To the editor:
In the 8 April 2010 issue of Blood, we reported characterization of IDH1 mutation in a large cohort of acute myeloid leukemia (AML) patients.1 Very recently, a series of reports about this mutation in AML have been published in a cluster of manuscripts.1-7 As IDH1 mutation brings prognostic information in glioma and possibly AML,4,8,9 identification of IDH1 R132 mutations will bring increasing clinical relevance. Moreover, the mutation seems quite stable and may serve as a marker for monitoring minimal residual disease.1 Hence, a sensitive and simple method for detecting this mutation will be highly desirable. We here report a very sensitive, single-tube, multiplex polymerase chain reaction (PCR)–based method, which has been verified by direct sequencing method. With this method, we then determined the stability of the R132 mutation in sequential samples of AML and measured the incidence of this mutation in a cohort of patients with myelodysplastic syndrome (MDS). This study has been approved by the Institutional Review Board of the National Taiwan University Hospital.
We designed 6 forward mutation-specific primers to cover all possible types of R132 mutations (Figure 1A), although only 5 of them had been reported until now.1-7 Another upstream forward primer was used to generate a product as an internal control. Combination of these 7 forward primers and a common reverse primer (Figure 1A) would generate a shorter mutant and a longer control product in any genomic DNA containing any type of R132 mutation, whereas only the longer product would be seen in samples without this mutation (Figure 1B). PCR was performed by heating at 95°C for 10 minutes, followed by 45 cycles of 95°C, 62°C, and 72°C for 30 seconds each in a total of 25 μL containing 200nM each dNTP, 1.5mM MgSO4, 50 ng of genomic DNA, 100nM upstream forward primer, 500nM each of the other 7 primers, and 1.5 units of PlatinumTaq polymerase (Invitrogen). A single tube combining 8 primers would be easy, economic, and fast in screening any possible type of IDH1 R132 mutation.
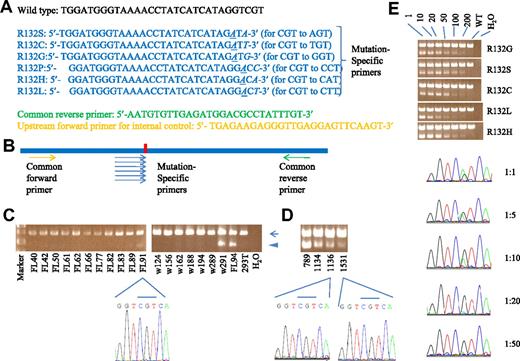
A multiplex, allele-specific PCR for rapid and sensitive screening of IDH1 R132 mutations. (A) The sequences of the primers. The 3rd last nucleotide was intentionally mutated to avoid background signals (italic and underlined). The last nucleotide matches only to mutant but not wild-type sequences (italic). Wild-type sequence was shown for reference. (B) Schematic representation of our multiplex, allele-specific PCR strategy. The red vertical bar represents the site of R132 mutation. (C) Multiplex PCR on genomic DNA from bone marrow cell of AML patients. The top band (arrow) indicated an internal control band. The bottom band (arrowhead) represented the mutant signal. Samples w291 and FL94 were positive for IDH1 mutation in both sequencing and multiplex methods. FL91 was negative by sequencing but yielded a mutant band in multiplex PCR. 293T represented a complex genomic DNA from this cell line without IDH1 mutation and served as a negative control. Results from other samples were not shown. (D) Mutant signals were evident in samples 789 (UPN 789, at diagnosis), 1134, 1136 (2 samples from this relapsed patient), and 1531 (a sample from another relapsed patient) by multiplex PCR, but sequencing did not reveal mutation in the samples 1136 and 1531. (E) Mutant bands could be seen in patients' DNA diluted with 293T genomic DNA up to 200-fold (top panel). On the other hand, the mutant signal was barely seen in direct sequencing at 20-fold dilution (bottom panel). In our previous report, only 5 types of IDH1 mutation were seen (no R132P).1 (Top panel) One, 10, and 200 mean dilution fold of mutant genomic DNA with 293T DNA. WT indicates 293T genomic DNA alone.
A multiplex, allele-specific PCR for rapid and sensitive screening of IDH1 R132 mutations. (A) The sequences of the primers. The 3rd last nucleotide was intentionally mutated to avoid background signals (italic and underlined). The last nucleotide matches only to mutant but not wild-type sequences (italic). Wild-type sequence was shown for reference. (B) Schematic representation of our multiplex, allele-specific PCR strategy. The red vertical bar represents the site of R132 mutation. (C) Multiplex PCR on genomic DNA from bone marrow cell of AML patients. The top band (arrow) indicated an internal control band. The bottom band (arrowhead) represented the mutant signal. Samples w291 and FL94 were positive for IDH1 mutation in both sequencing and multiplex methods. FL91 was negative by sequencing but yielded a mutant band in multiplex PCR. 293T represented a complex genomic DNA from this cell line without IDH1 mutation and served as a negative control. Results from other samples were not shown. (D) Mutant signals were evident in samples 789 (UPN 789, at diagnosis), 1134, 1136 (2 samples from this relapsed patient), and 1531 (a sample from another relapsed patient) by multiplex PCR, but sequencing did not reveal mutation in the samples 1136 and 1531. (E) Mutant bands could be seen in patients' DNA diluted with 293T genomic DNA up to 200-fold (top panel). On the other hand, the mutant signal was barely seen in direct sequencing at 20-fold dilution (bottom panel). In our previous report, only 5 types of IDH1 mutation were seen (no R132P).1 (Top panel) One, 10, and 200 mean dilution fold of mutant genomic DNA with 293T DNA. WT indicates 293T genomic DNA alone.
We verified the utility of this multiplex method in 103 AML marrow samples previously studied by PCR and direct sequencing.1 Such selection is based solely on availability of samples, without consideration of any other factors. Comparison of this multiplex PCR with direct sequencing revealed perfect concordance except for 1 patient whose IDH1 mutation was detected only by multiplex PCR but not by direct sequencing (FL91 in Figure 1C). Furthermore, in 9 patients with IDH1 mutation at diagnosis the same mutation could be detected by multiplex method in all 11 samples obtained at disease relapse, including the 4 in which mutant signals were no longer seen by sequencing (Figure 1D and data not shown). The sensitivity of this method was approximately 0.5%, obviously higher than sequencing (Figure 1E). We also screened 113 patients with MDS (22 refractory anemia [RA], 9 RA with ring sideroblasts, 19 RA with excess blasts-1 [RAEB-1], 11 RAEB-2, 26 RAEB with transformation, and 26 chronic myelomonocytic leukemia) by this method and found 3 (2.7%; 2 RAEB with transformation and 1 RAEB-2) bearing this mutation, which was then confirmed by direct sequencing. Thus, we provide a quick, economic, and sensitive method for screening and monitoring minimal residual disease of IDH1 R132 mutations, and conclude that this mutation is quite stable during disease evolution in AML and is rare in MDS.
Authorship
Acknowledgments: This work was supported by grants NSC 96-2628-B002-013-MY2 and 98-2314-B-002-033-MY3 from National Science Council (Taiwan), NHRI-EX97-9731BI from National Health Research Institute, DOH99-TD-C-111-001 (Taiwan), NTUH 98-S1052, NTUH 99-S1383, and YongLin Healthcare Foundation (to W.-C.C.), and NSC 97-2314-B002-015-MY3 and NSC-97-2628-B-002-002-MY3 (to H.-F.T.).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hwei-Fang Tien, Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, No. 7, Chung-Shan S Rd, Taipei, Taiwan, 100; e-mail: hftien@ntu.edu.tw; or Wen-Chien Chou, Department of Laboratory Medicine and Internal Medicine, National Taiwan University Hospital, No. 7, Chung-Shan S Rd, Taipei, Taiwan, 100; e-mail: wchou@ntu.edu.tw.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal