Analysis of overall and site-specific cancer risk in patients with primary immunodeficiency diseases (PIDs) may shed light on the role played by the immune system in controlling malignancies. The association of PID and cancer has been known for many years.1 However, until recently, data on cancer risk in patients with PID have mostly derived from volunteer reporting to PID registries1-3 or from single-center studies.4 In this issue of Blood, Vajdic et al report on 1132 patients with PID whose data were entered into the Australasian Society of Clinical Immunology and Allergy (ASCIA) PID Registry and matched with those of the Australian National Death Index and of the Australian National Cancer Database.5 Because registration of primary invasive cancers is mandatory in Australia, the study by Vajdic et al represents the first large population-based analysis of cancer risk in PID, relative to the general population.
Patients with PID were found to have a 1.6 excess standardized incidence rate (SIR) of cancer. The risk was higher for non-Hodgkin lymphoma, leukemia, and thymic and stomach cancer. When PIDs were divided into major subgroups based on the nature of the immune defect, an increased risk of cancer was confirmed only for patients with predominantly antibody deficiencies, immune-deficiency syndromes, and diseases of immune dysregulation.
Previous studies had shown that patients with iatrogenic or acquired immunodeficiency are at higher risk for a variety of solid tumors with an infectious etiology,6 indicating that cell-mediated immunity is essential in the surveillance against such tumor-promoting agents. In contrast, Vajdic et al found that of all cancers with possible infectious origin, an increased SIR in patients with PID was identified only for stomach cancer. An increased risk of stomach and colon cancer among PID patients with antibody deficiencies had been previously reported.7,8 Vajdic et al conclude that patients with primary antibody deficiency may be at higher risk for a narrower range of malignancies than patients with iatrogenic or acquired immune deficiency, presumably because their cell-mediated immunity is intact.
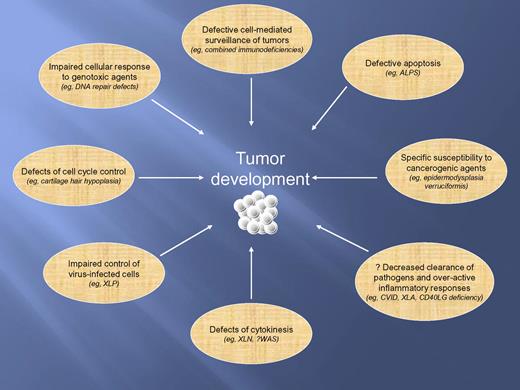
Overall, the report by Vajdic et al illustrates the importance of investigating patients with rare disorders to better understand the pathophysiology of cancer. However, this study has several limitations. Only a minority of patients with PID were reported to the ASCIA PID Registry, no active follow-up was performed, and no quality control of the specific PID diagnosis was attempted. Because of the rarity of PIDs, underreporting or misreporting of patients may introduce a significant bias in the assessment of risk of cancer. Furthermore, some forms of PIDs are very severe, and require radical intervention with hematopoietic cell transplantation early in life to prevent death. If successful, this treatment is curative, thus impacting on the actual incidence of cancer in PIDs.9 This may explain the difference of SIR of cancer between the cohort of PID patients studied by Vajdic et al and patients with iatrogenic or acquired immune deficiencies. Finally, the criteria in use to classify PIDs are not adequate to describe the mechanisms of susceptibility to cancer in various forms of PID (see figure). For example, PIDs with combined T- and B-cell deficiency include defects of DNA repair that carry a well-defined risk of transformation due to genomic instability, not just a lack of effective immune surveillance.10
Mechanisms contributing to susceptibility to tumors in patients with PIDs. ALPS indicates autoimmune lymphoproliferative syndrome; CVID, common variable immunodeficiency diseases; CD40LG, CD40 ligand gene; WAS, Wiskott-Aldrich syndrome; XLA, X-linked agammaglobulinemia; XLN, X-linked neutropenia (allelic to WAS); and XLP, X-linked lymphoproliferative disease.
Mechanisms contributing to susceptibility to tumors in patients with PIDs. ALPS indicates autoimmune lymphoproliferative syndrome; CVID, common variable immunodeficiency diseases; CD40LG, CD40 ligand gene; WAS, Wiskott-Aldrich syndrome; XLA, X-linked agammaglobulinemia; XLN, X-linked neutropenia (allelic to WAS); and XLP, X-linked lymphoproliferative disease.
Nonetheless, the study by Vajdic et al demonstrates the power of linking data derived from population-based and disease-specific registries to gain insights into the pathophysiology of cancer. With the development of PID registries in many areas of the world, the findings of this study are expected to be confirmed or challenged. Regional differences in the incidence of cancer among patients with PID might also emerge because of the distribution variability of various types of PID, exposure to tumor-promoting pathogens, and frequency of genetic variants conferring cancer susceptibility among different populations.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal