Abstract
Transforming growth factor-β1 (TGF-β1) is a pleiotropic cytokine with major in vitro effects on hematopoietic stem cells (HSCs) and lymphocyte development. Little is known about hematopoiesis from mice with constitutive TGF-β1 inactivation largely because of important embryonic lethality and development of a lethal inflammatory disorder in TGF-β1−/− pups, making these studies difficult. Here, we show that no sign of the inflammatory disorder was detectable in 8- to 10-day-old TGF-β1−/− neonates as judged by both the number of T-activated and T-regulator cells in secondary lymphoid organs and the level of inflammatory cytokines in sera. After T-cell depletion, the inflammatory disease was not transplantable in recipient mice. Bone marrow cells from 8- to 10-day-old TGF-β1−/− neonates showed strikingly impaired short- and long-term reconstitutive activity associated with a parallel decreased in vivo homing capacity of lineage negative (Lin−) cells. In addition an in vitro–reduced survival of immature progenitors (Lin− Kit+ Sca+) was observed. Similar defects were found in liver cells from TGF-β1−/− embryos on day 14 after vaginal plug. These data indicate that TGF-β1 is a critical regulator for in vivo homeostasis of the HSCs, especially for their homing potential.
Introduction
Transforming growth factor-β1 (TGF-β1) is a member of a large superfamily of growth factors that includes TGF-β, activins, and bone morphogenetic proteins (BMPs). Members of this superfamily exert pleiotropic effects on cell cycle, apoptosis and differentiation, adhesion, and migration, and they have been implicated in the control of many cellular responses, especially in the biology of hematopoietic stem cells (HSCs).1,2
In the hematopoietic system, 2 members of this superfamily, BMP4 and TGF-β1, have emerged as important regulators of HSCs. BMP4 plays a key role during embryogenesis in the development of the hemangioblast2,3 and may also act on self-renewal of HSCs from neonate and adult.4 However, the role of TGF-β1 on HSCs is more controversial. In vitro, it inhibits the growth of primitive hematopoietic progenitor cells5,6 and maintains HSC properties7 through different mechanisms. TGF-β1 down-regulates cytokine receptors such as c-Kit8 or transcription factors such as PU-1 and GATA-1.9 It regulates cell cycle molecules by inducing both expression of CDKi from the CIP/Kip or INK4 families10,11 and repression of CDK4 or c-myc.12 Finally, TGF-β1 modulates Bcl-2 and p27 Kip1 by a mechanism that is independent of cell cycle.9 In vivo experiments have shown that Smad4, the common mediator of the TGF-β family signaling, plays a critical role in HSCs,13 suggesting that the TGF-β family plays an important role on HSC properties.
Recently, elegant experiments performed at the unicellular level have shown that TGF-β1 maintains HSCs in quiescence by inhibiting cytokine-mediated lipid raft clustering and, thus, allowing HSCs to maintain their reconstitution potential when treated in vitro by cytokines. These data uncover a critical role for TGF-β1 as a niche signal in the control of HSC quiescence.14 It is shown that the action of TGF-β1 may be both paracrine through the bone marrow (BM) microenvironment and autocrine because TGF-β1 antisense oligonucleotides and neutralizing anti–TGF-β1 antibodies induce cycling of purified primitive hematopoietic progenitors.15,16
In vivo experiments that used knockout (KO) animals have failed to conclusively show the role of TGF-β1 on HSC properties. A large proportion of TGF-β1−/−, TGF-β type II receptor (TβRII−/−), and TGF-β type I receptor (ALK5; TβRI−/−) embryos die at mid gestation from anemia due to defects in both yolk sac vasculature and hematopoiesis. A detailed analysis of TβRI−/− yolk sac showed a reduced number of erythroid cells, but a normal number of hematopoietic progenitors, strongly suggesting that the hematopoietic defect is secondary to abnormalities in endothelial differentiation affecting vasculogenesis.17 Difficulties encountered with TGF-β1 KO models are an embryonic lethality highly dependent on the genetic background18,19 and the lethal wasting syndrome that develops in all pups during the first 3 weeks of life.20,21 The precise mechanism of the wasting syndrome is complex and mimics an autoimmune disorder with the presence of autoantibodies.22 Depletion of CD8+ or CD4+ cells can reverse the phenotype.23 More recently, the role of T regulator (Treg) lymphocytes has been underscored. It has been reported that Treg cells isolated from TGF-β1−/− mice have a decreased suppressive function on T and natural killer (NK) cells.24 To analyze the in vivo effects of TGF-β1 on hematopoiesis, conditional KO animals have been developed. Conditional disruption of TβRII in adult mice led to lethal transplantable inflammatory diseases with apparently no marked defect on hematopoiesis. Conditional disruption of TβRI did not impair primitive hematopoiesis because no abnormality in HSC quiescence, self-renewal, and reconstitutive properties was found in adult mice.25 Only a higher sensitivity to the proliferative effect of stem cell factor was observed in vitro.
However, experiments performed with TβRI conditional KO mice may have some limitations because they do not exclude a putative role for TGF-β1 during fetal hematopoiesis and a possible redundancy among TGF-β receptors. In endothelial cells, TGF-β1 mediates signaling via ALK5/TβRI and ALK126 with the requirement of endoglin to obtain an efficient TGF-β1 signaling.27 Endoglin is expressed on a subset of HSCs28 and may play a role in hemangioblast specification and hematopoietic commitment.29 In addition, TβRI conditional KO may also lead to signaling arrest of other TGF-βs, such as TGF-β2, that may favor HSC proliferation.30
Constitutive TGF-β1 inactivation in mice with a mixed Sv129 × CF-1 genetic background allows the birth of a relatively high proportion of homozygous TGF-β1−/− pups.19,31 We used these mice before the beginning of the multifocal inflammatory disease to investigate their hematopoiesis. We found defects in the HSC and progenitor compartments in both fetuses and neonates, indicating that TGF-β1 is a critical molecule in HSC regulation in vivo.
Methods
Mice
Heterozygous TGF-β1 breeders with a mixed Sv129 × CF-1 genetic background were provided by T. Doetschman (BIOS Institute, University of Arizona). Because 100% of homozygous TGF-β1−/− animals die before weaning, experiments were performed with cells obtained either from 8- to 10- or 15- to 18-day-old TGF-β1−/− mice or wild-type (wt) littermates. Pregnant females were killed on day 14 after vaginal plug (E14) to collect fetal liver (FL) cells. Approval of Institut Gustave Roussy, Unité Inserm 790 has been given for mice used in this study.
Polymerase chain reaction genotyping
Cell preparation
Cells from BM, spleen, lymph nodes, thymus, and E14 FL were harvested as described previously.32 Erythrocytes were lysed in ammonium chloride potassium buffer.
γ-Interferon and interleukin-6 quantification
γ-Interferon (IFN-γ) and interleukin-6 (IL-6) levels were determined in serum with an enzyme-linked-immunosorbent assay (murine IFN-γ and murine IL-6; Quantikine Kit; R&D Systems) according to the manufacturer's instructions.
Cell sorting and flow cytometry
BM and E14 FL cells were stained with a cocktail of biotinylated lineage antibodies as described,32 and then with an anti–Sca-1-Pe7 (E13-161-7), an anti–c-Kit-allophycocyanin (APC; 2B8), and a streptavidin APC–indocyanine 7 to isolate Lin− Sca-1+ c-Kit+ (LSK) cells. Among the LSK population, signaling lymphocyte activation molecule (SLAM) cells were analyzed as CD150+ CD48low33,34 with an anti–CD48-fluorescein isothiocyanate (FITC; HM48-1) and an anti–CD150-phycoerythrin (TC15-12F-12.2).
For culture and transplantation assays, BM and E14 FL cells were enriched for Lin− cells and labeled with an anti–Sca-1-Pe and an anti–c-Kit-APC as described previously.32,35
Activated T lymphocytes and Treg lymphocytes were analyzed by labeling spleen, lymph nodes, and thymus cells with anti–CD3-FITC (2C11), anti–CD4-APC-indocyanine 7 (L3T4), and anti–CD25-phycoerythrin (PC61) antibodies. For Tregs, cells were fixed, permeabilized as described by the manufacturer, and incubated with the anti–FOXP3-APC (FJK-16s) antibody (eBioscience).
All antibodies were purchased from PharMingen, and 1 μg/mL of 7 amino actinomycin (Sigma-Aldrich) was added 5 to 10 minutes before analysis to exclude dead cells.
Cells were sorted with the use of a FACSDiva cytometer (Becton Dickinson) or analyzed with a FACSort and LSR II cytometer (Becton Dickinson).
Liquid culture assays
For survival and proliferation assays, LSK cells were sorted and plated at 1 cell/well in Terasaki plates and at 1000 cells/well in 96-well plates containing a serum-free medium (Stem Span medium; StemCell Technologies) supplemented with recombinant cytokines: 10 ng/mL recombinant murine IL-3, 10 ng/mL recombinant murine IL-6, 50 ng/mL recombinant murine stem cell factor, 3 U/mL recombinant human erythropoietin, 10 ng/mL recombinant human thrombopoietin, and 20 ng/mL recombinant murine Flt3-L for the BM and E14 FL of TGF-β1−/− and TGF-β1+/+ mice, respectively. Cultures were grown at 37°C with 5% CO2 in air during 5 days. Survival and cell division of HSCs were monitored by microscopy.
For proliferation, LSK-sorted cells were labeled during 2 hours with the cytoplasmic dye carboxyfluorescein diacetate succinimidyl diester (CFSE) according to the manufacturer's instructions (Molecular Probes). Cells (1 × 105 cells) were plated in 6-well plates in serum-free medium supplemented with cytokines as described in the previous paragraph. The number of cell divisions was assessed daily during 4 days by flow cytometry, taking CSFE+ cells treated by nocodazole as controls.
Myeloid colony assays
Colony-forming cells were grown in methylcellulose M3234 medium supplemented with recombinant cytokines according to the manufacturer's instructions (StemCell Technologies). Granulocyte-macrophage colony-forming unit (CFU)–, erythroid burst-forming unit–, and granulocyte-erythroid-megakaryocyte-macrophage CFU (CFU-GEMM)–derived colonies were counted after 7 to 10 days on morphology. Cultures were established with 2 × 105 cells/mL for E14 FL cells.
In vivo homing assay
BM and E14 FL cells were labeled with CFSE according to the manufacturer's instructions (Molecular Probes). In vivo homing assays were performed as described by Foudi et al.36
Long-term repopulation assays
Lethally irradiated (10 Gy delivered by a x-ray apparatus) female wt mice (8-10 weeks old) were used as hosts and received a transplant with 1 × 105, 2 × 105, 4 × 105, and 8 × 105 Lin− cells from 8 to 10-day-old male TGF-β1+/+ or TGF-β1−/− mice. In a second set of repopulation experiments, 5 × 106, 1 × 106, and 5 × 105 E14 FL male cells were injected alone or together with 1 × 106 female BM competitor cells (ratio, 0.5:1, 1:1, and 5:1) in irradiated wt female hosts. The hematopoietic reconstitution was assessed on genomic DNA obtained from peripheral blood collected from the retro-orbital sinus of anesthetized mice. DNA was extracted with high pure PCR template preparation kit (Roche Applied Science) as described by the manufacturer. Quantification of DNA products was realized in real time with a Gene Amp 5700 sequence detector (Applied Biosystems). Y chromosome–specific primers (forward, 5′-GTGCTAAGGAGTAGAGCGGAGAA; reverse, 5′-CATGGTAACTGCTCAAGCGGT). To compare loading, titine was used as a control gene. Primers were synthesized by MWG-Biotech AG (forward, 5′-AAAACGAGCAGTGACGTGAGC; reverse, 5′-TTCAGTCATGCTGCTAGCGC). Analysis was conducted with specific hybridization probes with the use of double-labeled fluorogenic probes (Y probe, 5′-TTCTCCAGGACCAGTGACTGGAGAYCA; titine, 5′-TGCACGGAAGCGTCTCGTCTCAGTC). PCR reactions were run with 50 ng of DNA, 1 μL of each primer at 10 pmol/μL, 0.5 μL of hybridization probe at 10 pmol/μL, 2 μL of H2O, and 2X Master Mix up to a total volume of 25 μL. The following program conditions were applied for the specific hybridization probe method: step 1: 50°C, 1 minute, 1 repetition; step 2: 95°C, 10 minutes, 1 repetition; step 3: 95°C, 0.15 minute, 60°C, 1 minute, 40 repetitions).
Detection of antinuclear autoantibodies
Serum (1:40 to 1:1280 diluted in phosphate-buffered saline) from hosts that received a transplant with TGF-β1+/+ or TGF-β1−/− Lin− BM cells was hybridized to Hep2 antigen substrate slide (Ambroise Paré Hospital) for 30 minutes at 25°C. Slides were washed with phosphate-buffered saline/1% bovine serum albumin, stained with an anti–mouse immunoglobulin G FITC-conjugated (1:10; Sigma-Aldrich), mounted, and visualized with the use of a Nikon Eclipse 600 fluorescence microscope equipped with a 40×/1.30 NA oil objective and an RS photometrics Cool Snap charge-coupled device camera and LSMS image software. MRL/lpr mice were used as a positive control (The Jackson Laboratory).
Statistical analysis
The Student t test was used with data obtained from at least 3 repeated experiments to determine the P value. Significant results are defined with an asterisk as P less than .05.
Results
Immunologic status of TGF-β1−/− mice
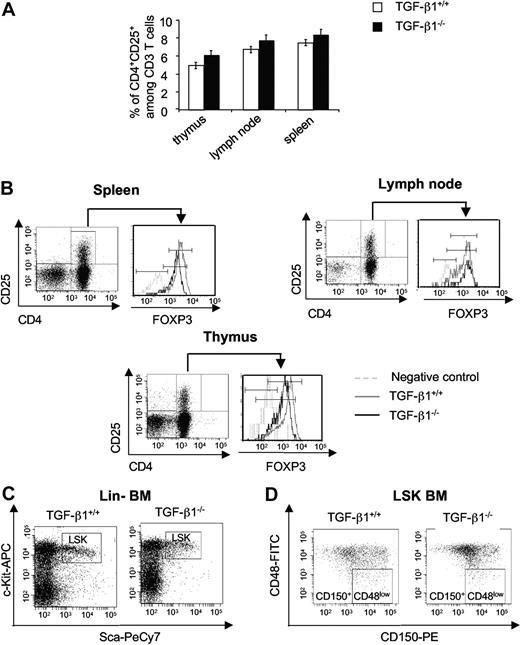
We first analyzed hematologic parameters of TGF-β1−/− mice in comparison to age-matched wt littermates. No major difference was seen (data not shown) as reported previously.20,21 With the use of CD25 as a marker of activation, no increase of CD4+ CD25+ cells was found in the spleen, lymph nodes, and thymus of 8- to 10-day-old TGF-β1−/− mice compared with wt mice (Figure 1A). The inflammatory disorder of TGF-β1−/− mice is considered as an autoimmune-like disease and is linked to a Treg defect. Previous works have shown the role of TGF-β1 in Treg homeostasis. We thus examined if Treg cells were present in our TGF-β1−/− animals. Analysis of FOXP3 expression in CD4+CD25+ T cells from spleen, lymph nodes, and thymus cells from 8- to 10-day-old TGF-β1−/− animals showed a slightly diminished level compared with wt mice (Figure 1B). By contrast, the number of Tregs was decreased in 15- to 18-day-old TGF-β1−/− mice (data not shown). Furthermore, levels of IL-6 and IFN-γ measured in sera from 8- to 10-day-old TGF-β1−/− and wt littermates were not significantly different (Table 1) in contrast with the 1.6-fold increase in IFN-γ level seen in 15- to 18-day-old TGF-β1−/− mice (data not shown).
Immunologic status of 8- to 10-day-old TGF-β1−/− mice. (A) Analysis of CD4+ CD25+ expression in CD3 T cell of thymus, lymph node, and spleen cells from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice. Histograms are the mean ± SD of 3 independent experiments, *P < .05. (B) Analysis of FOXP3 expression in CD4+CD25+ cells from spleen, lymph node, and thymus. The flow cytometric profiles are the representative histograms of 2 experiments (6 mice per experiment). (C) Analysis of the Lin− Sca-1+ c-Kit+ (LSK) population in BM cells from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice. The flow cytometric profiles are representative of 3 independent experiments with 6 mice per experiment. (D) Analysis of the CD150+ CD48low (signaling lymphocyte activation molecule) population in LSK BM cells from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice. The flow cytometric profiles are representative of 3 independent experiments with 6 mice per experiment, *P < .05. PE indicates phycoerythrin; Cy7, indocyanine 7.
Immunologic status of 8- to 10-day-old TGF-β1−/− mice. (A) Analysis of CD4+ CD25+ expression in CD3 T cell of thymus, lymph node, and spleen cells from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice. Histograms are the mean ± SD of 3 independent experiments, *P < .05. (B) Analysis of FOXP3 expression in CD4+CD25+ cells from spleen, lymph node, and thymus. The flow cytometric profiles are the representative histograms of 2 experiments (6 mice per experiment). (C) Analysis of the Lin− Sca-1+ c-Kit+ (LSK) population in BM cells from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice. The flow cytometric profiles are representative of 3 independent experiments with 6 mice per experiment. (D) Analysis of the CD150+ CD48low (signaling lymphocyte activation molecule) population in LSK BM cells from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice. The flow cytometric profiles are representative of 3 independent experiments with 6 mice per experiment, *P < .05. PE indicates phycoerythrin; Cy7, indocyanine 7.
Inflammatory cytokines IL-6 and IFN-γ levels between TGF-β1+/+ and TGF-β1−/− mice
| . | IL-6, ng/mL . | IFN-γ, ng/mL . |
|---|---|---|
| 8- to 10-day-old TGF-β1+/+ mice | 19.7 ± 9.6 | 1.3 ± 0.5 |
| 8- to 10-day-old TGF-β1−/− mice | 11.1 ± 4.3 | 2 ± 1.2 |
| Mice that received transplant with TGF-β1+/+ Lin− cells | 6.3 ± 1.5 | 1.05 ± 0.7 |
| Mice that received transplant with TGF-β1−/− Lin− cells | 6.6 ± 1.9 | 0.6 ± 0.4 |
| . | IL-6, ng/mL . | IFN-γ, ng/mL . |
|---|---|---|
| 8- to 10-day-old TGF-β1+/+ mice | 19.7 ± 9.6 | 1.3 ± 0.5 |
| 8- to 10-day-old TGF-β1−/− mice | 11.1 ± 4.3 | 2 ± 1.2 |
| Mice that received transplant with TGF-β1+/+ Lin− cells | 6.3 ± 1.5 | 1.05 ± 0.7 |
| Mice that received transplant with TGF-β1−/− Lin− cells | 6.6 ± 1.9 | 0.6 ± 0.4 |
Levels of IL-6 and IFN-γ were quantified by enzyme-linked immunoabsorbent assay in serum from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice (data represent the mean of 3 independent experiments with 6 mice per experiment) and in serum from mice that received a transplant with TGF-β1+/+ and TGF-β1−/− T-depleted Lin− cell after 3 months (data represent the mean of 2 experiments with 5 mice per experiment).
Sca-1 is a usual hematopoietic differentiation marker transcriptionally regulated by IFN.37 We thus checked the frequency of Sca+ c-Kit+ cells in the Lin− BM cells of both genotypes. The frequency was similar in 8- to 10-day-old TGF-β1−/− and wt mice (1.6% ± 0.18% vs 1.76% ± 0.14%; Figure 1C). By contrast, a marked increase in cells expressing Sca-1 was found in 15- to 18-day-old TGF-β1−/− mice (5.6% ± 1.2% vs 0.7% ± 0.07%; data not shown).
To better characterize the HSCs, we used the CD48 and CD150 signaling lymphocyte activation molecule markers. The number of CD150+CD48low cells in LSK population was significantly reduced in BM from 8- to 10-day-old TGF-β1−/− mice (6.3% ± 0.4% vs 3.1% ± 0.3%; Figure 1D).
Collectively, these results suggest that the inflammatory disease has not begun in 8- to 10-day-old TGF-β1−/− mice, and the phenotypic analyses show that the HSC compartment as defined by CD150+CD48low LSK cells was quantitatively altered in TGF-β1−/− mice.
Decrease in the hematopoietic stem/progenitor pool in 8- to 10-day-old TGF-β1−/− neonates
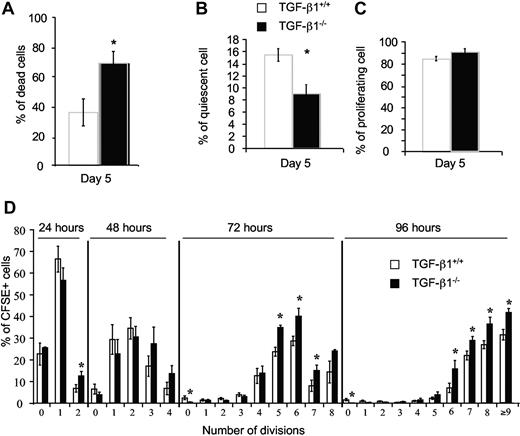
We first evaluated the in vitro effect of a disruption of the TGF-β1 gene on survival and proliferation of LSK cells isolated from the BM of 8- to 10-day-old TGF-β1−/− mice. LSK cells were sorted and cultured at 1 cell/well during 5 days in serum-free medium supplemented with cytokines (as described in “Liquid culture assays”). We scored the number of wells containing no cells, 1 cell and 2 cells or more than 2 cells by an inverted microscope. In these conditions, few TGF-β1−/− LSK cells survived (69% ± 8% of wells with dead cells) in contrast to TGF-β1+/+ LSK cells (35.6% ± 9.1% of wells with dead cell; Figure 2A). The number of wells containing only 1 living cell was markedly decreased for TGF-β1−/− LSK (9% vs 16% for wt; Figure 2B). Consequently, wells with 2 or more cells were more frequent for TGF-β1−/− LSK cells (Figure 2C).
TGF-β1 played a major role in preventing HSC death and maintained HSCs in quiescence. LSK BM cells from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice were single cell sorted into wells of Terasaki plates and incubated in serum-free medium supplemented with cytokines. At 5 days of culture, we determined (A) cell viability as a percentage of wells containing no cell over the total number of wells plated, (B) cell quiescence as the percentage of wells containing 1 living cell over the total number of wells containing viable cells, and (C) proliferation as the percentage of wells containing 2 or more cells over the total wells containing viable cells. Wells were examined under an inverted microscope. (D) Sorted LSK BM cells (1 × 105) from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice were stained with CFSE. CFSE+ LSK cells were sorted into a 6-well plate and incubated in serum-free medium supplemented with cytokines. Number of cell divisions was determined by the percentage of CFSE+ cells by flow cytometry during 96 hours. Graphs show the mean ± SD of 3 experiments (6 mice per experiment); *P < .05.
TGF-β1 played a major role in preventing HSC death and maintained HSCs in quiescence. LSK BM cells from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice were single cell sorted into wells of Terasaki plates and incubated in serum-free medium supplemented with cytokines. At 5 days of culture, we determined (A) cell viability as a percentage of wells containing no cell over the total number of wells plated, (B) cell quiescence as the percentage of wells containing 1 living cell over the total number of wells containing viable cells, and (C) proliferation as the percentage of wells containing 2 or more cells over the total wells containing viable cells. Wells were examined under an inverted microscope. (D) Sorted LSK BM cells (1 × 105) from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice were stained with CFSE. CFSE+ LSK cells were sorted into a 6-well plate and incubated in serum-free medium supplemented with cytokines. Number of cell divisions was determined by the percentage of CFSE+ cells by flow cytometry during 96 hours. Graphs show the mean ± SD of 3 experiments (6 mice per experiment); *P < .05.
These results were confirmed in a kinetic experiment in which 1 × 105 CFSE-labeled LSKs were plated. As measured by flow cytometry, the number of divisions was increased in the TGF-β1−/− culture as early as day 2 (Figure 2D).
This enhanced number of divisions was associated with a quicker and increased differentiation into the myeloid and erythroid lineages and a major increase in cell death (trypan blue–positive cells) compared with wt cultures (data not shown). These data show that TGF-β1 plays a major role in the survival of primitive progenitors and may be implicated in their quiescence.
Impaired reconstitutive ability of Lin− BM cells from TGF-β1−/− neonates
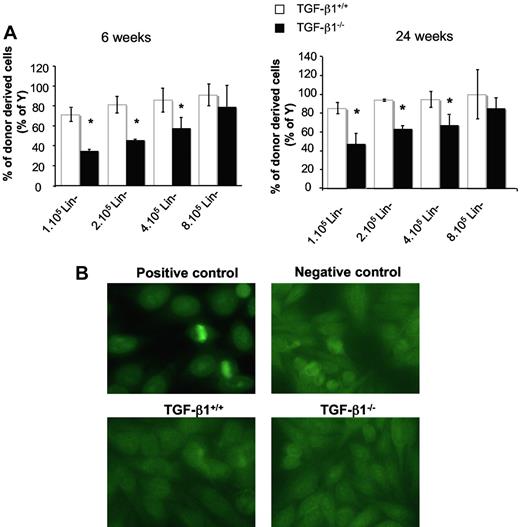
To obtain evidence that TGF-β1 depletion had a direct effect on HSC properties, we performed reconstitution experiments with male cells engrafted into female hosts. To bypass the risk of transplanting the inflammatory disease,20,21,38 we transplanted Lin− cells from BM that was depleted in mature T cells. At almost all cell concentrations tested (1 × 105, 2 × 105, and 4 × 105 Lin− cells/mouse) and whether analyses were performed at 6 or 24 weeks after transplantation, blood chimerism was lower in recipients reconstituted with TGF-β1−/− than with TGF-β1+/+ BM cells (Figure 3A). Moreover, the chimerism level increased with the quantity of TGF-β1−/− cells injected, whereas saturation was almost reached at the lowest concentration (1 × 105 Lin−) of TGF-β1+/+ cells. At week 6 or 24 after transplantation, approximately 8-fold more TGF-β1−/− Lin− cells were necessary to reach a level of chimerism similar to that observed when TGF-β1+/+ cells were engrafted (Figure 3A). These results show that the compartment of HSCs of BM is altered in TGF-β1−/− mice.
Transplanted TGF-β1 deficient progenitors have decreased reconstitutive capacity with no sign of inflammatory/autoimmune disease. (A) Analysis of reconstitutive capacities of Lin− BM cells from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice. T-depleted Lin− cells (1 × 105, 2 × 105, 4 × 105, and 8 × 105) from male TGF-β1+/+ and TGF-β1−/− mice were transplanted into lethally irradiated CF-1 × Sv129 female mice (10 mice per group). Graphs represent the percentage of donor-derived cells (percentage of Y) in peripheral blood (PB) at 6 weeks (left) and 24 weeks (right) after transplantation (5 mice per group). An example of 2 independent experiments is shown (5 mice per dose of cells; *P < .05). The contribution of donor-derived cells is determined by quantifying chromosome Y sequence in PB of reconstituted irradiated mice in comparison to an autosomal sequence (titine). (B) Serum reactivity from mice that received a transplant with T-depleted Lin− BM cells from TGF-β1+/+ and TGF-β1−/− mice. Sera were collected at week 3 after transplantation. Reactivity was tested in an ANA stain as described by Oak et al39 on the human epithelial cell line Hep2. Antibody binding activity was shown with an anti–mouse immunoglobulin G FITC conjugate. Fluorescence was analyzed with an immunofluorescence microscope. MRL/lpr lupic mice were used as a positive control and TGF-β1+/+ mice as a negative control.
Transplanted TGF-β1 deficient progenitors have decreased reconstitutive capacity with no sign of inflammatory/autoimmune disease. (A) Analysis of reconstitutive capacities of Lin− BM cells from 8- to 10-day-old TGF-β1+/+ and TGF-β1−/− mice. T-depleted Lin− cells (1 × 105, 2 × 105, 4 × 105, and 8 × 105) from male TGF-β1+/+ and TGF-β1−/− mice were transplanted into lethally irradiated CF-1 × Sv129 female mice (10 mice per group). Graphs represent the percentage of donor-derived cells (percentage of Y) in peripheral blood (PB) at 6 weeks (left) and 24 weeks (right) after transplantation (5 mice per group). An example of 2 independent experiments is shown (5 mice per dose of cells; *P < .05). The contribution of donor-derived cells is determined by quantifying chromosome Y sequence in PB of reconstituted irradiated mice in comparison to an autosomal sequence (titine). (B) Serum reactivity from mice that received a transplant with T-depleted Lin− BM cells from TGF-β1+/+ and TGF-β1−/− mice. Sera were collected at week 3 after transplantation. Reactivity was tested in an ANA stain as described by Oak et al39 on the human epithelial cell line Hep2. Antibody binding activity was shown with an anti–mouse immunoglobulin G FITC conjugate. Fluorescence was analyzed with an immunofluorescence microscope. MRL/lpr lupic mice were used as a positive control and TGF-β1+/+ mice as a negative control.
To understand whether this defect was related to the development of an inflammatory disease, determination of IL-6 and IFN-γ levels were performed in serum. No difference was observed (Table 1). In addition, because the inflammatory disease is associated with autoantibodies, especially antinuclear immunoglobulin G antibodies, we used immunofluorescence microscopy to show antibodies that could react with the nucleus of Hep2 cells (see “Detection of antinuclear autoantibodies”). No antinuclear antibodies were detected in sera from mice reconstituted with TGF-β1−/− cells (Figure 3B). By contrast, they were present in sera from MRL/lpr mice used as a positive control (Figure 3B). These data suggest that the TGF-β1−/− BM cell reconstitutive defect is probably intrinsic to HSCs and is not related to the development of an inflammatory disorder.
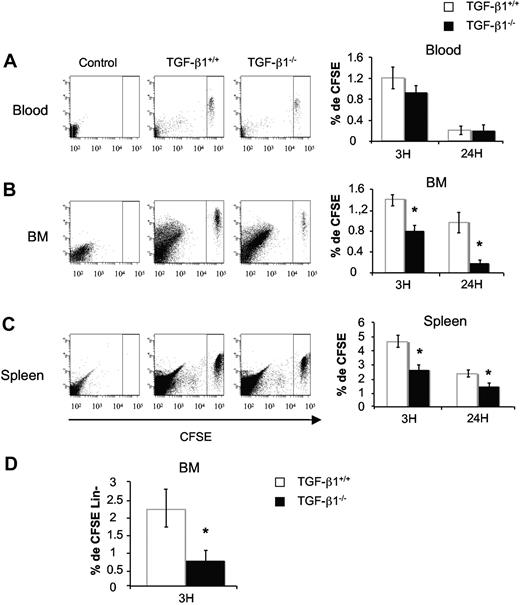
Next, we asked if the defect in reconstitutive activity could be related to a homing defect. Indeed, it has been reported that TGF-β1 can facilitate stromal cell–derived factor 1–mediated retention of stem/progenitor cells in the BM39 and may enhance chemotaxis of naive T cells by up-regulating CXCR4.40 To test this hypothesis, total BM cells from TGF-β1−/− and TGF-β1+/+ mice were stained with the CFSE dye before being transplanted into lethally irradiated hosts. Homing into BM and spleen was evaluated by flow cytometry 3 and 24 hours after the injection of 15 × 106 CFSE-stained cells per host. Whatever the genotype, CFSE+ cells homed rapidly within the BM (Figure 4B) and the spleen (Figure 4C). Compared with controls, homing of CSFE+ cells from TGF-β1−/− donors was significantly reduced in BM and spleen at 3 hours (1.7-fold). This was even more pronounced after 24 hours (4-fold in BM and 1.6-fold in spleen; Figure 4B-C). To identify the cell population that exhibited altered homing, homed CFSE+ cells from TGF-β1+/+ and TGF-β1−/− mice were isolated from the BM after 3 hours and incubated with a cocktail of lineage monoclonal antibodies: anti–GR-1, anti–MAC-1, anti-B220, anti-CD4, anti-CD8, and anti–TER-119. The percentage of CFSE+ Lin− cells was then analyzed by flow cytometry. A marked decrease (3-fold) in Lin− cells was observed for TGF-β1−/− cells in comparison to the control (0.72% ± 0.29% vs 2.19% ± 0.51%; Figure 4D). The number of LSK cells was too low to be investigated.
Defect of in vivo homing of BM cells from 8- to 10-day-old TGF-β1−/− mice. Total BM cells from TGF-β1+/+ and TGF-β1−/− mice were stained with CFSE dye as described in “In vivo homing assay.” CFSE+ cells were injected in lethally irradiated mice (15 × 106/mouse). Recipients were killed 3 and 24 hours after injection. Peripheral blood (A), BM (B), and spleen (C) were harvested, and the percentage of homed CFSE+ cells was analyzed by flow cytometry. Shown are flow cytometric profiles for a representative analysis and histograms showing the mean ± SD of 3 independent experiments with 4 mice per experiment (*P < .05). (D) Percentage of CFSE+ Lin− cells in BM. Irradiated mice were injected as above and killed after 3 hours. Cells were stained with antibodies against lineage markers, and CFSE+ Lin− cells were analyzed by flow cytometry. The histogram represents the mean ± SD of 3 independent experiments with 4 mice per experiment; *P < .05.
Defect of in vivo homing of BM cells from 8- to 10-day-old TGF-β1−/− mice. Total BM cells from TGF-β1+/+ and TGF-β1−/− mice were stained with CFSE dye as described in “In vivo homing assay.” CFSE+ cells were injected in lethally irradiated mice (15 × 106/mouse). Recipients were killed 3 and 24 hours after injection. Peripheral blood (A), BM (B), and spleen (C) were harvested, and the percentage of homed CFSE+ cells was analyzed by flow cytometry. Shown are flow cytometric profiles for a representative analysis and histograms showing the mean ± SD of 3 independent experiments with 4 mice per experiment (*P < .05). (D) Percentage of CFSE+ Lin− cells in BM. Irradiated mice were injected as above and killed after 3 hours. Cells were stained with antibodies against lineage markers, and CFSE+ Lin− cells were analyzed by flow cytometry. The histogram represents the mean ± SD of 3 independent experiments with 4 mice per experiment; *P < .05.
Then, 3-fold higher CFSE-stained TGF-β1−/− cells in parallel with TGF-β1+/+ were injected into lethally irradiated hosts, and homing into BM and spleen was evaluated to help clarify the issue of defective hematopoiesis. In these conditions, homing of CSFE+ cells from TGF-β1−/− donors in BM (supplemental Figure 1B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and spleen (supplemental Figure 1C) at 3 hours was equivalent to controls. Furthermore, the percentage of CFSE+ Lin− cells that homed in BM was the same for TGF-β1−/− cells in comparison to the control (supplemental Figure 1D).
Overall, these last results show that TGF-β1 is an important mediator, which may be involved in the regulation of homing.
Defects in hematopoietic stem cells in TGF-β1−/− E14 FL
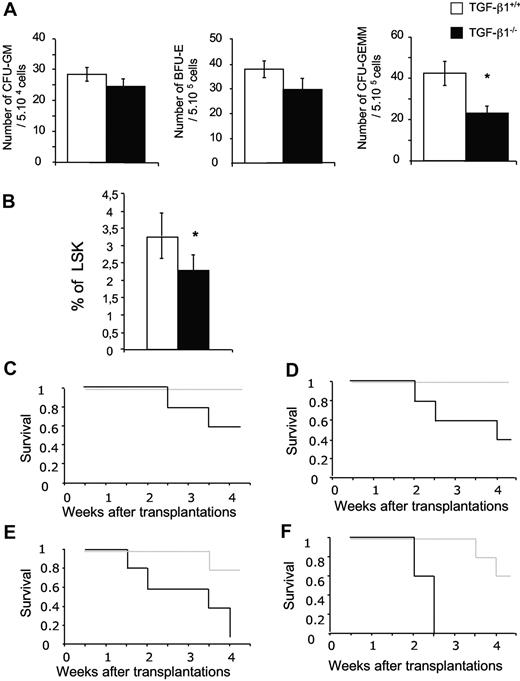
To test whether the defect in HSCs occurred during fetal life, we analyzed the hematopoiesis of TGF-β1−/− and TGF-β1+/+ E14 FL. No difference was seen in size, cellularity, and cellular composition (data not shown). In TGF-β1−/− FL, the number of CFU-GEMMs was decreased 2-fold, whereas erythroid burst-forming unit and granulocyte-macrophage CFU numbers were nearly normal, although a trend toward a decrease was seen (Figure 5A). In agreement with the reduced number of CFU-GEMMs, the proportion of LSK cells was significantly lower in TGF-β1−/− E14 FL (Figure 5B).
TGF-β1−/− E14 FL cells show a defect in hematopoietic stem/progenitor cell compartment. (A) E14 FL cells (5 × 104) from TGF-β1+/+ and TGF-β1−/− mice were plated in methylcellulose. Colonies were scored between 7 and 10 days in culture. The average ± SD of 5 experiments is shown (6 mice per experiment); *P < .05. (B) Analysis of LSK population in E14 FL from TGF-β1+/+ and TGF-β1−/− mice. The histograms are the average ± SD of 6 independent experiments; *P < .05. (C-F) Lethally irradiated hosts were injected with 4 dilutions of E14 TGF-β1+/+ and TGF-β1−/− FL cells: (C) 10 × 106, (D) 5 × 106, (E) 1 × 106, and (F) 5 × 105 male E14 FL cells (n = 5 mice per cell dose and per genotype). Recipients were monitored daily. Survival data were analyzed with the use of a log-rank nonparametric test, and the results are expressed as Kaplan-Meier survival curves.
TGF-β1−/− E14 FL cells show a defect in hematopoietic stem/progenitor cell compartment. (A) E14 FL cells (5 × 104) from TGF-β1+/+ and TGF-β1−/− mice were plated in methylcellulose. Colonies were scored between 7 and 10 days in culture. The average ± SD of 5 experiments is shown (6 mice per experiment); *P < .05. (B) Analysis of LSK population in E14 FL from TGF-β1+/+ and TGF-β1−/− mice. The histograms are the average ± SD of 6 independent experiments; *P < .05. (C-F) Lethally irradiated hosts were injected with 4 dilutions of E14 TGF-β1+/+ and TGF-β1−/− FL cells: (C) 10 × 106, (D) 5 × 106, (E) 1 × 106, and (F) 5 × 105 male E14 FL cells (n = 5 mice per cell dose and per genotype). Recipients were monitored daily. Survival data were analyzed with the use of a log-rank nonparametric test, and the results are expressed as Kaplan-Meier survival curves.
Next, we studied the functional proliferative capacity of HSCs from E14 FL. We also used the LSK population from FL because at day 14, FL HSCs already display this phenotype as in adult.41,42 LSK cells were sorted, and 1000 cells were cultured during 7 days in serum-free medium supplemented with cytokines. Proliferation and the presence of dead cells were evaluated by counting the cells with the use of the trypan blue exclusion test. We observed a poor proliferative response of TGF-β1−/− LSK cells in comparison to wt LSK cells. Indeed, we saw a marked significant decrease (67%; P < .05) in the number of cells derived from TGF-β1−/− LSK cells after 7 days with an increase in cell death (data not shown). These results support the assumption that TGF-β1 is a major survival factor for immature progenitors.
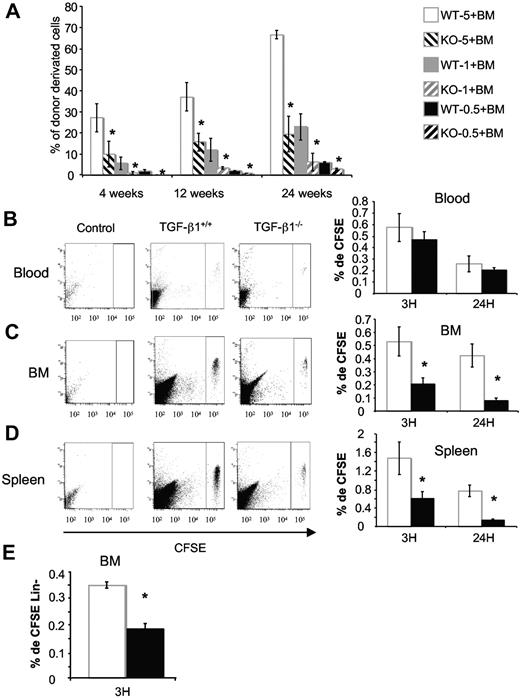
To investigate stem cell activity in FL, we performed transplantation experiments. We first tested the radioprotection capacity of TGF-β1−/− and TGF-β1+/+ E14 FL cells.43 Lethally irradiated hosts were engrafted with increasing numbers of FL cells (5 × 105 to 1 × 107), and host survival was monitored during 4 weeks. At all cell concentrations tested, a higher rate of mortality was observed in mice engrafted with TGF-β1−/− FL cells (Table 2). Moreover, mortality was recorded in groups of mice reconstituted with the 2 highest cell concentrations (5 × 106 and 1 × 107) of TGF-β1−/− FL cells, whereas none was observed in the control group. In addition, at the lowest cell concentration (5 × 103), mice reconstituted with TGF-β1−/− FL cells died at approximately 2 weeks compared with 4 weeks for controls (Figure 5C-F). Thus, TGF-β1−/− E14 FL showed a marked short-term reconstitutive defect in comparison to wt FL. To study the long-term reconstitutive ability of FL cells, male E14 FL cells from both genotypes were tested in competitive reconstitution assay. Two experiments were performed with Y chromosome PCR quantification to measure chimerism. Whatever the dose of cells injected, both short-term and long-term reconstitutive abilities of TGF-β1−/− E14 FL cells were markedly impaired (∼ 3- to 5-fold; Figure 6A).
Short-term radioprotection by limiting injection of FL cells
| No. of cells injected/mouse . | No. of mice surviving until week 4 after transplantation . | |
|---|---|---|
| TGF-β1+/+ . | TGF-β1−/− . | |
| 10 × 106 | 5/5 | 3/5 |
| 5 × 106 | 5/5 | 2/5 |
| 1 × 106 | 4/5 | 0/5 |
| 0.5 × 106 | 3/5 | 0/5 |
| No. of cells injected/mouse . | No. of mice surviving until week 4 after transplantation . | |
|---|---|---|
| TGF-β1+/+ . | TGF-β1−/− . | |
| 10 × 106 | 5/5 | 3/5 |
| 5 × 106 | 5/5 | 2/5 |
| 1 × 106 | 4/5 | 0/5 |
| 0.5 × 106 | 3/5 | 0/5 |
Freshly isolated total FL cells were engrafted to lethally irradiated recipient mice at the concentrations indicated. Values represent the number of living mice, 4 weeks after transplantation, of 5 animals injected per cell dose and per genotype.
Competitive repopulation assays with TGF-β1−/− E14 FL cells show defects in hematopoietic repopulation. (A) 5 × 106, 1 × 106, 5 × 105 male E14 FL TGF-β1+/+ (wt-5, wt-1, and wt-0.5, respectively) or TGF-β1−/− (KO-5, KO-1, and KO-0.5, respectively) cells were mixed with 1 × 106 female BM cells and transplanted into lethally irradiated CF-1 × Sv129 mice. Graphs represent the percentage of donor-derived cells (percentage of Y) in peripheral blood (PB) of reconstituted mice at 6 weeks, 12 weeks, and 24 weeks after injection (6 mice per group); *P < .05. An example of 2 independent experiments is shown. The contribution of donor-derived cells to each lineage is determined by analyzing the percentage of chromosome Y in the PB of reconstituted mice. (B-D) Total E14 FL cells from TGF-β1+/+ and TGF-β1−/− mice were stained with CFSE dye as described in “In vivo homing assay.” CFSE+ cells were injected in lethally irradiated mice (15 × 106/mouse). Recipients were killed 3 and 24 hours after injection. Peripheral blood (B), BM (C), and spleen (D) were harvested, and the percentage of homed CFSE+ cells was analyzed by flow cytometry. Shown ae flow cytometric profiles for a representative analysis and histograms showing the mean ± SD of 3 independent experiments with 4 mice per experiment (*P < .05). (E) Percentage of CFSE+ Lin− cells in BM. Irradiated mice were injected as above and killed 3 hours after injection. Cells were stained with antibodies against lineage markers, and CFSE+ Lin− cells were analyzed by flow cytometry. The histogram represents the mean ± SD of 3 independent experiments with 4 mice per experiment (*P < .05).
Competitive repopulation assays with TGF-β1−/− E14 FL cells show defects in hematopoietic repopulation. (A) 5 × 106, 1 × 106, 5 × 105 male E14 FL TGF-β1+/+ (wt-5, wt-1, and wt-0.5, respectively) or TGF-β1−/− (KO-5, KO-1, and KO-0.5, respectively) cells were mixed with 1 × 106 female BM cells and transplanted into lethally irradiated CF-1 × Sv129 mice. Graphs represent the percentage of donor-derived cells (percentage of Y) in peripheral blood (PB) of reconstituted mice at 6 weeks, 12 weeks, and 24 weeks after injection (6 mice per group); *P < .05. An example of 2 independent experiments is shown. The contribution of donor-derived cells to each lineage is determined by analyzing the percentage of chromosome Y in the PB of reconstituted mice. (B-D) Total E14 FL cells from TGF-β1+/+ and TGF-β1−/− mice were stained with CFSE dye as described in “In vivo homing assay.” CFSE+ cells were injected in lethally irradiated mice (15 × 106/mouse). Recipients were killed 3 and 24 hours after injection. Peripheral blood (B), BM (C), and spleen (D) were harvested, and the percentage of homed CFSE+ cells was analyzed by flow cytometry. Shown ae flow cytometric profiles for a representative analysis and histograms showing the mean ± SD of 3 independent experiments with 4 mice per experiment (*P < .05). (E) Percentage of CFSE+ Lin− cells in BM. Irradiated mice were injected as above and killed 3 hours after injection. Cells were stained with antibodies against lineage markers, and CFSE+ Lin− cells were analyzed by flow cytometry. The histogram represents the mean ± SD of 3 independent experiments with 4 mice per experiment (*P < .05).
We then studied whether the homing defect seen in neonates could be shown with TGF-β1−/− FL cells. FL cells were labeled by CSFE and injected in irradiated hosts. Our observations confirmed published data showing that FL cells exhibit very low homing efficiency compared with BM cells.44 Nevertheless, significantly fewer CFSE+ TGF-β1−/− FL cells than CFSE+ wt FL cells were found in the host BM at both 3 hours (2.5-fold) and 24 hours (5-fold; Figure 6C). Similarly, the homing of CFSE+ TGF-β1−/− cells in the spleen was significantly reduced (2.5-fold) at 3 hours (5-fold) and at 24 hours (Figure 6D). As shown in Figure 6E, the percentage of Lin− CFSE+ cells that homed to the BM was 2- to 3-fold reduced when the transplant was TGF-β1−/− FL (0.72% ± 0.29% vs 2.2% ± 0.5%), a result similar to that observed with BM cells from neonates.
Collectively, these results show that TGF-β1 deficiency alters HSC properties far before the occurrence of a multifocal inflammatory disease and thus provide evidence that TGF-β1 is a major regulator of stem cells in vivo, especially of their homing capacities.
Discussion
In this study, we aimed to solve the discrepancy between in vivo and in vitro experiments on the role of TGF-β1 on the regulation of HSCs. Although in vitro experiments have shown that TGF-β1 was a major regulator of proliferation of HSCs and immature progenitors by mediating quiescence, mice deficient in TβRI (ALK5), and presumably in TGF-β1 signaling, had no defect in HSC reconstitutive activity despite an increased proliferation in vitro.25 We have used constitutive TGF-β1 KO mice and studied their hematopoiesis. This approach had not been performed in detail because of 2 pitfalls of this model. First, because TGF-β1 plays an important role during embryogenesis, an embryonic lethality is observed with a penetrance depending on the genetic background of the mice,18 because of the presence of a genetic modifier mapped on chromosome 5.19 Second, in the TGF-β1 KO model, a multifocal inflammatory disease develops quickly and is lethal usually between weeks 3 and 4 after birth. In this study, we have used the mixed Sv129 × CF-1 background that allows the birth of approximately 50% of the expected TGF-β1−/− pups.18 We could show that TGF-β1 KO mice have developed the inflammatory disease at 15 to 18 days after birth as shown by an increase in activated T cells, high levels of interferon-γ in sera, as well as an increase of Sca-1 on hematopoietic cells, a cell marker regulated by interferon-γ. However, no sign of the onset of multifocal inflammatory disease was observed at days 8 to 10 after birth. Treg cells play an important role in the peripheral tolerance. Their number is decreased or their function is altered in patients with SLE45 as well as in TGF-β1 mice with inflammatory disorder. In the thymus, spleen, and lymph nodes of 8- to 10-day-old TGF-β1 KO mice, the number of Treg cells was not significantly decreased, further indicating that the inflammatory disorder was not onset. Thus, we provide strong evidence that the hematopoietic phenotype observed in 8- to 10-day-old mice was the result of the depletion of TGF-β1 on hematopoiesis and not mediated by an autoimmune disorder. Moreover, we found that 8- to 10-day-old TGF-β1 KO neonates exhibit a deficiency in the hematopoietic progenitor/stem cells that includes a loss of quiescence associated with a quicker differentiation, an increased cell death, and a decreased proliferation in response to hematopoietic cytokines.
As previously described, transplantation of purified Lin− TGF-β1 KO BM cells can be performed in irradiated Sv129 × CF-1wt animals without rejection and/or development of an inflammatory disease.31 In this mouse model we found a loss of reconstitutive activity after injection of TGF-β1−/− BM cells, which paralleled a homing defect. Indeed the homing defect was restored after transplantation of 3-fold higher BM cells from TGF-β1−/− than from TGF-β1+/+ mice (supplemental Figure 1), and injection of 3- to 6-fold more TGF-β1−/− cells was required to correct the long-term reconstitutive activity. These results indicate a profound defect in homing capacity of TGF-β1−/− BM unseparated cells. This defect was also found for purified TGF-β1−/− Lin− BM cells. However, we could not test by this technique if more primitive cells such as LSK had a similar homing defect because this cell fraction is too rare in the homed cells. Transplantation experiments with FL cells confirmed this defect. They showed that 5-fold more TGF-β1−/− FL cells compared with wt FL cells could almost rescue the chimerism, whereas the homing capacities of TGF-β1−/− Lin− FL was 5-fold reduced at 24 hours. This affords evidence that FL HSCs had a defect in homing, which correlates with their defect in reconstitutive potential. However, this was not the sole abnormality found because a defect in cytokine response of TGF-β1−/− FL LSK and neonate BM LSK cells was found in in vitro experiments.
Altogether, these results show that, in this mouse model, the repopulation defect of TGF-β1−/− BM and FL HSCs is mainly caused by a homing defect. However, we cannot exclude that another mechanism such as a defect in cytokine response also participates in this loss of reconstitutive activity.
These results are different from those reported with a conditional disruption of the TβRI in the adult mice25 where no defect in the hematopoietic progenitor/stem cell compartment, including self-renewal capacities of TβRI−/− HSC, was shown.
One main difference between the 2 models concerns the possible involvement of a major role of TGF-β1 in hematopoiesis during embryogenesis in our model. It has been shown that TGF-β1 or TβRII KO mice have a profound defect in the development of blood islands.46,47 However, this defect is the consequence of an abnormal vasculogenesis.17 The role of TGF-β1 on HSCs in definitive hematopoiesis, especially at the level of the aorta-gonad-mesonephros, is presently unknown. Thus, during embryogenesis, TGF-β1 might be involved in the specification of HSCs for definitive hematopoiesis, in the migration of HSCs into the fetal liver, and/or in the amplification of fetal HSCs and preservation of their functions. In this study, we found a profound and similar defect in short-term and long-term reconstitutive activity from E14 FL cells and neonate BM. It is therefore possible that part of the quantitative defect in neonate HSCs is the consequence of a homing defect of HSCs during development.
By contrast, it is difficult to explain the defects in homing and cell survival in vitro by a developmental abnormality. The major impairment of the survival of TGF-β1−/− LSK cells was unexpected, although a recent study has underscored the role of TGF-β1 as a survival factor for HSCs.14 It was shown that mice deficient in TGF-β activated kinase 1, a downsteam key effector of TGF-β/BMP signaling, displayed BM failure because of HSC apoptosis.48 Moreover, it has been reported that HSCs produce latent TGF-β1, which can regulate their properties in a manner independent of cell cycle. Therefore, we cannot exclude that rupture in this autocrine loop may play a role in the HSC defects of TGF-β1 KO mice.
The role of TGF-β1 in the homing capacities of HSC/progenitors has not been previously described. However, in T cells it has been shown that TGF-β1 positively regulates homing receptors and in different cellular models that TGF-β1 can regulate CXCR4 expression.39,40,49 Thus, it is possible that a defect in the CXCR4/stromal cell–derived factor 1 axis may be involved in the homing defect of TGF-β1−/− hematopoietic cells. This hypothesis will require further experiments.
The differences between the conditional TβRI KO and the constitutive TGF-β1 KO models may have at least 4 nonexclusive explanations. First, a major role of TGF-β1 in the ontogeny of HSCs; second, an important function of autocrine TGF-β1 by a not classical signaling pathway; third, the possibility that TβRI KO does not only disrupt TGF-β1 signaling in HSC but also the activity of other TGF-β, such as TGF-β2 that can induce HSC proliferation.30 Fourth, it has been shown recently that TβRI transcript was at the threshold of detection in HSCs,14 although TGF-β1 could inhibit cell cycle entry by cytokines. This may suggest that another receptor may mediate TGF-β1 in HSCs.
Our present study underscores for the first time the important role of TGF-β1 on HSC functions in vivo as previously shown in vitro. However, the main role of TGF-β1 signaling in vivo may concern the homing capacities of HSCs. Further determination of the in vivo role of TGF-β1 on HSCs will require the development of new mouse models, such as conditional TGF-β1 KO mice, which will allow the definition of its precise role in both fetal and adult hematopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the late Patrice Ardouin, Annie Rouches, and all personnel at the Institut Gustave Roussy animal facilities for their precious help in maintaining the mice. We thank Dr Françoise Wendling and Dr Sylvain Clauser for critical reading of the manuscript.
This work was supported by grants from the Insem and a grant from la Ligue Nationale contre le Cancer to WV (équipe labellisée 2004).
Authorship
Contribution: C.C. performed the experiments, analyzed the data, and wrote the manuscript; C.L. performed mice breeding and genotyping and part of the experiments; Y.L. was in charge of the FACS experiments and analyses; V.J. performed enzyme-linked immunoabsorbent assay and immunofluorescence assays; H.C. established the mouse colony at IGR and performed part of the experiments; S.C. and A.G. developed the quantitative PCR; A.B.-G. participated in early studies on 3- to 4-week-old animals; E.C.-B. corrected the paper; and W.V. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William Vainchenker, Inserm U1009, Université Paris XI, Institut Gustave Roussy, 39 rue Camille Desmoulins, 94805 Villejuif, France; e-mail: verpre@igr.fr.
References
Author notes
C.L. and Y.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal