selectin, P-selectin, and L-selectin constitute the selectin gene family. E-selectin is exclusively expressed in the vascular endothelium. Early studies in E-selectin knockout (E-sel−/−) mice revealed that peripheral blood neutrophils rolled at higher velocities in TNF-α–treated venules of the cremaster muscle4 and that neutrophils exhibited a significant decrease in stable adhesion to inflamed dermal and mesenteric microvessels.5 But E-selectin null animals did not exhibit a leukocyte recruitment defect in a bacterial or thioglycollate peritonitis or delayed-type hypersensitivity models of inflammation, which is due to compensation by endothelial- expressed P-selectin.6,7 E-selectin–dependent slow rolling led to longer transit times of leukocytes rolling in inflamed venules and longer transit time was postulated to facilitate recruitment of neutrophils to sites of inflammation.6 Meanwhile, work in other labs with different models discovered leukocyte PSGL-1 engagement by E- or P-selectin–transmitted signals through its cytoplasmic tail to prime integrin activation and initiate gene transcription.8,9 These data and other accumulating evidence led to the idea that leukocyte integrins interact with endothelial cell ICAM-1 to support slower leukocyte rolling on inflamed venules and facilitate arrest in the presence of appropriate chemokines presented by the apical surface of the endothelium.
However, the molecular mechanism underlying E-selectin–dependent “slow” rolling velocity remained undefined until 2007 when studies by Zarbock, Ley, and colleagues shed light on the problem.1,2 Their elegant studies used a combination of gene knockout animals and an ex vivo whole blood perfusion chamber to reveal E-selectin engagement of neutrophil PSGL-1–triggered LFA-1 binding of ICAM-1 through sequential activation of Syk, DAP12/FcRγ, and p38 kinase in combination with chemokine receptor signaling pathways to promote leukocyte slow rolling. In this issue, Yago and colleagues sought to extend these observations by exploring the role of CD44 in signal transduction and activation of LFA-1 integrins in leukocyte to enable slow rolling in venules in vivo and in vitro on immobilized E-selectin and ICAM-1 adhesion molecules. The new data shows that either leukocyte CD44 or PSGL-1 mediate slow rolling of bone marrow neutrophils in in vivo and in vitro models, and all 3 neutrophil Src family kinases Hck, Lyn, and Fgr play a role in Syk activation, with the Tec kinase Bruton tyrosine kinase, Btk, contributing to Syk-dependent activation of p38 kinase for LFA-1 activation and slow rolling on E- or P-selectin molecules coimmobilized with ICAM-1. Interestingly, a selective role for membrane lipid rafts was demonstrated not for rolling on P- or E-selectins, but for slow rolling on selectins plus ICAM-1, which differs from an earlier report as noted by the authors. In addition, slow rolling on E- or P-selectin was independent of PI3 Kinase (and PI3Kγ and PI3Kδ) even though this pathway is required for integrin-mediated arrest and spreading in chemokine-stimulated neutrophils.
There are some differences in these datasets and lack of experiments probing a role for ESL-1 that merit discussion. Yago et al observe a defect in slow rolling only in PSGL-1/CD44 double knockout mice whereas the ex vivo perfusion assay by Zarbock detected an obvious defect in slow rolling in PSGL-1-/- cells and a statistical difference in CD44-/- leukocytes, albeit the defect was very small compared with that of PSGL-1-/- neutrophils (Zarbock,2 Figure 1A). In addition, Zarbock and colleagues have just published a follow-up paper in Blood10 that identified Btk and a combination of PLCγ and PI3Kγ pathways that control LFA-1–mediated slow rolling on E-selectin plus ICAM-1 substrates. As noted above, Yago et al did not observe a role for PI3 Kinase in neutrophil slow rolling. Such differences in results from these 2 groups may be related to the differences in experimental approaches. Yago et al use neutrophils purified from bone marrow and perfused across immobilized substrates in an in vitro flow chamber while Zarbock employs an ex vivo whole blood perfused chamber that exposed peripheral blood to immobilized proteins in presence of all other formed elements (platelets, plasma proteins, etc). Lastly, what about ESL-1 and slow rolling? Studies by Hidalgo and colleagues have evaluated the role of ESL-1, PSGL-1, and CD44 in neutrophil interactions with E-selectin using a combination of knockout mice (CD44 and PSGL-1−/− mice) and shRNA approaches to knockdown ESL-1. They have not examined E-selctin–dependent flow rolling per se, but did find that ESL-1 is the more dominant ligand, that each of the 3 ligands encompass all the E-selectin activity, and each participates in specialized functions of rolling, adhesion, and signaling.11
Model for E-selectin–mediated slow rolling. The circled numbers represent new signaling components identified in this paper. Neutrophils rolling on E-selectin engage both CD44 and PSGL-1 to initiate signaling through a common pathway that requires lipid rafts, the cytoplasmic domain of PSGL-1, all three SFKs, the ITAM adaptors DAP12 and FcRγ, the Tec kinase Btk, and p38. This signaling cascade activates integrin LFA-1 to a conformation that enables slow rolling but not arrest on ICAM-1. PSGL-1 and CD44 may not be located in the same raft domains as depicted in the figure. See the complete figure in the article by Yago et al on page 485.
Model for E-selectin–mediated slow rolling. The circled numbers represent new signaling components identified in this paper. Neutrophils rolling on E-selectin engage both CD44 and PSGL-1 to initiate signaling through a common pathway that requires lipid rafts, the cytoplasmic domain of PSGL-1, all three SFKs, the ITAM adaptors DAP12 and FcRγ, the Tec kinase Btk, and p38. This signaling cascade activates integrin LFA-1 to a conformation that enables slow rolling but not arrest on ICAM-1. PSGL-1 and CD44 may not be located in the same raft domains as depicted in the figure. See the complete figure in the article by Yago et al on page 485.
Taken together, these exciting new results establish a more detailed model of the mechanisms that underlie neutrophil slow rolling in the context of neutrophil recruitment in vivo and raise a number of new questions that need to be addressed. Do other granulocytic, myelocytic, or lymphoid cells engage in slow rolling on E- and P-selectins? If so, what role do E- and P-selectin ligands play and is the process of slow rolling essential for recruitment to sites of inflammation? Is the cytoplasmic tail of CD44 involved in activation of the tyrosine kinase cascade, or does PSGL-1 constitute the intracellular “node” for signaling by recruitment to membrane lipid rafts? Could ESL-1 participate in slow rolling, given the work by Hidalgo et al? Lastly, a recent report identified Basigin (CD147) as an E-selectin ligand on neutrophils.12 This begs the question of whether basigin is involved in neutrophil slow rolling.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal