Abstract
The effectiveness of antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia (ET) is not proven. In this study, the incidence rates of arterial and venous thrombosis were retrospectively analyzed in 300 low-risk patients with ET treated with antiplatelet drugs as monotherapy (n = 198) or followed with careful observation (n = 102). Follow-up was 802 and 848 person-years for antiplatelet therapy and observation, respectively. Rates of thrombotic events were 21.2 and 17.7 per 1000 person-years for antiplatelet therapy and observation, respectively (P = .6). JAK2 V617F–positive patients not receiving antiplatelet medication showed an increased risk of venous thrombosis (incidence rate ratio [IRR]: 4.0; 95% CI: 1.2-12.9; P = .02). Patients with cardiovascular risk factors had increased rates of arterial thrombosis while on observation (IRR: 2.5; 95% CI: 1.02-6.1; P = .047). An increased risk of major bleeding was observed in patients with platelet count greater than 1000 × 109/L under antiplatelet therapy (IRR: 5.4; 95% CI: 1.7-17.2; P = .004). In conclusion, antiplatelet therapy reduces the incidence of venous thrombosis in patients with JAK2-positive ET and the rate of arterial thrombosis in patients with associated cardiovascular risk factors. In the remaining low-risk patients, this therapy is not effective as primary prophylaxis of thrombosis, and observation may be an adequate option.
MedscapeCME Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians. Medscape, LLC designates this educational activity for a maximum of 0.75 AMA PRA Category 1 credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://cme.medscape.com/journal/blood; and (4) view/print certificate. For CME questions, see page 1387.
Disclosures
The authors, the Associate Editor Martin S. Tallman, and the CME questions author Charles P. Vega, University of California, Irvine, CA, declare no competing interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the diagnosis of essential thrombocythemia (ET)
Gauge the efficacy of antiplatelet therapy in reducing the overall risk for thrombosis in low-risk patients with ET
Distinguish patient factors that make antiplatelet therapy more effective in preventing venous and arterial thrombosis in low-risk patients with ET
Introduction
Essential thrombocythemia (ET) is a myeloproliferative neoplasm associated with an increased tendency to thrombosis and hemorrhage.1,2 Advanced age (> 60 years) and previous vascular events predispose patients with ET to thrombotic complications and define a “high-risk” group requiring cytoreductive therapy.2,3 On the contrary, younger patients (< 60 years) without a history of prior thrombosis are considered at low risk of vascular complications and are usually managed conservatively. Some investigators segregate an “intermediate-risk” group of patients with ET on the basis of age (40-60 years) and/or the presence of cardiovascular risk factors, such as smoking, hypertension, dyslipidemia, and diabetes,4-6 but data supporting this segregation are lacking; therefore, this intermediate risk category is not universally accepted.7 The JAK2 V617F mutation has been identified in most patients with polycythemia vera (PV) and half of those with ET and primary myelofibrosis.8-10 It has been reported that patients with ET with the JAK2 V617F mutation would display some features similar to those of patients with PV, including higher hemoglobin values and a higher frequency of venous thrombosis.11
Antiplatelet therapy with low-dose acetylsalicylic acid has been shown to reduce the risk of thrombosis in patients with PV.12 In addition, hydroxyurea plus low-dose acetylsalicylic acid is the treatment of choice in high-risk ET, resulting in a significant reduction of thrombotic events.13 These observations have fostered the widespread use of low-dose aspirin as primary prophylaxis of thrombosis in patients with low-risk ET, despite the lack of evidence-based data from either randomized clinical trials or high-quality observational studies. However, although cardiovascular risk factors, presence of the JAK2 mutation, and leukocytosis have been found to be associated with an increased risk of thrombosis in ET, the clinical management of patients presenting with these features has not yet been established.14-16
The objective of the present study was to assess the effect of antiplatelet therapy in the prevention of thrombosis in patients with low-risk ET.
Methods
Study design
The medical charts of all patients diagnosed with ET in 11 institutions in Spain were reviewed. In every case, the diagnosis of ET was reassessed with the use of the updated criteria of the World Health Organization.17 Patients were eligible for inclusion in the study if they were younger than 60 years and had no prior history of thrombosis at ET diagnosis. Patients receiving cytoreductive drugs as the initial therapy for ET were excluded from the study. Antiplatelet therapy or observation was decided according to the criterion of the attending hematologist. Informed consent for the scientific use of the patients' clinicohematologic data and biologic samples was obtained in accordance with the requirements of and with approval from the local ethics committees from all participating institutions.
In all patients the main clinicohematologic data at presentation of ET were collected, including age, sex, cardiovascular risk factors (smoking, arterial hypertension, hypercholesterolemia defined as a serum cholesterol level > 200 mg/dL, and diabetes), hemoglobin value, and leukocyte and platelet counts. Evolutive data recorded included the therapeutic approach (reason for initiation, time of start, and duration); occurrence of thrombosis and bleeding; evolution to PV, myelofibrosis, or acute leukemia; and causes of death.
Outcomes
The primary outcome of the study was the occurrence of thrombosis, either arterial or venous. As a secondary outcome, the incidences of arterial and venous events were taken into account separately. The safety outcome was major hemorrhage.
Thrombosis was defined according to the International Classification of Diseases (ninth revision). Arterial thromboses included stroke, transient ischemic attacks, retinal artery occlusion, coronary arterial disease, and peripheral arterial disease. Venous thromboses included cerebral venous sinus thrombosis, deep vein thrombosis, pulmonary thromboembolism, Budd-Chiari syndrome, and splenoportal vein thrombosis. Minor occlusive events, such as erythromelalgia and superficial thrombophlebitis of the extremities, were not considered. Severe hemorrhage was defined as a symptomatic bleeding in a critical organ or an overt hemorrhage requiring transfusion or associated with a hemoglobin decrease greater than 20 g/L without transfusion.
Statistical methods
For the purpose of the present study, patients were considered to exit the time at risk of thrombosis or bleeding when they died; progressed to myelofibrosis, PV, or acute leukemia; were started on cytolytic therapy; or evolved into high-risk ET, whichever occurred first. The incidence rate of thrombosis or bleeding while the patients remained in the low-risk status was calculated as the number of events per 1000 patient-years of follow-up. The incidence rate method allows accounting for periods without antiplatelet therapy in those patients who were started on this therapy later after diagnosis or withdrew from it at some time during follow-up.
Multivariate analyses of factors influencing the incidence rate of thrombosis or bleeding were done by Poisson regression with correction for overdispersion when needed. In Poisson regression, the exponentiated coefficients of covariates can be regarded as incidence rate ratios (IRRs) and are comparable to the hazard ratios in Cox models. Variables analyzed for their independent association with the incidence rate of thrombosis or hemorrhage included age, sex, cardiovascular risk factors, hematologic values at diagnosis, and whether the patient was on antiplatelet therapy. Cutoffs for continuous variables were established at the median value for the whole series. Leukocyte count greater than 10 × 109/L and platelet count greater than 1000 × 109/L were also explored as cutoffs predictive of thrombosis or bleeding. We did not attempt to statistically determine the most discriminating cutoffs, because this practice would have probably led to over-fitting our data, thereby eroding the external validity of the results. Because the JAK2 mutational status was not available in some patients, additional Poisson models were fitted for the 243 patients with these data. Further models were fitted with interactions of antiplatelet therapy with selected patient clinical or biologic features. Because the physician's decision to initiate antiplatelet medication was not random but influenced by the patient's characteristics, a propensity score was calculated from the binary logistic regression of the initial clinical and laboratory features predicting antiplatelet therapy. The propensity score assigns to every patient a probability of being in the observation or the antiplatelet therapy groups conditional to their presenting features, and it was forced into the Poisson models to control for confounding.
Overall and event-free survival curves were drawn by the Kaplan-Meier method. The cumulative incidence curve was calculated by taking exit from the time at risk as a competing risk for thrombosis. Because exit from the time at risk is a kind of informative censoring, the competing risks framework provides an unbiased estimate of the actual incidence of thrombosis, which would have otherwise been overestimated by actuarial methods such as Kaplan-Meier.18
All the statistical analyses were done with Stata Version 10 (www.stata.com). The time-span splitting method19 was used to calculate the incidence rates and fitting the Poisson models. The cumulative incidence of thrombosis within the framework of competing risks was calculated with the “stcompet” Stata module developed by Coviello.20
Results
Characteristics of the patients and follow-up data
A total of 300 patients with low-risk ET were included in the study. Their main clinical and hematologic data at diagnosis are shown in Table 1. One hundred thirteen patients (38%) had 1 cardiovascular risk factor, 25 (8%) had 2 factors, and 5 (1.5%) had 3 or more factors.
Main clinicohematologic characteristics at diagnosis in 300 patients with low-risk ET
| . | Observation (n = 102) . | Antiplatelet therapy (n = 198) . |
|---|---|---|
| Median age, y (range) | 35 (5-59) | 41 (13-59) |
| Male/female, n/n | 36/66 | 65/133 |
| Cardiovascular risk factors | ||
| Smoking, n (%) | 28 (27) | 47 (24) |
| Hypertension, n (%) | 8 (8) | 29 (15) |
| Hypercholesterolemia, n (%) | 12 (12) | 45 (23) |
| Diabetes, n (%) | 4 (4) | 7 (4) |
| Median hemoglobin level, g/L (range) | 140 (110-170) | 141 (115-171) |
| Median WBC count, ×109/L (range) | 8.6 (4.1-18.8) | 9.1 (3.9-16.5) |
| Median platelet count, ×109/L (range) | 817 (498-2328) | 767 (457-2400) |
| JAK2 V617F mutation, n (%)* | 30 (40) | 82 (48) |
| . | Observation (n = 102) . | Antiplatelet therapy (n = 198) . |
|---|---|---|
| Median age, y (range) | 35 (5-59) | 41 (13-59) |
| Male/female, n/n | 36/66 | 65/133 |
| Cardiovascular risk factors | ||
| Smoking, n (%) | 28 (27) | 47 (24) |
| Hypertension, n (%) | 8 (8) | 29 (15) |
| Hypercholesterolemia, n (%) | 12 (12) | 45 (23) |
| Diabetes, n (%) | 4 (4) | 7 (4) |
| Median hemoglobin level, g/L (range) | 140 (110-170) | 141 (115-171) |
| Median WBC count, ×109/L (range) | 8.6 (4.1-18.8) | 9.1 (3.9-16.5) |
| Median platelet count, ×109/L (range) | 817 (498-2328) | 767 (457-2400) |
| JAK2 V617F mutation, n (%)* | 30 (40) | 82 (48) |
ET indicates essential thrombocythemia; and WBC, white blood cell.
Of 74 and 169 assessable patients for observation and antiplatelet groups, respectively.
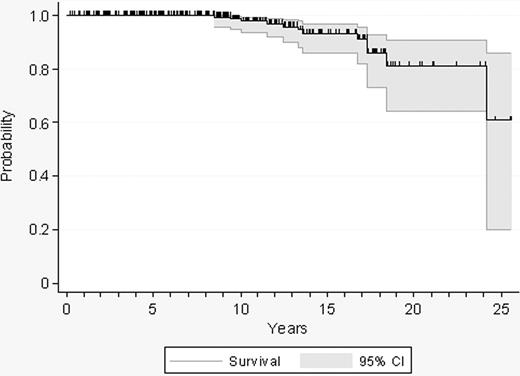
Twelve patients died after a median follow-up of 9 years (range, 0.2-25.5 years), resulting in a projected 10-year survival of 98% (Figure 1). Causes of death included acute leukemia (n = 5), bone marrow transplantation–related complications (n = 2), neoplasia (n = 2), infection after transformation of ET (n = 1), thrombosis (n = 1), and bleeding (n = 1). Other reasons for exiting the study time were disease transformation, initiation of cytolytic therapy, and progression to high-risk ET.
Probability of survival in 300 patients with low-risk essential thrombocythemia.
Probability of survival in 300 patients with low-risk essential thrombocythemia.
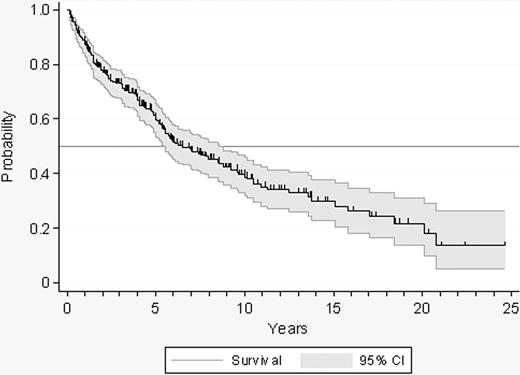
Evolution to PV, myelofibrosis, or acute leukemia was documented in 10, 19, and 6 patients, respectively. Cytolytic therapy was started in 142 patients. The indication of cytoreduction was as follows: thrombosis (n = 26), hemorrhage (n = 13), age older than 60 years (n = 19), microvascular symptoms not responding to antiplatelet drugs (n = 37), extreme thrombocytosis (n = 39), and others (n = 8). A total of 57 patients reached the age of 60 during follow-up. The overall probability of remaining in the low-risk status and being, therefore, assessable for the incidence of thrombosis and hemorrhage is shown in Figure 2.
Probability of survival free of transformation, cytolytic therapy, or evolution to high-risk ET in 300 patients with low-risk ET.
Probability of survival free of transformation, cytolytic therapy, or evolution to high-risk ET in 300 patients with low-risk ET.
Outcomes
Over the 1650 person-years of follow-up time in which the patients with ET were in the low-risk status, 102 patients were managed on a wait-and-see approach exclusively, and 198 received antiplatelet therapy (acetylsalicylic acid, 100 mg daily, n = 185; dipyridamole, 150 mg daily, n = 10; triflusal, 600 mg daily, n = 2; ticlopidine, 250 mg daily, n = 1). Seventy-eight patients were on antiplatelet therapy since the time of diagnosis, whereas antiplatelet medication was started in 120 patients, after a median of 14 months from diagnosis. Overall, 80% of the 198 patients on antiplatelet therapy started on this therapy at ET diagnosis or within the following 2 years. Ten of the 198 patients receiving antiplatelet drugs stopped this therapy at some time during the period under study. The follow-up time on antiplatelet therapy or on observation was 802 and 848 person-years, respectively.
By binary logistic regression, antiplatelet therapy was significantly more frequently administered in patients older than 40 years (P = .049), with leukocyte counts greater than 8.7 × 109/L (P = .02), and platelet counts less than 800 × 109/L (P = .001). The predicted probability of starting antiplatelet therapy for each of the 300 patients ranged from 0.52 to 0.88, and it was used as a propensity score in subsequent analyses.
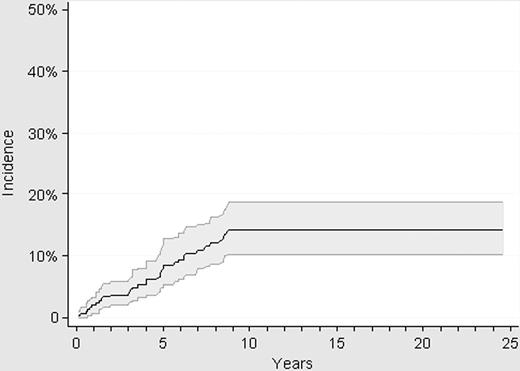
Thirty-two thrombotic events were registered while the patients were in the low-risk status, resulting in a cumulative incidence of thrombosis of 14% (95% CI: 10.2-19.1) at 10 years (Figure 3). All the thrombotic events occurred within the first 9 years after diagnosis, with no episode of thrombosis being registered in the 63 patients with a follow-up longer than 10 years. Pulmonary embolism was the cause of death in 1 patient with low-risk ET after an urgent traumatologic operation while on antiplatelet therapy.
Cumulative incidence of thrombosis in 300 patients with low-risk ET.
Cumulative incidence of thrombosis in 300 patients with low-risk ET.
A total of 32 thrombotic events were recorded in 15 and 17 patients of the observation and antiplatelet groups, respectively, with this resulting in an incidence rate of 17.7 per 1000 person-years while on observation and 21.2 per 1000 person-years while under antiplatelet therapy (P = .6 for the comparison; Table 2). None of the patients' initial features included in the multivariate analysis (age, sex, cardiovascular risk factors, hemoglobin level, leukocytosis, thrombocytosis) or antiplatelet therapy was significantly associated with the occurrence of thrombosis (Table 3). Leukocyte count greater than 10 × 109/L and platelet count greater than 1000 × 109/L were also explored in the multivariate model, with the results being similar with regard to the incidence rate ratios of total, arterial, and venous thrombosis (data not shown). The JAK2 mutation showed no association with the rate of thrombosis, neither in the whole population of patients with ET (IRR: 0.9; 95% CI: 0.4-1.9; P = .7) nor in the subgroup not receiving antiplatelet drugs (IRR: 1.3; 95% CI: 0.6-3.1; P = .5).
Outcomes in 300 patients with low-risk ET followed with careful observation or treated with antiplatelet therapy
| . | Observation (848 person-years) . | Antiplatelet therapy (802 person-years) . | P . | ||
|---|---|---|---|---|---|
| No. of events . | Incidence rate* (95% CI) . | No. of events . | Incidence rate* (95% CI) . | ||
| Thrombosis (arterial and venous) | 15 | 17.7 (10.7-29.3) | 17 | 21.2 (13.2-34.1) | .6 |
| Arterial thrombosis | 8 | 9.4 (4.7-18.9) | 13 | 16.2 (9.4-27.9) | .2 |
| Venous thrombosis | 7 | 8.2 (3.9-17.3) | 4 | 4.9 (1.9-13.3) | .4 |
| Bleeding | 5 | 6.0 (2.5-14.5) | 10 | 12.6 (6.8-23.4) | .09 |
| . | Observation (848 person-years) . | Antiplatelet therapy (802 person-years) . | P . | ||
|---|---|---|---|---|---|
| No. of events . | Incidence rate* (95% CI) . | No. of events . | Incidence rate* (95% CI) . | ||
| Thrombosis (arterial and venous) | 15 | 17.7 (10.7-29.3) | 17 | 21.2 (13.2-34.1) | .6 |
| Arterial thrombosis | 8 | 9.4 (4.7-18.9) | 13 | 16.2 (9.4-27.9) | .2 |
| Venous thrombosis | 7 | 8.2 (3.9-17.3) | 4 | 4.9 (1.9-13.3) | .4 |
| Bleeding | 5 | 6.0 (2.5-14.5) | 10 | 12.6 (6.8-23.4) | .09 |
ET indicates essential thrombocythemia.
Events per 1000 person-years.
Multivariate analysis for the primary and secondary endpoints according to the presenting features in 300 patients with low-risk ET followed with observation or treated with antiplatelet therapy
| . | Total thrombosis . | Arterial thrombosis . | Venous thrombosis . | Major bleeding . | ||||
|---|---|---|---|---|---|---|---|---|
| IRR (95% CI) . | P . | IRR (95% CI) . | P . | IRR (95% CI) . | P . | IRR (95% CI) . | P . | |
| Age older than 40 y | 1.7 (0.8-3.8) | .2 | 1.7 (0.6-4.4) | .3 | 1.7 (0.4-6.9) | .4 | 1.4 (0.4-4.9) | .6 |
| Male sex | 1.9 (0.9-3.9) | .09 | 2.8 (1.5-7.0) | .02 | 0.85 (0.2-3.4) | .8 | 1.1 (0.3-3.4) | .9 |
| Cardiovascular risk factors | 1.7 (0.8-3.7) | .2 | 2.6 (0.98-6.9) | .056 | 0.7 (0.2-2.9) | .7 | 1.0 (0.3-3.3) | .9 |
| Hemoglobin level greater than 140 g/L | 1.1 (0.5-2.3) | .8 | 0.6 (0.3-1.6) | .3 | 3.0 (0.8-11.4) | .1 | 0.6 (0.2-2.0) | .4 |
| Leukocyte count greater than 8.7 × 109/L* | 0.8 (0.4-1.8) | .6 | 0.95 (0.4-2.3) | .9 | 0.7 (0.2-3.1) | .6 | 3.8 (0.8-17.8) | .09 |
| Platelet count greater than 800 × 109/L* | 1.5 (0.7-3.2) | .3 | 1.6 (0.65-4.0) | .3 | 1.0 (0.3-4.1) | .9 | 10.6 (1.6-69.3) | .01 |
| Absence of antiplatelet therapy | 0.9 (0.4-1.8) | .7 | 0.6 (0.2-1.6) | .3 | 1.7 (0.5-6.2) | .4 | — | — |
| Antiplatelet therapy | — | — | — | — | — | — | 1.9 (0.6-6.0) | .3 |
| . | Total thrombosis . | Arterial thrombosis . | Venous thrombosis . | Major bleeding . | ||||
|---|---|---|---|---|---|---|---|---|
| IRR (95% CI) . | P . | IRR (95% CI) . | P . | IRR (95% CI) . | P . | IRR (95% CI) . | P . | |
| Age older than 40 y | 1.7 (0.8-3.8) | .2 | 1.7 (0.6-4.4) | .3 | 1.7 (0.4-6.9) | .4 | 1.4 (0.4-4.9) | .6 |
| Male sex | 1.9 (0.9-3.9) | .09 | 2.8 (1.5-7.0) | .02 | 0.85 (0.2-3.4) | .8 | 1.1 (0.3-3.4) | .9 |
| Cardiovascular risk factors | 1.7 (0.8-3.7) | .2 | 2.6 (0.98-6.9) | .056 | 0.7 (0.2-2.9) | .7 | 1.0 (0.3-3.3) | .9 |
| Hemoglobin level greater than 140 g/L | 1.1 (0.5-2.3) | .8 | 0.6 (0.3-1.6) | .3 | 3.0 (0.8-11.4) | .1 | 0.6 (0.2-2.0) | .4 |
| Leukocyte count greater than 8.7 × 109/L* | 0.8 (0.4-1.8) | .6 | 0.95 (0.4-2.3) | .9 | 0.7 (0.2-3.1) | .6 | 3.8 (0.8-17.8) | .09 |
| Platelet count greater than 800 × 109/L* | 1.5 (0.7-3.2) | .3 | 1.6 (0.65-4.0) | .3 | 1.0 (0.3-4.1) | .9 | 10.6 (1.6-69.3) | .01 |
| Absence of antiplatelet therapy | 0.9 (0.4-1.8) | .7 | 0.6 (0.2-1.6) | .3 | 1.7 (0.5-6.2) | .4 | — | — |
| Antiplatelet therapy | — | — | — | — | — | — | 1.9 (0.6-6.0) | .3 |
ET indicates essential thrombocythemia; and IRR, incidence rate ratio.
Cut points for leukocyte count greater than 10 × 109/L and platelet count greater than 1000 × 109/L were also explored by multivariate analysis but yielded no relevant change in the incidence rate ratios (data not shown).
The incidence rate of arterial thrombosis was 9.4 events per 1000 person-years during observation versus 16.2 per 1000 person-years while on antiplatelet therapy (P = .2). In the multivariate analysis, there was a trend for a higher rate of arterial thrombosis in those patients with any cardiovascular risk factor (P = .056; Table 3) with this risk attaining statistical significance in smokers (IRR: 2.75; 95% CI: 1.2-6.5; P = .02). At the interaction analyses, patients with cardiovascular risk factors had a significantly higher rate of arterial thrombosis while they were on observation (IRR: 2.5; 95% CI: 1.02-6.1; P = .047) but not when they were on antiplatelet therapy (IRR: 1.2; 95% CI: 0.4-3.2; P = .7).
Arterial thrombosis occurred at a significantly higher rate in men than in women (IRR: 2.8; 95% CI: 1.5-7.0). Interaction analysis of antiplatelet therapy in male patients showed that male patients with ET receiving antiplatelet therapy still had an increased risk of arterial thrombosis, although the difference was in the limit of statistic signification (IRR: 2.5; 95% CI: 0.9-6.4; P = .06). The presence of the JAK2 mutation was not associated with an increased risk of arterial thrombosis (IRR: 0.45; 95% CI: 0.2-1.3; P = .1). Patients with JAK2-positive ET not receiving antiplatelet therapy did not experience a higher rate of arterial thrombosis (IRR: 0.5; 95% CI: 0.1-2.1; P = .3).
Venous thrombosis occurred at a rate of 8.2 per 1000 patient-years in the observation group and 4.9 in the antiplatelet group (P = .4; Table 2). No clinical or hematologic feature was associated with an increased rate of venous thrombosis at the multivariate analysis (Table 3). The presence of the JAK2 mutation was not associated with a higher rate of venous thrombosis (IRR: 2.7; 95% CI: 0.7-10.5; P = .2). However, when the analysis was performed according to the therapy, JAK2-positive patients not receiving antiplatelet drugs had a significant higher rate of venous thrombosis (IRR: 4.0; 95% CI: 1.2-12.9; P = .02).
Severe bleeding was observed in 15 patients (5%) and was the ultimate cause of death in 1 case. At the time of the hemorrhagic event, 10 patients were on antiplatelet therapy and 5 were on observation. Thrombocytosis greater than 800 × 109/L was the only feature independently associated with an increased rate of bleeding (IRR: 10.6; 95% CI: 1.7-69; P = .01; Table 3), and a similar result was observed when the cutoff was set at a platelet count greater than 1000 × 109/L (data not shown). The incidence rate of bleeding was 2 times higher while on antiplatelet medication than while under observation (12.6 versus 6 hemorrhages per 1000 person-years, respectively), but the difference did not attain statistical significance in the whole group of patients (Table 2). Patients with platelet counts greater than 1000 × 109/L who were on antiplatelet therapy had a significantly higher rate of major bleeding than the remainder of the patients (IRR: 5.4; 95% CI: 1.7-17.2; P = .004). The presence of the JAK2 mutation was not associated with an increased rate of bleeding (IRR: 1.4; 95% CI: 0.4-5.3; P = .6), even after interaction with antiplatelet therapy (IRR: 2.7; 95% CI: 0.7-9.8; P = .1).
Discussion
The present study was aimed at evaluating the effectiveness of antiplatelet therapy in the primary prevention of thrombosis in patients with low-risk ET not receiving cytoreductive therapy. It constitutes the largest study on the subject and the one with the longest follow-up, as well as the first to include data on the JAK2 mutational status. Therapeutic algorithms based on expert recommendations consider antiplatelet therapy in young patients with ET with cardiovascular risk factors, hereditary thrombophilia, or even for all patients unless contraindicated.4-6,14,21 However, it is currently unknown whether antiplatelet therapy results in any clinical benefit in low-risk ET, because no randomized controlled trials have been performed in this group of patients. In this sense, a previous study by van Genderen et al22 on 68 patients with ET concluded that antiplatelet drugs, either in monotherapy or associated with cytoreduction, resulted in a lower incidence of thrombosis without a significant increase in major bleeding. However, the results of the that study are difficult to interpret, given the low number of patients, the inclusion of minor thrombosis, and the mixture of patients with low- and high-risk ET.22
The present series involves true low-risk patients with ET, as shown by the good long-term survival and the low cumulative incidence of thrombosis. The incidence rate of thrombosis observed in the present study is comparable to the figures reported by other investigators in low-risk patients.23-25 When the incidence rate of thrombosis was analyzed according to whether the patients were on observation or on antiplatelet therapy, the latter strategy showed no benefit in reducing the overall risk of thrombosis or the specific risks for arterial or venous events. Nevertheless, it must be pointed out that we could identify 2 groups of patients with ET who obtained a clinical benefit from antiplatelet therapy. The first group was integrated by patients with cardiovascular risk factors in whom antiplatelet therapy offset the increased risk of arterial thrombosis associated with cardiovascular factors. The second group included those patients carrying the JAK2 V617F mutation, who experienced a higher rate of venous thrombosis if they were not on antiplatelet medication. Lack of a control group of persons without ET precluded us to ascertain whether the protective role of antiplatelet therapy was higher in patients with ET than the one expected in the general population with similar cardiovascular risk factors. Even so, our results provide some support to those who segregate an “intermediate-risk” group from the remaining low-risk patients with ET on the basis of the presence of cardiovascular factors.5,26 This intermediate-risk group might include not only patients with cardiovascular risk factors but also those positive for the JAK2 V617F mutation.
A recent meta-analysis has shown that the JAK2 mutation is associated with an increased risk of both arterial and venous thrombosis in ET, with the risk being higher for venous thrombosis.15 In the present study, antiplatelet therapy reduced the risk of venous thrombosis associated with the JAK2 mutation in low-risk patients with ET. The mechanisms involved in such an effect are uncertain, because platelets are currently considered not to play an important role in venous thrombosis. However, it is worth noting that in patients without ET at high risk for venous thromboembolic complications, antiplatelet therapy has been reported to reduce the incidence of deep vein thrombosis by 27% and that of symptomatic pulmonary embolisms by 50%, compared with placebo.27 In addition, whether patients with leukocytosis would benefit from antiplatelet therapy or other treatments remains largely unknown. We did not find any association between the rate of thrombosis and the leukocyte count at diagnosis. In this regard, note that, unlike in previous studies supporting a prognostic significance for leukocytosis, in which the whole lifespan of patients with ET was taken into account,16 we restricted the analysis to the period in which patients remained in the low-risk category.
The main limitation for the systematic use of antiplatelet drugs in patients with ET is the potentially increased risk of major bleeding. The incidence rate of severe bleeding observed in the present study, 6 and 12.6 per 1000 person-years under observation and antiplatelet therapy, respectively, is clearly higher than the 0.7 hemorrhages per 1000 person-years reported from studies on antiplatelet drugs in the general population,28 which highlights the hemorrhagic diathesis typical of ET. Because our results show that low-risk patients with ET without either cardiovascular risk factors or the JAK2 mutation do not obtain any measurable benefit from antiplatelet therapy with regard to prevent thrombosis, the risk of severe hemorrhage prevails; therefore, antiplatelet drugs should be used with caution. On the contrary, in patients with cardiovascular risk factors or those with the JAK2 mutation, antiplatelet therapy reduces the risk of thrombosis and should therefore be used unless the platelet count exceeds 1000 × 109/L, in which case the increased risk of bleeding may counterbalance the benefits of preventing thrombosis.
The best option to assess the benefit of antiplatelet therapy in low-risk ET would be a controlled clinical trial. However, the low prevalence of ET in young patients, the scarce number of vascular events, and the long follow-up that would be necessary to obtain reliable results make this option costly and unfeasible. In fact, our results show that follow-up of patients enrolled in such a study should be extended for a decade at least to capture most of the thrombotic events. In the absence of controlled clinical trials, rigorously conducted observational studies provide information that may prove to be useful in the daily clinical decision-making process. Nevertheless, possible biases arising from the selection of patients and uncontrolled drug prescription are important limitations in retrospective studies such as the present one. In this sense, we minimized the bias associated with drug prescription by including a propensity score in every multivariate model, so that outcomes were adjusted for the clinical characteristics that made the patient more prone to be on antiplatelet therapy. In addition, the incidence rates of thrombosis and bleeding were restricted to the period of time in which patients remained in the low-risk category without receiving cytoreductive drugs or transformation, in an attempt to assess the time at risk in a population of truly untreated patients with ET.
In conclusion, in patients with low-risk ET, primary prophylaxis with antiplatelet therapy lowers the increased risk of arterial thrombosis associated with cardiovascular factors, irrespective of the JAK2 mutation status, and reduces the rate of venous thrombosis in patients who are JAK2 V617F positive. In the remaining low-risk patients with ET (ie, patients JAK2 negative and without cardiovascular risk factors), observation may be an adequate option, because antiplatelet therapy does not reduce the incidence of thrombotic events and might increase the bleeding risk if platelets are greater than 1000 × 109/L.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.A.-L. designed the study, collected the data, performed the statistical analysis, analyzed and interpreted the results, and wrote the paper; F.C. designed the study, interpreted the results, and wrote the paper; A.P. designed the study, performed the statistical analysis, analyzed and interpreted the results, and wrote the paper; E.A.-R. and J.-C.H.-B. collected the data, interpreted the results, and approved the final version; V.P.-A., R.A., C.S., A.M., V.V., L.H.-N., C. Burgaleta, and B.X. collected the data and approved the final version; B.B. performed the molecular studies; and C. Besses designed the study, interpreted the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alberto Alvarez-Larrán, Hematology Department, Hospital del Mar, Passeig Marítim 25-29, 08003 Barcelona, Spain; e-mail: 95967@parcdesalutmar.cat.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal