Abstract
Autoimmunity is a surprisingly common complication of primary immunodeficiencies, yet the molecular mechanisms underlying this clinical observation are not well understood. One widely known example is provided by Wiskott-Aldrich syndrome (WAS), an X-linked primary immunodeficiency disorder caused by mutations in the gene encoding the WAS protein (WASp) with a high incidence of autoimmunity in affected patients. WASp deficiency affects T-cell antigen receptor (TCR) signaling and T-cell cytokine production, but its role in TCR-induced apoptosis, one of the mechanisms of peripheral immunologic tolerance, has not been investigated. We find that WASp-deficient mice produce autoantibodies and develop proliferative glomerulonephritis with immune complex deposition as they age. We also find that CD4+ T lymphocytes from WASp-deficient mice undergo reduced apoptosis after restimulation through the TCR. While Fas-induced cell death is normal, WASp deficiency affects TCR-induced secretion of Fas ligand (FasL) and other components of secretory granules by CD4+ T cells. These results describe a novel role of WASp in regulating TCR-induced apoptosis and FasL secretion and suggest that WASp-deficient mice provide a good model for the study of autoimmune manifestations of WAS and the development of more specific therapies for these complications.
Introduction
Wiskott-Aldrich Syndrome (WAS) is an X-linked primary immunodeficiency that affects the development and function of multiple hematopoietic cell lineages, including, T, B, and natural killer (NK) cells, dendritic cells, and platelets.1,2 Clinically, WAS is characterized by the clinical triad of thrombocytopenia, eczema, and susceptibility to infection. Autoimmune complications are also surprisingly common in WAS, occurring in 40% to 70% of patients in retrospective cohort studies, and are becoming increasingly frequent clinical management issues as these patients live longer due to more effective prophylaxis and treatment of infectious complications.2,3 Frequent autoimmune disorders in WAS patients can include autoimmune hemolytic anemia, thrombocytopenia, nephritis, vasculitis, and inflammatory bowel disease.2–4 Interestingly, autoimmune disorders are also common complications in posthematopoietic cell transplant WAS patients when mixed chimerism is obtained.5
Most cases of WAS can be linked to loss of function mutations in the WAS protein (WASp).6 WASp is a multidomain 502 amino acid cytoplasmic protein expressed specifically in hematopoietic cells.6–8 In T cells, WASp is activated by the T-cell antigen receptor (TCR) through the small G protein Cdc42 or tyrosine phosphorylation. WASp induces branched-actin polymerization through interactions with the ARP2/3 complex. WASp deficiency results in defective formation of the immunologic synapse in WASp-deficient T cells and NK cells.6,9–12 WASp-deficient T cells proliferate poorly after T-cell receptor stimulation, but this can be largely rescued by addition of exogenous interleukin-2.13
We and others have found a defect in regulatory T-cell (Treg) homeostasis and function in WAS deficiency,14–17 providing one possible mechanism that could predispose WAS patients to develop autoimmunity. Whether TCR-induced cell death, another mechanism of peripheral immune tolerance, is affected by WASp deficiency has not been investigated. Activated T cells can undergo apoptosis in response to stimulation through the TCR, a process termed restimulation-induced cell death (RICD).18–20 This pathway can eliminate T cells responding to chronically expressed antigens, such as autoantigens and pathogens in persistent infections.21–23 In CD4+ T cells, much of this RICD depends on autocrine interactions of the tumor necrosis factor (TNF) family member Fas ligand (FasL) and its receptor Fas/CD95.20,24 Fas or FasL deficiency results in systemic autoimmunity in humans and mice,25,26 and more recently it has been found that deficiency of Fas in T cells, B cells, or dendritic cell lineages can independently lead to autoantibody production in animal models.19,27 Given the T-cell signaling defects described in WASp-deficient T lymphocytes, we hypothesized that in addition to affecting aspects of T-cell activation, WASp deficiency may impair the TCR-induced RICD pathway, contributing to the breakdown of self-tolerance and autoimmunity. Indeed, we show here that T cells from Was knockout (KO) mice have defective production of biologically active FasL after restimulation through the TCR. These defects may contribute to the development of age-dependent production of autoantibodies and immune-complex nephritis that we have seen in these animals and play a role in the onset of autoimmunity disease in patients with WAS.
Methods
Animals
WASP-deficient mice on the 129 background (129S6/SvEvTac-Wastm1Sbs/J) were obtained from The Jackson Laboratory). Some mice were backcrossed for 3 generations to C3H/HeJ and for 8 generations to C57Bl/6J, and sera were sampled as described. Mice were maintained in SPF conditions, and experiments were carried out according to the Animal Care guidelines of the National Institutes of Health (NIH) Intramural Research Program (IRP) and the Memphis VA Medical Center. All mouse experiments were approved by the NIH IRP.
Measurement of autoantibodies and circulating immune complexes
The presence of antinuclear antibodies (ANA) was determined by immunofluorescence staining of fixed HEp-2 cells (Antibodies). Cells were incubated with 1:40 diluted sera and then with Alexa 488–conjugated goat anti–mouse immunoglobulin G (IgG) antiserum (Invitrogen). Fluorescence was evaluated using fluorescent microscopy by 3 blinded observers (N.P.N., D.B., and R.M.S.) with very good interobserver reproducibility. Positive staining was defined as a distinct staining within the nucleus brighter than the staining evident in the cytoplasm. Positive sera were further serially diluted until they became negative for nuclear immunofluorescence. ANA-positive sera were tested for anti-double–stranded DNA (anti-dsDNA) using mouse enzyme-linked immunosorbent assay (ELISA; Alpha Diagnostic International), following the manufacturer's instructions. The cutoff for a positive anti-dsDNA was 2 times the absorbance of the negative control corrected for blank values. Serum circulating immune complexes were measured by ELISA according to the manufacturer's protocol (Alpha Diagnostic International).
Kidney immunofluorescence and measurement of proteinuria
Sections of one of the kidneys were fixed in buffered formalin. Five-micron sections were stained with periodic acid–Schiff (PAS) and were processed for light microscopic evaluation. Immunofluorescence was performed on 4-μm cryostat sections of the other cryopreserved kidney with the use of goat anti–mouse fluorescein isothiocyanate (FITC)–conjugated polyclonal antibody to IgG, IgA, IgM (K&P Laboratories), and rat anti-mouse FITC-conjugated monoclonal antibody to C3 (Cedarlane Laboratories, Ltd). At least 30 glomeruli from each sample were examined, and a semiquantitative grading from 0 to 4+ was given as previously described.28 Examples are shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Ig deposits were scored independently by 3 observers blinded to the origin of the samples and averaged. Urine albumin was measured by using a mouse Albumin ELISA Quantitation kit (Alpha Diagnostic International) according to the manufacturer's protocol. Urine creatinine was determined by using a urine Creatinine ELISA Quantitation kit (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol, and albumin/creatinine ratio was calculated.
T-cell activation, proliferation, and apoptosis assays
Single cell suspensions were prepared from spleen and lymph nodes from 6- to 8-week-old 129Sv WASp-deficient mice9 and age- and sex-matched 129/SvEv controls (Taconic). CD4+ T lymphocytes were prepared using CD4+ T-cell enrichment columns (R&D Systems) and were routinely > 90% pure. CD4+ T cells were primed with plate-bound anti-CD3 antibody (2C11; BD Pharmingen) at 5 μg/mL and anti-CD28 antibody (BD Pharmingen) at 5 μg/mL at 37°C in complete RPMI medium (RPMI 1640, supplemented with 10% fetal calf serum [FCS], pen/strep, 2.5mM L-glutamine, 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 1mM sodium pyruvate, and nonessential amino acids) in the presence of 50 U/mL recombinant human interleukin-2 (IL-2; National Cancer Institute [NCI]–Frederick). After 3 days, cells were washed twice and replated at 1 × 106/mL in complete medium with 50 U/mL IL-2. After another 3 days, activation status was assessed by flow cytometry for CD4, CD25, and Fas on a FACSCalibur flow cytometer (Becton Dickinson). Proliferation was assayed by pulsing activated T cells for 24 hours with 1 uCi/well of 3H-thymidine and measuring incorporation of 3H-thymidine into DNA by standard methods. For pre- and postactivation monitoring of proliferation, CD4+ T cells were labeled with 5μM carboxyfluorescein diacetate, succinimidyl ester (CFSE), and examined for CFSE dilution every 24 hours. The division index (average number of cell divisions in the population) was calculated with FlowJo Version 8.8.6 software (TreeStar). To induce apoptosis, activated CD4+ T cells were incubated for 6 hours with plate-bound anti-CD3 Ab (2C11; BD Pharmingen) or soluble Mega FasL (a generous gift from Apotech, Lausanne, Switzerland). Cells were then analyzed for viability and apoptosis by staining for annexin V and propidium iodide (PI) uptake on a FACSCalibur as previously described.29 Anti-FasL (MFL3; BD Pharmingen) was used to determine whether apoptosis was specifically caused by FasL. For inducing cell death due to cytokine withdrawal, after initial activation for 48 hours with anti-CD3/CD28 and culture with IL-2 for 3 days, T cells were washed 3 times and placed in complete media with 50 U/mL IL-2 or without exogenous cytokines. Viable cell number was determined by trypan blue exclusion, and cell viability was determined by flow cytometry using PI and the mitochondrial dye DiOC (Invitrogen). Specific cell death was determined as follows: % specific death = 1 − (%viable treated/%viable untreated) × 100%.
Measurement of FasL mRNA, protein, and function
RNA was prepared using Trizol (Invitrogen) and RNeasy mini kit (QIAGEN) and amplified using one-step Superscript II RT-PCR (Invitrogen). Applied Biosystems primer/probe sets for FasL and β-2 microglobulin as standard were used. RNA induction was quantified using the ΔCT method. To evaluate FasL secretion, supernatants from the cell death assays were collected at 6 hours, and the amount of FasL was quantified using mouse ELISA (Fas Ligand/TNFSF6 Duoset; R&D Systems) according to the manufacturer's recommendations. FasL surface levels were tested by FACS using anti-CD178-PE (BD Pharmingen). FasL was collected from activated T cells cultured at 4 × 106 cells/mL with anti-CD3 for 6 hours in the presence of IL-2. Supernatants were prespun at 10 000g for 30 minutes to remove cellular debris, and cell-free supernatant was then fractionated by centrifugation through Microcon YM-100 membranes after prewetting with a 1% bovine serum albumin (BSA) solution in phosphate-buffered saline (PBS). The concentrated fraction (> 100 kDa) was rediluted to the original volume. FasL activity was assayed on WEHI-279 B cell lymphoma cells (ATCC) at 2.5 × 105 cells/mL mixed at a 1:3 ratio with T-cell supernatants for 23 hours. Apoptosis was measured with the Cell Titer Glo cell viability assay (Promega). Anti-FasL (10 μg/mL, MFL3; BD Pharmingen) in the presence of Fc receptor blockade with 2.4G2 was added to some samples to determine whether apoptosis was caused by FasL. Purified soluble FasL was obtained from R&D Systems, and vesicular FasL was obtained from Upstate/Millipore.
β-Hexosaminidase release assay
Degranulation induced via TCR restimulation was determined by β-hexosaminidase release. CD4+ or CD8+ T cells were incubated at 5.0 × 105 cells/mL in Tyrode buffer (135mM NaCl, 5mM KCl, 1mM MgCl2, 1.8mM CaCl2, 5.6mM glucose, 20mM HEPES, pH 7.4). Cells were then restimulated with plate-bound murine anti-CD3e in the presence of 50 U/mL IL-2 for 4 hours at 37°C. The reaction was terminated by centrifugation at 4°C. The supernatant was collected, and the total hexosaminidase concentration was obtained by cell lysis in 1% Triton X-100. Aliquots of the supernatants and total cell lysates were incubated with 50μL 1mM p-nitrophenyl-N-acetyl-D-glucopytanoside (p-NAG; Sigma-Aldrich) substrate for 1 hour at 37°C in 0.1 M sodium citrate buffer (pH 4.5) at 37°C. The reaction was terminated by the addition of 100 μL 0.1 M carbonate/bicarbonate buffer. The release of the product 4-p-nitrophenol was read by optical absorbance at 405 nm. Percentage of β-hexosaminidase release was calculated as follows: (stimulated sup − unstimulated sup)/unstimulated total) × 100.
Statistical analysis
Quantitative statistics were computed by Student t test. Categorical variables were assessed using Fisher exact and χ2 tests. Two-sided P values were used for all analyses, and the level of statistical significance was set a priori at .05. Statistical analyses were performed using SAS E-Guide, Version 3 for Windows (SAS Institute) and Prism Version 4 for Macintosh (GraphPad Software).
Results
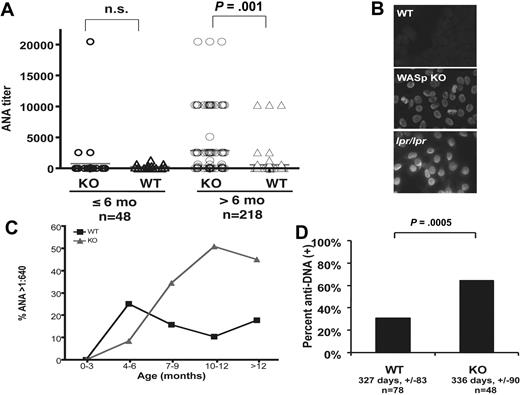
To screen for manifestations of autoimmunity in a genetically defined animal model of WAS, we first evaluated autoantibody production in WASp-deficient mice. Sera from WASp-deficient mice on a 129 SvEv/Tac background were screened for ANA and compared with those from wild-type (WT) controls. A total of 266 serum samples were tested. Forty-seven percent of WASp-deficient mice more than 6 months old had an ANA titer of greater than 1:640 (as high as 1:20 480) vs 25% in 129 age-matched controls, with significantly higher ANA titers in older WASp-deficient mice (Figure 1A). The prevalence of ANA seropositivity remained significantly higher in WASp-deficient mice compared with WT controls at all titers higher than 1:640 (P < .002, Wilcoxon 2-sample test). The pattern of ANA observed was typically homogeneous, similar to that seen in Fas-deficient lpr/lpr control sera (Figure 1B). There was a clear age-dependence of ANA production (Figure 1C), and logistic regression analysis revealed significant independent contributions of WASP genotype (P = .0138) and age (P = .046).
Autoantibody production WASp-deficient mice. (A) Fluorescent ANA titers in WASp-deficient and age-matched control mice on 129SvEv genetic background divided into 2 groups based on age (> 6 months and < 6 months). Statistical comparison was performed using the Fisher exact test. A total of 266 serum samples were tested. For the group less than or equal to 6 months old (n = 48), the mean age ± SD in days was WASp-deficient, 119 ± 35; WT controls, 104 ± 33 (P = .18). For the group more than 6 months old (n = 218), the mean age ± SD in days was WASp-deficient, 325 ± 98; WT controls, 303 ± 83 (P = .08). (B) Representative images of 129 background WASp ANA (serum dilution 1:640) with positive (B6.lpr/lpr 1:640) and negative 129 SvEv WT controls (serum dilution 1:40). Note the staining of mitotic figures by serum from the WASp-deficient mouse, suggesting the presence of anti-chromatin antibodies. (C) Kaplan-Meier analysis of the proportion of mice becoming ANA-positive at 1:640 cutoff titers over time (solid line, WT controls; dashed line, WASp-deficient mice). (D) Sera from ANA-positive mice tested for anti-dsDNA specificity by ELISA and expressed as a percent of the total tested samples per genotype. Mean age in days ± SD (not statistically significantly different) and number of mice per group are indicated (anti-dsDNA–negative, □; anti-dsDNA–positive, ■).
Autoantibody production WASp-deficient mice. (A) Fluorescent ANA titers in WASp-deficient and age-matched control mice on 129SvEv genetic background divided into 2 groups based on age (> 6 months and < 6 months). Statistical comparison was performed using the Fisher exact test. A total of 266 serum samples were tested. For the group less than or equal to 6 months old (n = 48), the mean age ± SD in days was WASp-deficient, 119 ± 35; WT controls, 104 ± 33 (P = .18). For the group more than 6 months old (n = 218), the mean age ± SD in days was WASp-deficient, 325 ± 98; WT controls, 303 ± 83 (P = .08). (B) Representative images of 129 background WASp ANA (serum dilution 1:640) with positive (B6.lpr/lpr 1:640) and negative 129 SvEv WT controls (serum dilution 1:40). Note the staining of mitotic figures by serum from the WASp-deficient mouse, suggesting the presence of anti-chromatin antibodies. (C) Kaplan-Meier analysis of the proportion of mice becoming ANA-positive at 1:640 cutoff titers over time (solid line, WT controls; dashed line, WASp-deficient mice). (D) Sera from ANA-positive mice tested for anti-dsDNA specificity by ELISA and expressed as a percent of the total tested samples per genotype. Mean age in days ± SD (not statistically significantly different) and number of mice per group are indicated (anti-dsDNA–negative, □; anti-dsDNA–positive, ■).
We also examined ANA in sera from WASp-deficient mice backcrossed for 8 generations to C57Bl/6. On this background, we found statistically significantly higher ANA prevalence (P = .005, Fisher exact test, n = 16) and titers (P = .031, Wilcoxon 2-sample test, n = 16) in WASp-deficient mice compared with WT controls older than 6 months. Similar trends were seen in sera from WASp-deficient mice backcrossed for 3 generations to C3H (50% ANA-positive WASp-KO sera vs 0% WT controls, n = 13, P = .07) in animals more than 6 months old. To determine the specificity of the autoantibodies produced in WASp-deficient mice, we measured anti-dsDNA antibody titers in ANA-positive mice. ANA-positive sera from WASp-deficient mice were positive for anti-dsDNA in 64% vs 31% in WT controls (Figure 1D, P < .001, 2-tailed Fisher exact test, n = 116). Interestingly, female WASp-deficient mice were more likely to have positive anti-DNA titers compared with controls (85% vs 33%, P = .003, Fisher exact test, n = 62), but this difference did not reach statistical significance in males (48% vs 28%, P = .16, Fisher exact test, n = 54).
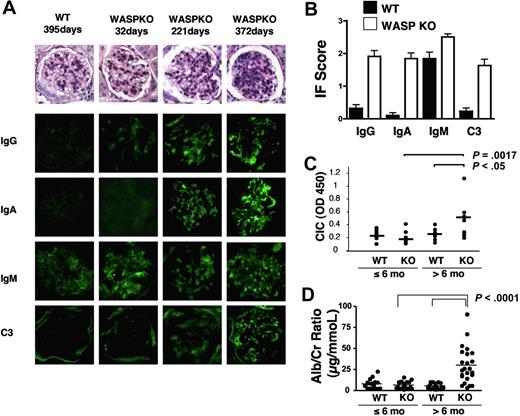
To determine whether these autoantibodies are pathogenic, we examined kidneys from 129SvEv WASp-deficient mice for evidence of immune-complex nephritis. Mild mesangial cell proliferation and glomerular basement membrane thickening was observed in PAS-stained sections from WASp-deficient mice older than 6 months, increasing in severity with age (Figure 2A). Indirect immunofluorescence showed significantly more intense glomerular IgA, IgG, and C3 complement deposition in the kidneys of WASp-deficient mice compared with WT controls (Figure 2A). Although IgM deposition was also seen, this was nonspecific as IgM deposition could also be seen in kidneys from normal mice. Quantitation of immunofluorescence results from 20 WASp-deficient mice and an equal number of matched controls confirmed these findings (Figure 2B). Circulating immune complexes capable of binding C1q were also found in the serum of WASp-deficient mice older than 6 months (Figure 2C). Measurement of the urinary albumin/creatinine ratio in WASp-deficient mice more than 6 months old revealed greater than 5-fold elevation of this measure of proteinuria (Figure 2D). These results suggest that autoantibody production in WASp-deficient mice has pathologic consequences.
Immune complex deposition and mesangial cell proliferation in WASp-deficient mice. (A) Representative PAS-stained glomeruli from WASp-deficient and 129 SvEv control mice and immunofluorescence images of IgA, IgG, IgM, and C3 deposition in glomeruli from the same animals. (B) Quantitation of immunofluorescence results from mice of the indicated genotype more than 6 months old measured as in panel B. (C) Circulating immune complexes in mice of the indicated genotypes and ages. (D) Urinary albumin/creatinine ratios in a cohort of WASp-deficient and 129 age-matched control mice.
Immune complex deposition and mesangial cell proliferation in WASp-deficient mice. (A) Representative PAS-stained glomeruli from WASp-deficient and 129 SvEv control mice and immunofluorescence images of IgA, IgG, IgM, and C3 deposition in glomeruli from the same animals. (B) Quantitation of immunofluorescence results from mice of the indicated genotype more than 6 months old measured as in panel B. (C) Circulating immune complexes in mice of the indicated genotypes and ages. (D) Urinary albumin/creatinine ratios in a cohort of WASp-deficient and 129 age-matched control mice.
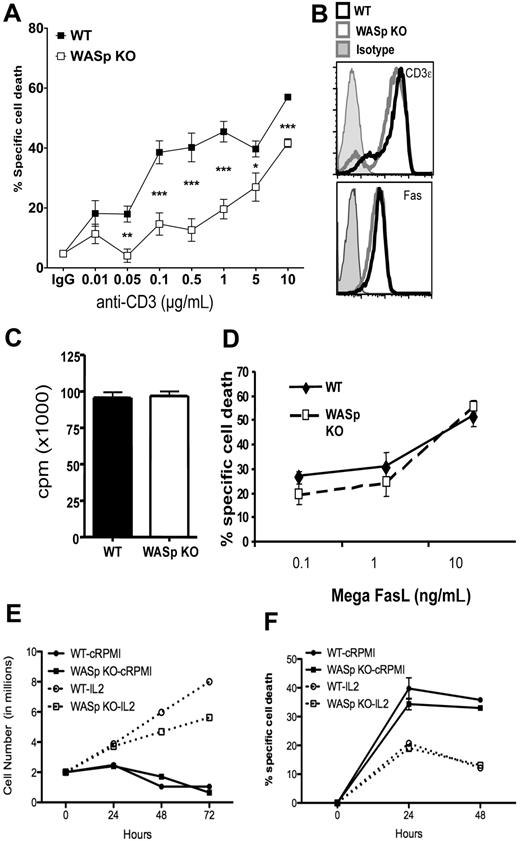
Defects in T-cell apoptosis can contribute to loss of self-tolerance and autoantibody formation.20 To determine whether WASp contributes to RICD, we examined the ability of activated CD4+ T cells from WASp-deficient mice to undergo apoptosis in response to TCR stimulation (Figure 3A). Purified CD4+ T cells were activated for 2 days and expanded in the presence of IL-2 for 3 days. Then RICD was induced by restimulation with plate-bound anti-CD3. Cell death under these conditions was dependent on FasL-Fas interactions, as blocking antibodies against FasL completely abrogated cell death (data not shown). TCR-induced cell death was significantly reduced in WASp-deficient T cells over a wide range of concentrations of anti-CD3. This was not due to differences in the surface expression of Fas or the TCR, because FACS staining revealed similar levels of these molecules on the surface of activated T cells from WASp-deficient and control mice (Figure 3B). TCR-induced apoptosis requires cell cycle progression through G1/S phase,30,31 so reduced apoptosis of WASp-deficient T cells could be due to reduced cycling. However, thymidine incorporation by WASp-deficient cells was similar to controls after 3 days of activation (Figure 3C). Cell division in WASp-deficient cells as assayed by CFSE dilution was initially delayed at 48 hours after initial activation, but was then comparable to controls during the expansion phase of culture when RICD is measured (supplemental Figure 2). Apoptosis induced by addition of a biologically active form of oligomerized FasL was intact (Figure 3D), indicating that the reduced TCR-induced cell death of WASp-deficient T cells is not due to a failure of Fas signaling. Previous studies have noted accelerated spontaneous apoptosis of cells from WAS patient lymphocytes.32,33 This may be ascribed to enhanced apoptosis in the absence of survival promoting cytokines. To determine the sensitivity of WASp-deficient T cells to this type of cell death, we cultured activated CD4+ T cells in the absence of cytokines and monitored viable cell number and viability (Figure 3E-F). Cell numbers were slightly lower, but viability was comparable between WASp and control T cells, with both showing significant cell death after withdrawal of IL-2. Thus, changes in the intrinsic cell death pathway do not appear to compensate for defective TCR-induced death of WASp-deficient T cells.
Impaired TCR-mediated apoptosis of activated WASp-deficient CD4+ T lymphocytes. (A) Specific cell death of activated CD4+ T cells from WT and WASp deficient mice restimulated with the indicated concentrations of plate-bound anti-CD3 mAb for 6 hours and measured by annexin V and PI staining. The data are average and SEM of 5 independent experiments with age- and sex-matched mice on the 129 backgrond. Apoptosis measurements were performed in triplicate for each sample. Results of unpaired t test comparisons of cell death at each dose of anti-CD3 are shown as *P < .05, **P < .005, ***P < .001. (B) Surface TCR, Fas on activated CD4+ T cells from WASp-deficient and control mice activated as in panel A. (C) Proliferation of WT and WASp-deficient T cells activated in the presence of exogenous IL-2 as in panel A measured by 3H-thymidine incorporation at 72 hours. (D) Cell death of WASp-deficient and control T-cell blasts after addition of the indicated concentrations of Mega-FasL (an oligomerized biologically active form of soluble FasL). (E) Viable cell number during IL-2 cytokine deprivation (cRPMI) or with IL-2 measured by trypan blue exclusion. (F) Cell viability measured by PI and DiOC of WASp-deficient and control CD4+ T cells during IL-2 cytokine withdrawal. (E-F) Data are representative of 2 independent experiments.
Impaired TCR-mediated apoptosis of activated WASp-deficient CD4+ T lymphocytes. (A) Specific cell death of activated CD4+ T cells from WT and WASp deficient mice restimulated with the indicated concentrations of plate-bound anti-CD3 mAb for 6 hours and measured by annexin V and PI staining. The data are average and SEM of 5 independent experiments with age- and sex-matched mice on the 129 backgrond. Apoptosis measurements were performed in triplicate for each sample. Results of unpaired t test comparisons of cell death at each dose of anti-CD3 are shown as *P < .05, **P < .005, ***P < .001. (B) Surface TCR, Fas on activated CD4+ T cells from WASp-deficient and control mice activated as in panel A. (C) Proliferation of WT and WASp-deficient T cells activated in the presence of exogenous IL-2 as in panel A measured by 3H-thymidine incorporation at 72 hours. (D) Cell death of WASp-deficient and control T-cell blasts after addition of the indicated concentrations of Mega-FasL (an oligomerized biologically active form of soluble FasL). (E) Viable cell number during IL-2 cytokine deprivation (cRPMI) or with IL-2 measured by trypan blue exclusion. (F) Cell viability measured by PI and DiOC of WASp-deficient and control CD4+ T cells during IL-2 cytokine withdrawal. (E-F) Data are representative of 2 independent experiments.
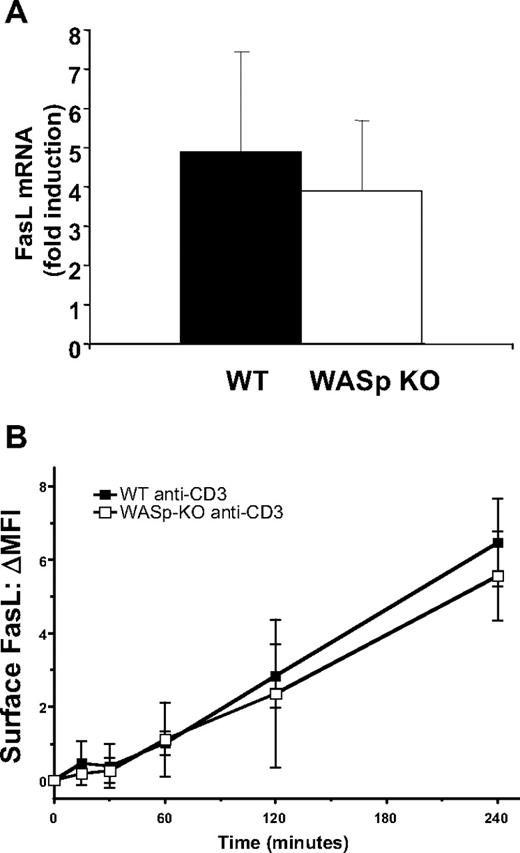
Because FasL-induced apoptosis was normal in WASp-deficient cells, WASp may instead regulate FasL production or secretion after TCR stimulation. We measured FasL mRNA induction in response to TCR stimulation of activated CD4+ T cells from WASp-deficient mice and controls. As shown in Figure 4A, induction of FasL mRNA was not significantly different between T cells from WASp-deficient mice and controls. FasL was also normally up-regulated on the plasma membrane after TCR restimulation in WASp-deficient T cells (Figure 4B). These results indicate that the components of TCR signaling responsible for the transcriptional induction of FasL and transport to the plasma membrane are not dependent on WASp.
Normal up-regulation of FasL mRNA and surface expression in WASp-deficient T cells. (A) Induction of FasL mRNA measured by real-time quantitative polymerase chain reaction (RT-qPCR) after exposure of activated CD4+ T lymphocytes to 1 μg/mL plate-bound anti-CD3 antibody for 6 hours. Induction of mRNA is shown relative to stimulation with isotype control antibodies. Average and SD of mRNA induction is shown. Similar results were observed in 2 independent experiments. (B) WT and WASp-deficient T cells were restimulated with anti-CD3 for the indicated number of hours, and surface FasL was quantitated by FACS. The mean change in geometric mean fluorescence is shown for WT and WASp-KO T cells restimulated for the indicated periods of time with anti-CD3. The data are the average ± SEM of 3 independent experiments.
Normal up-regulation of FasL mRNA and surface expression in WASp-deficient T cells. (A) Induction of FasL mRNA measured by real-time quantitative polymerase chain reaction (RT-qPCR) after exposure of activated CD4+ T lymphocytes to 1 μg/mL plate-bound anti-CD3 antibody for 6 hours. Induction of mRNA is shown relative to stimulation with isotype control antibodies. Average and SD of mRNA induction is shown. Similar results were observed in 2 independent experiments. (B) WT and WASp-deficient T cells were restimulated with anti-CD3 for the indicated number of hours, and surface FasL was quantitated by FACS. The mean change in geometric mean fluorescence is shown for WT and WASp-KO T cells restimulated for the indicated periods of time with anti-CD3. The data are the average ± SEM of 3 independent experiments.
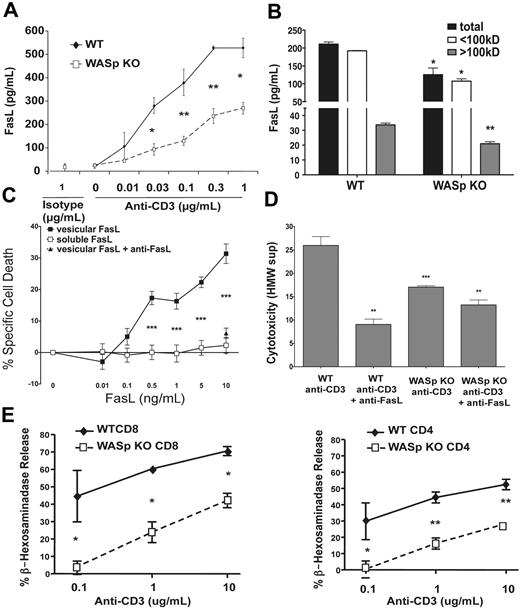
In addition to expression on the plasma membrane as a type II transmembrane protein, FasL can also be secreted as a soluble molecule or in secretory vesicles. Soluble FasL is produced after cleavage of the extracellular domain by metalloproteinases, and this form of FasL has generally been found to be nonfunctional in inducing cell death.34,35 FasL is secreted in membrane-bound form on the surface of exosomal vesicles derived from secretory lysosomes that contain enzymes such as granzymes and β-hexosaminidase.36 This form of FasL has been shown to be potently cytotoxic for Fas-expressing target cells.37 To test whether WASp might affect FasL secretion through either of these pathways, we measured FasL present in the supernatant of restimulated T cells from WASp-deficient and control T cells (Figure 5A). We found reduced amounts of FasL in the supernatant of restimulated WASp-deficient T cells. To determine whether WASp is required for the secretion of FasL in exosomes or in soluble form, we filtered the supernatants of restimulated WASp-deficient or control T cells through 100-kDa cutoff membranes that concentrate FasL in high-molecular weight vesicles but allow flow through of soluble FasL (supplemental Figure 3). WASp-deficient restimulated T cells secreted less FasL in both of these fractions (Figure 5B). To determine whether the reduced FasL secretion was functionally significant, we added T-cell supernatants to the Fas-sensitive WEHI-279 lymphoma cell line. As previously described,37 purified vesicular, but not soluble, FasL induced cell death in these cells, and a FasL blocking antibody could inhibit the cell death induced by vesicular FasL (Figure 5C). We then applied the supernatants from restimulated WT or WASp-deficient T cells to WEHI-279 cells to measure the cytotoxicity of these fractions (Figure 5D). Significant cyotoxicity could be found in the high-molecular weight fraction of WT T-cell supernatants and this could be blocked by anti-FasL, indicating that FasL was the principal cytotoxic molecule present in this fraction. The cytotoxicity of the high-molecular weight fraction of supernatants from WASp-deficient T cells was significantly reduced compared with WT supernatants in this assay. The residual cytotoxicity in the supernatant of WASp-deficient cells was partially inhibited by FasL blockade, but not as strongly as in WT supernatants. The low molecular weight fraction of both WT and WASp-deficient T cells induced less than 10% cytotoxicity (data not shown). To determine whether WASp more generally affects release of secretory lysosomes, we measured specific release of β-hexosaminidase by WASp-deficient and control CD4+ and CD8+ T cells after TCR restimulation. Secretion of β-hexosaminidase was significantly reduced in both CD4+ and CD8+ activated, WASp-deficient T cells (Figure 5E), suggesting that the defect in FasL secretion was tied to reduced secretion of exosomes by restimulated WASp-deficient T cells. Taken together, this data suggests that the decreased cell death in restimulated WASp-deficient T cells results at least in part from a deficiency in the production of biologically active FasL.
Reduced bioactive FasL and granule secretion by WASp-deficient T cells. (A) Secreted FasL measured by ELISA in supernatants after 6 hours of stimulation of activated CD4+ T lymphocytes with the indicated concentrations anti-CD3 antibody. Values are the average ± SEM of data from 2 mice per group, and similar results were obtained in 3 independent experiments. (B) Supernatants from CD4+ T cells restimulated for 6 hour with anti-CD3 were fractionated by centrifugation through 100-kDa cutoff membranes, and FasL was quantitated in each fraction by ELISA. No FasL was detected in the < 100-kDa fraction when purified vesicular FasL was filtered through identical membranes. (C) The indicated concentrations of purified vesicular and soluble FasL were added to WEHI-279 cells, and cytotoxicity was quantitated by a luminescent cell viability assay. Anti-FasL antibody was added to demonstrate specificity of this assay for bioactive FasL. Specific cell death was quantitated as described in the methods. (D) FasL-dependent apoptosis-inducing activity of the indicated fractions of supernatants collected from cells WT and WASp-KO T cells. Supernatants from cells restimulated in C were assayed on WEHI-279 cells for apoptosis-inducing activity. Anti-FasL was added to the indicated samples to neutralize FasL activity. Asterisks mark the results of comparisons of WASp-KO with the identical WT cell supernatant fractions, and anti-FasL–treated samples compared with the same samples without anti-FasL. Results of P values from comparisons using Student unpaired t test are denoted as *P < .05, **P < .005, ***P < .001. (E) β-Hexosaminadase release from activated WASp-deficient and control T cells restimulated with the indicated concentrations of anti-CD3 mAb. The curve of percent specific release was significantly different in WASp-deficient mice for both CD4 and CD8 than controls (P < .001, 2-way analysis of variance).
Reduced bioactive FasL and granule secretion by WASp-deficient T cells. (A) Secreted FasL measured by ELISA in supernatants after 6 hours of stimulation of activated CD4+ T lymphocytes with the indicated concentrations anti-CD3 antibody. Values are the average ± SEM of data from 2 mice per group, and similar results were obtained in 3 independent experiments. (B) Supernatants from CD4+ T cells restimulated for 6 hour with anti-CD3 were fractionated by centrifugation through 100-kDa cutoff membranes, and FasL was quantitated in each fraction by ELISA. No FasL was detected in the < 100-kDa fraction when purified vesicular FasL was filtered through identical membranes. (C) The indicated concentrations of purified vesicular and soluble FasL were added to WEHI-279 cells, and cytotoxicity was quantitated by a luminescent cell viability assay. Anti-FasL antibody was added to demonstrate specificity of this assay for bioactive FasL. Specific cell death was quantitated as described in the methods. (D) FasL-dependent apoptosis-inducing activity of the indicated fractions of supernatants collected from cells WT and WASp-KO T cells. Supernatants from cells restimulated in C were assayed on WEHI-279 cells for apoptosis-inducing activity. Anti-FasL was added to the indicated samples to neutralize FasL activity. Asterisks mark the results of comparisons of WASp-KO with the identical WT cell supernatant fractions, and anti-FasL–treated samples compared with the same samples without anti-FasL. Results of P values from comparisons using Student unpaired t test are denoted as *P < .05, **P < .005, ***P < .001. (E) β-Hexosaminadase release from activated WASp-deficient and control T cells restimulated with the indicated concentrations of anti-CD3 mAb. The curve of percent specific release was significantly different in WASp-deficient mice for both CD4 and CD8 than controls (P < .001, 2-way analysis of variance).
Discussion
We describe here a high incidence of autoantibodies and immune-complex nephritis in WASp-deficient mice. The pathologic features or renal impairment in WASp-deficient mice are not as severe as seen in complete Fas deficiency on a Murphy Roths Large (MRL) background or the NZB/NZW mouse model, but are reminiscent of human IgA nephropathy that has been reported in WAS.38 Anti-dsDNA antibodies were also increased in the serum of ANA-positive WASp-deficient mice. Since we see apoptosis defects in mice less than 3 months of age, before the development of autoantibodies, and autoantibodies are not seen in every animal, additional environmental factors likely play a role in the production of autoantibodies in the setting of WASp deficiency. Although our findings delineate an apoptosis defect in T cells, other cell lineages may have reduced apoptosis in response to the Fas ligand defect we describe here and also play a role in the pathogenesis of autoimmunity in WASp deficiency. Other mechanisms, such as defects in regulatory T cells in WAS deficiency14–17 may also contribute to the development of autoimmunity. Nevertheless, our findings suggest that WASp-deficient mice represent a useful model of the autoimmune and renal manifestations in WAS.
These results show for the first time that WASp is important not only in antigen-driven primary T-cell activation, but also in TCR-mediated restimulation and apoptotic cell death of previously activated T cells. Unlike the situation in lpr mice or the autoimmune lymphoproliferative syndrome, Fas-induced apoptosis is intact in WASp-deficient T cells, and the defect in FasL function is not as severe as is seen in FasL mutant gld mice.26 For this reason, it is not surprising that WASp-deficient mice do not show all the features of Fas or FasL deficiency, such as generalized lymphadenopathy and accumulation of peripheral CD4−CD8− “double-negative” T cells. We have looked for but not observed anti-red blood cell (RBC) antibodies that are seen in WAS patients nor the colitis that has been observed by others in WASp-deficient mice. However, it is interesting that the colitis associated with WASp deficiency has recently been shown to be transferable by T cells.39 We are currently performing experiments to specifically re-express WASp in T cells of WASp-deficient mice to determine whether the autoantibody production we see in WASp deficent mice stems from a T-cell intrinsic defect.
Our data suggests that reduced secretion of biologically active FasL contributes to inefficient TCR-induced apoptosis in the absence of WASp. Soluble FasL was also reduced in the supernatants of WASp-deficient T cells, suggesting that metalloprotease-mediated cleavage of FasL may also be reduced in WASp deficiency, although this did not result in measurable increases in surface FasL. Reduced apoptosis of WASp-deficient T cells is more likely attributable to the deficiency in membrane-bound FasL, since only this form of FasL induces significant cytotoxicity and protects against autoimmunity.35 The fact that surface expression of FasL was normal despite reduced RICD suggests that the vesicular form of FasL may be important in autocrine T-cell death.
WASp may influence trafficking of FasL into secretory lysosomes or may more generally affect trafficking of secretory lysosomes into multivesicular bodies and fusion of these structures with the plasma membrane.36,40 The reduced release of the secretory lysosome component β-hexosaminidase by WASp-deficient T cells supports the notion of a more generalized granule secretion defect in WASp deficiency. WASp-deficient T cells have been found to have defects in secretion of other cytokines such as IL-2 and interferon-γ (IFN-γ).41 Whether or not these defects result from the same mechanism that impairs vesicular FasL secretion is not known. Other cell types such as mast cells and NK cells have been shown to have defects likely related to abnormal granule secretion in the setting of WASp deficiency.10,42 Although B cell–specific deletion of Fas can result in autoantibody production,43 the mechanism we have uncovered here of defective FasL production is unlikely to affect B cells directly, as B cells are generally not thought to produce Fas ligand in sufficient quantity to induce autocrine apoptosis within the B cell compartment. Interestingly, other immunodeficiencies with reduced granule exocytosis, such as Griscelli syndrome associated with Rab27a deficiency and Hermansky-Pudlak syndrome due to AP3 deficiency, predispose patients to immunopathologic complications such as the hemophagocytic syndrome.44,45 Whether secretion of FasL is defective in these diseases is also not known.
Other mechanisms may also contribute to the pathogenesis of autoimmunity in WASp deficiency. Delayed phagocytosis of apoptotic cells has been reported in WASp deficiency and might be another potential mechanism of loss of peripheral tolerance.46 However, we have not found significant cell uptake defects in macrophages from WASp-deficient mice (supplemental Figure 4). The lymphopenia reported in WAS patients and WASp-deficient mice may also play an independent role in predisposing toward autoimmunity,47 although the degree of lymphopenia in WAS is not as severe as other human syndromes or mouse models associated with autoimmunity. The function and homeostasis of Treg are also affected by WASp deficiency.14–17 Since Treg may exert some of their suppressive effects through secretory lysosome components,48,49 WASp may regulate Treg function through similar mechanisms by which it controls TCR-induced cell death. Better understanding of these mechanisms may aid in the design of specialized therapies for autoimmune complications in WAS that avoid generalized immunosuppression, which is especially important in patients with concomitant immunodeficiency.
The online version of this article contains a data supplement.
Acknowledgments
We thank the animal care staffs of the NHGRI and NIAMS for their assistance, particularly Joseph Woo and Robin Handon. We also thank Mike Lenardo and Silvia Bolland for helpful discussion and comments and Jeffrey Kopp for assistance with immunofluorescence studies. We wish to acknowledge Rochelle Wallace, who performed initial experiments related to this work, and Taizo Wada, whose initial observations stimulated these investigations.
This work was supported by intramural research program of NIAMS and NHGRI at the NIH, and also by a Bench-to-Bedside award funded by the NIH Office of Rare Diseases, NHGRI, and NIAMS. J.A. was a Howard Hughes Medical Institute–NIH scholar.
National Institutes of Health
Authorship
Contribution: N.P.N., M.S., D.B., S.C., and J.A. designed and carried out experiments and analyzed data; T.S. provided reagents and analyzed data; P.L.S., F.C., and R.M.S. interpreted data, designed experiments, and supervised the project; N.P.N., D.B., S.C., and R.M.S. wrote the paper; and all authors participated in editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Nikolay P. Nikolov is Molecular Physiology and Therapeutics Branch, National Institute of Dental and Craniofacial Research (NIDCR), NIH, Bethesda, MD. The current affiliation for Masaki Shimizu is Department of Pediatrics, School of Medicine, Institute of Medical, Pharmaceutical, and Health Sciences, Kanazawa University, Japan.
Correspondence: Richard M. Siegel, NIH, 10 Center Dr, Bldg 10, Rm 13C103, Bethesda, MD 20892; e-mail: siegelr@mail.nih.gov.
References
Author notes
N.P.N., M.S., and S.C. contributed equally to this work.