Abstract
Natural killer (NK) cells and polymorphonuclear cells (PMNs) play a critical role in the first line of defense against microorganisms. Upon host infection, PMNs phagocytose invading pathogens with subsequent killing by oxidative or nonoxidative mechanisms. NK cells are known to have immunoregulatory effects on T cells, B cells, dendritic cells (DCs), and monocytes through secretion of various soluble products and cell-cell contact. However, their impact on PMN survival and function is not well known. We found that soluble factors derived from cytokine-activated NK cells delay PMN apoptosis and preserve their ability to perform phagocytosis and produce reactive oxygen species (ROS). The expression patterns of CD11b and CD62L on PMNs differed according to the cytokine combination used for NK-cell stimulation. Irrespective of the NK-cell treatment, however, PMN survival was prolonged with sustained functional capacity. We found that interferon γ, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor α produced by NK cells upon stimulation with cytokines played a crucial role in NK cell–mediated effects on PMNs. Our study demonstrates that soluble factors derived from cytokine-activated NK cells send survival signals to PMNs, which would promote their accumulation and function at the site of inflammation in vivo.
Introduction
Natural killer (NK) cells constitute approximately 10% to 15% of the total blood lymphocytes, and are defined by the absence of CD3 and presence of CD56 on their surface.1 Based on the expression of CD56 and CD16, they can be divided into 2 subsets: CD56dimCD16hi and CD56brightCD16lo/−.2,3 In peripheral blood, CD56dim cells constitute 90% of the total NK cells. They are potent killers and are capable of destroying virus-infected cells and tumor cells without prior sensitization. In contrast, the cytotoxic potential of CD56bright cells is limited, but they are known to produce large amounts of cytokines and chemokines such as interferon γ (IFNγ), tumor necrosis factor α (TNFα), macrophage inflammatory proteins (MIPs) MIP-1α and MIP-1β, and granulocyte-macrophage colony-stimulating factor (GM-CSF), which in turn can recruit other immune cells and exhibit immunomodulatory effects on their functional activities.4-6
Polymorphonuclear cells (PMNs) phagocytose either directly or via various receptors (such as Fc receptors, complement receptors, and others) and subsequently kill pathogens by producing enzymes, such as defensins, proteases, permeability inducing factors, and toxic reactive oxygen and nitrogen species.7-9 They have a short half-life of 6 to 8 hours in circulation. Senescent PMNs and PMNs that have accomplished their task of killing invading microorganisms undergo apoptosis to prevent the release of toxic components into the surroundings that would damage the host tissue.10 However, removal of PMNs implies a reduction in the ability of the first line of defense to quickly eliminate pathogens. Various inflammatory cytokines such as interleukin–1β (IL-1β), IL-2, TNFα, IL-15, IFNγ, granulocyte-colony stimulating factor (G-CSF), GM-CSF, IL-6, and IL-8, and other mediators like lipopolysaccharide (LPS) have been shown to enhance PMN survival.10
NK cells are known to have immunoregulatory effects on immune cells, such as T cells, B cells, dendritic cells (DCs), and monocytes through secretion of various soluble products and cell-cell contact.11-14 Previous in vitro studies in human and murine models have addressed the effects of PMNs on NK-cell functions.15-18 However, to our knowledge, the impact of NK cells on PMNs has not been studied. Because NK cells and PMNs are among the first cells to be recruited to the site of inflammation, reciprocal interaction between them is highly plausible. In this study, we investigated the impact of NK cells on PMN survival, activation, and function. We show that soluble factors released by cytokine-activated NK cells send survival signals to PMNs, which may promote their accumulation for extended time periods at the site of inflammation and support their functions.
Methods
Cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) were separated from blood filters obtained from the Institute of Transfusion Medicine, Hannover Medical School, by Ficoll density gradient centrifugation (Biochrom). PBMCs were stained with monoclonal antibodies against CD3 PerCP, CD14 Pacific blue, and CD56 APC (BD Biosciences) for gating on NK cells, defined as CD3−CD14−CD56+. For sorting CD56dim and CD56bright NK cells, PBMCs were stained with CD3 PerCP, CD14 PerCP, CD19 PerCP, CD16 APC-H7 (BD Biosciences), and CD56 PE-Cy7 (Beckman Coulter). CD56dim NK cells were defined as CD3−CD14−CD19−CD56dimCD16hi, and CD56bright NK cells were defined as CD3−CD14−CD19−CD56brightCD16lo/−. NK cells were sorted on a FACSAria (Becton Dickinson) at the Hannover Medical School sorting facility. The purity of NK cells achieved ranged from 95% to 98%.
This study was approved by the ethical review board of the Hannover Medical School. Heparinized blood was obtained from healthy adult volunteers, and PMNs were isolated as described elsewhere.19 The purity was more than 95% as assessed by size and granularity on flow cytometry and examination of microscopic cytocentrifuge slides.
NK cells were stimulated in R10 (RPMI 1640 medium supplemented with 10% fetal calf serum, l-glutamine, penicillin/streptomycin, and sodium pyruvate) with IL-2 (100 U/mL; R&D Systems), IL-15 (100 ng/mL), IL-21 (50 ng/mL; Immunotools), IL-12 (100 ng/mL), and/or IL-18 (100 ng/mL; MBL International) at a concentration of 1 × 106/mL for 18 to 24 hours. PMNs were incubated in the absence or presence of supernatants derived from 1 × 106 cells/mL unstimulated or stimulated NK cells at a concentration of 1 × 106 cells/mL for 18 to 24 hours, and evaluated for apoptosis, activation, and function.
Flow cytometric analysis for apoptosis and activation markers
After overnight culture, approximately 100 000 PMNs were stained with either CD62L PC5 (Beckman Coulter) and CD11b PE (Immunotools) for evaluation of activation, or with annexin APC and viaprobe (BD Biosciences) for apoptosis. Annexin binding buffer (BD Biosciences) was added to PMNs for assessing apoptosis on FACSCalibur within 4 hours. PMNs stained with CD62L, CD11b, and isotype-matched antibodies were washed and resuspended in phosphate-buffered saline with 0.05% bovine serum albumin and analyzed on FACSCalibur.
Phagocytosis (antigen uptake assay)
PMNs were washed, resuspended in R10 medium, and incubated with 1-μm fluoresbrite YO latex beads (Polysciences) at a concentration of 10 beads per cell. The tubes were incubated at 37°C or on ice (control to rule out nonspecific binding) for 30 minutes, followed by wash and resuspension in phosphate-buffered saline plus 2% fetal calf serum. The uptake of fluorescent beads by PMNs was analyzed on FACSCalibur.
ROS production
The amount of reactive oxygen species (ROS) produced by PMNs was determined by electron spin resonance (ESR) spectroscopy with the use of the spin-trap 1-hydroxy-3-carboxy-pyrrolidine (CP-H; Alexis Corporation) as described elsewhere.20 Samples were loaded in 50-μL capillaries and the ESR spectra of the superoxide produced by PMNs as a result of CP-H oxidation to paramagnetic 3-carboxyl-proxyl (CP) were recorded using a MiniScope ESR spectrometer (Magnettech).
Cytokine bead array
Quantitative measurement of TNFα and IFNγ in the culture supernatants of cytokine-activated NK cells was performed using cytokine bead array (CBA) for human Th1/Th2 cytokines according to the manufacturer's specifications (BD Biosciences). GM-CSF was measured using the CBA Flex set (BD Biosciences). The samples were measured on FACSCalibur and analyzed using the CBA analysis software (BD Biosciences).
Statistical analysis
The statistical significance of the observed differences was evaluated using 1-way ANOVA, followed by Bonferroni posttests. Statistical analysis was carried out using GraphPad Prism 5 software (GraphPad Software, Inc). A P value less than .05 was considered significant.
Results
Inhibition of PMN apoptosis by cytokine-activated NK-cell supernatant
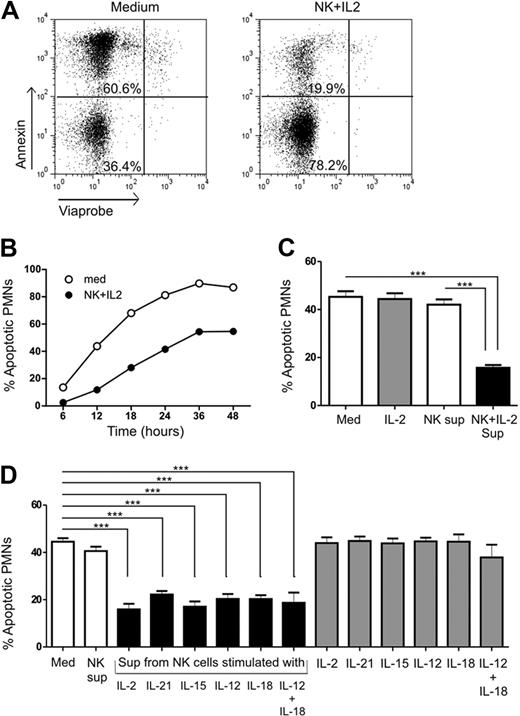
PMNs have a short half-life of 6 to 8 hours in peripheral blood, which can be enhanced once they have entered the site of inflammation.10 We observed that direct coculture of PMNs and NK cells in the presence of IL-2 delayed PMN apoptosis and enhanced activation (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To investigate whether the effect observed was cell-cell contact–dependent or soluble factor–mediated, we cultured PMNs with supernatants derived from unstimulated or cytokine-activated NK cells. PMNs were incubated with supernatants of IL-2–activated NK cells for different time periods from 6 to 48 hours, and assessed PMN apoptosis by flow cytometry using annexin and viaprobe staining. Figure 1A shows representative flow cytometric analysis of PMN apoptosis where apoptotic cells were defined as annexin+viaprobe− cells and living cells as annexin−viaprobe− cells. No significant difference in cell death was observed during the first 6 hours of PMN culture (Figure 1B). The percentage of PMNs undergoing spontaneous apoptosis increased throughout between 12 and 48 hours. However, the presence of IL-2–stimulated NK-cell supernatant inhibited PMN apoptosis at every time point, with the maximum occurring between 18 and 24 hours, indicating that the effect was soluble factor–mediated. There was an increase in PMN survival by almost 2-fold. Based on these observations, PMN cultures in all subsequent experiments were conducted with supernatants from cytokine-activated NK cells for 18 to 24 hours to ascertain the factors involved in the effects observed on PMNs.
Evaluation of PMN apoptosis in the presence of cytokine-activated NK-cell supernatant. (A) PMNs were cultured in the absence (Med) or presence (NK + IL-2) of IL-2–activated NK-cell supernatant, and stained with annexin and viaprobe to determine apoptosis. Annexin+viaprobe− cells (top left quadrant) were defined as apoptotic and annexin−viaprobe− cells (bottom left quadrant) as living. (B) The percentage of apoptotic PMNs was determined at different time points in the absence (Med) or presence (NK + IL-2) of IL-2–activated NK-cell supernatant (n= 1). The data points represent the percentage of apoptotic PMNs when cultured alone or with supernatants from cytokine-activated NK cells. (C) Quantitative representation of the percentage of apoptotic PMNs when cultured alone (Med) or with supernatants from unstimulated (NK sup) or IL-2–activated (NK + IL-2 sup) NK cells for 18-24 hours, from 24 individual experiments totally. (D) PMNs cultured with supernatants from NK cells stimulated with IL-2, IL-21, IL-15, IL-12, IL-18, or a combination of IL-12 plus IL-18 compared with control medium (Med) and PMNs treated with cytokines alone (n= 5). The results are expressed as mean ± SEM. ***P < .001.
Evaluation of PMN apoptosis in the presence of cytokine-activated NK-cell supernatant. (A) PMNs were cultured in the absence (Med) or presence (NK + IL-2) of IL-2–activated NK-cell supernatant, and stained with annexin and viaprobe to determine apoptosis. Annexin+viaprobe− cells (top left quadrant) were defined as apoptotic and annexin−viaprobe− cells (bottom left quadrant) as living. (B) The percentage of apoptotic PMNs was determined at different time points in the absence (Med) or presence (NK + IL-2) of IL-2–activated NK-cell supernatant (n= 1). The data points represent the percentage of apoptotic PMNs when cultured alone or with supernatants from cytokine-activated NK cells. (C) Quantitative representation of the percentage of apoptotic PMNs when cultured alone (Med) or with supernatants from unstimulated (NK sup) or IL-2–activated (NK + IL-2 sup) NK cells for 18-24 hours, from 24 individual experiments totally. (D) PMNs cultured with supernatants from NK cells stimulated with IL-2, IL-21, IL-15, IL-12, IL-18, or a combination of IL-12 plus IL-18 compared with control medium (Med) and PMNs treated with cytokines alone (n= 5). The results are expressed as mean ± SEM. ***P < .001.
The percentage of apoptotic PMNs was reduced by 3-fold when cultured with supernatants from IL-2–stimulated NK cells compared with the control medium (Med vs NK + IL-2; P < .001) or in the presence of unstimulated NK cell supernatant after 18 to 24 hours (NK vs NK + IL-2; P < .001; Figure 1C). NK cells, upon stimulation with different cytokines, display different cytokine and chemokine profiles.21-23 Therefore, in addition to IL-2, we stimulated NK cells with IL-15, IL-21, IL-12, IL-18, and a combination of IL-12 and IL-18 to study whether PMN survival, activation, and function vary upon differential cytokine stimulation of NK cells. PMNs cultured with supernatants from NK cells that had been stimulated with different cytokines showed enhanced survival compared with PMNs cultured in control medium or with supernatant from unstimulated NK cells (Figure 1D). Treatment of PMNs with cytokines alone did not have any effect on apoptosis, making direct effects of these cytokines on PMN survival very unlikely. In conclusion, these data suggest that soluble factors released by cytokine-activated NK cells significantly delay PMN apoptosis.
Activation of PMNs
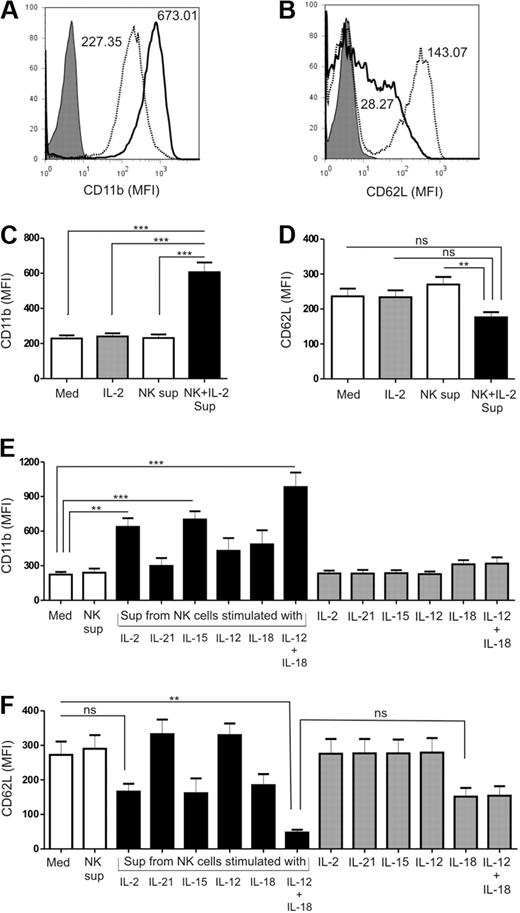
Activated PMNs play a very important role in the elimination of invading pathogens. Loss of CD62L and CD11b up-regulation are considered markers of in vitro PMN activation, and regulate several PMN functions.24-27 We therefore analyzed the expression of these 2 surface molecules on PMNs by flow cytometry, after culturing them with and without supernatants from cytokine-activated NK cells. Figure 2A and B shows representative staining patterns of CD11b and CD62L expression, respectively. The level of CD11b expression on PMNs increased by 3-fold when cultured with IL-2–activated NK-cell supernatants compared with the respective control medium (Med vs NK + IL-2; P < .001) or in the presence of supernatant from unstimulated NK cells (NK vs NK + IL-2; P < .001; Figure 2C). On the other hand, expression of CD62L was downmodulated from the surface of PMNs in the presence of IL-2–activated NK-cell supernatants compared with the control medium, though the difference was not statistically significant (Figure 2D). We observed that activation of PMNs in terms of CD11b (Figure 2E) and CD62L (Figure 2F) expression in response to conditioned supernatants from NK cells varied depending on the cytokine used for NK-cell stimulation. PMNs cultured with supernatants of NK cells stimulated with IL-2, IL-15, and IL-12 plus IL-18 showed up-regulation of CD11b expression, whereas supernatants from NK cells stimulated with IL-21, IL-12, or IL-18 had no effect (Figure 2E). Stimulation of NK cells with a combination of IL-12 and IL-18 resulted in a major loss of CD62L and enhanced CD11b expression on the surface of PMNs. However, supernatant derived from NK cells stimulated with either IL-12 or IL-18 separately did not produce the same effect on PMNs (Figure 2F). CD62L expression remained unaltered when stimulated with supernatant from IL-21– or IL-12–activated NK cells. These results indicate that in addition to the prosurvival properties, supernatants derived from cytokine-stimulated NK cells also promote PMN activation.
Expression of activation markers on PMNs when cultured with supernatants from cytokine-activated NK cells. Representative histograms of (A) CD11b and (B) CD62L expression on PMNs when cultured in the absence (dotted profile) or presence (solid profile) of cytokine-activated NK-cell supernatant. Matched isotype controls are shown by the shaded profile. Mean fluorescence intensity (MFI) is indicated. Quantitative representation of the mean fluorescence intensity of (C) CD11b and (D) CD62L on the surface of PMNs when cultured alone (Med) or with supernatants from unstimulated (NK sup) or IL-2–activated (NK + IL-2 sup) NK cells for 18 to 24 hours, from 24 individual experiments. Evaluation of (E) CD11b and (F) CD62L surface expression on PMNs when cultured with supernatants from NK cells stimulated with IL-2, IL-21, IL-15, IL-12, IL-18, or a combination of IL-12 plus IL-18 compared with control medium (Med) and PMNs treated with cytokines alone (n= 5). The results are expressed as mean ± SEM. **P < .01; ***P < .001.
Expression of activation markers on PMNs when cultured with supernatants from cytokine-activated NK cells. Representative histograms of (A) CD11b and (B) CD62L expression on PMNs when cultured in the absence (dotted profile) or presence (solid profile) of cytokine-activated NK-cell supernatant. Matched isotype controls are shown by the shaded profile. Mean fluorescence intensity (MFI) is indicated. Quantitative representation of the mean fluorescence intensity of (C) CD11b and (D) CD62L on the surface of PMNs when cultured alone (Med) or with supernatants from unstimulated (NK sup) or IL-2–activated (NK + IL-2 sup) NK cells for 18 to 24 hours, from 24 individual experiments. Evaluation of (E) CD11b and (F) CD62L surface expression on PMNs when cultured with supernatants from NK cells stimulated with IL-2, IL-21, IL-15, IL-12, IL-18, or a combination of IL-12 plus IL-18 compared with control medium (Med) and PMNs treated with cytokines alone (n= 5). The results are expressed as mean ± SEM. **P < .01; ***P < .001.
Preserved functional properties
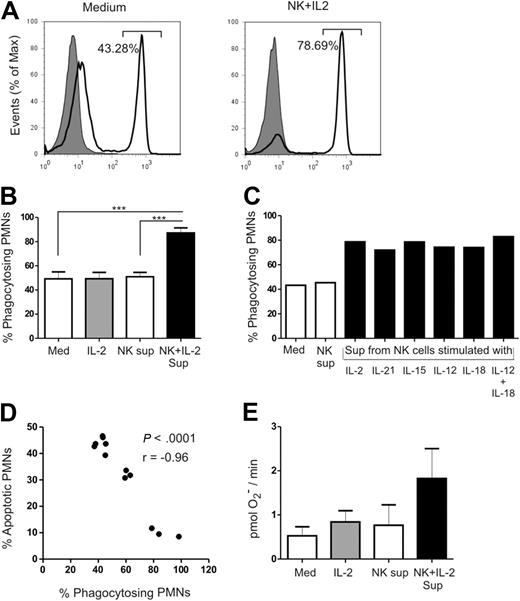
Upon host infection, PMNs ingest invading pathogens and destroy them via oxidative or nonoxidative mechanisms. To study the effect of culture supernatants of NK cells on the functional capabilities of PMNs, we measured phagocytosis and production of ROS. Phagocytosis was evaluated using a flow cytometer–based assay, which determined the ability of PMNs to ingest fluorescently labeled 1-μm latex beads (Figure 3A). Roughly 50% of PMNs were able to take up 1-μm latex beads when cultured alone or in the presence of supernatant of unstimulated NK cells. In contrast, 87% of the PMNs cultured with supernatant of IL-2–stimulated NK cells displayed phagocytic activity (Med vs NK + IL-2; P < .001 and NK vs NK + IL-2; P < .001; Figure 3B). Supernatants from NK cells stimulated with different cytokines were also able to preserve the phagocytic property of PMNs (Figure 3C). The percentage of apoptotic PMNs inversely correlated with the percentage of PMNs that were able to perform phagocytosis (P < .001; r = −0.96; Figure 3D). ROS production by PMNs was detected by the spin trap 1-hydroxy-3-carboxy-pyrrolidine. Although the difference was not statistically significant, there was an almost 2-fold increase in the amount of superoxide produced by PMNs in the presence of IL-2–stimulated NK-cell supernatant compared with the control medium (Figure 3E). Treatment of PMNs with cytokines alone did not have any effect on phagocytosis or ROS production, indicating that the effects observed on PMNs were a result of NK cell–derived factors. Therefore, culture supernatants of cytokine-activated NK cells not only rescued PMNs from apoptosis, but also retained their functional competence.
Evaluation of functional properties of PMNs. Functional properties of PMNs were assessed in the absence or presence of IL-2–activated NK-cell supernatant after 18 to 24 hours of culture. (A) Representative FACS analysis of PMNs coincubated with 1-μm fluorescent labeled latex beads in the absence (left panel) or presence of IL-2–activated NK cells supernatant (right panel). Shaded profiles represent uptake when PMNs were incubated on ice, and open profiles represent active uptake at 37°C. The percentage of PMNs phagocytosing 1-μm latex beads is indicated. (B) Quantitative representation of bead uptake by PMNs from 4 individual experiments. (C) Evaluation of the phagocytic capacity of PMNs when cultured with supernatants from NK cells stimulated with IL-2, IL-21, IL-15, IL-12, IL-18, or a combination of IL-12 plus IL-18 (n= 1). (D) Correlation analysis between the percentage of apoptotic PMNs and the percentage of phagocytosing PMNs. (E) Quantitative representation of the amount of superoxide produced by PMNs from 3 individual experiments. Amount of superoxide produced by PMNs was determined by measuring the electron spin of a superoxide radical. Results are expressed as mean ± SEM. ***P < .001.
Evaluation of functional properties of PMNs. Functional properties of PMNs were assessed in the absence or presence of IL-2–activated NK-cell supernatant after 18 to 24 hours of culture. (A) Representative FACS analysis of PMNs coincubated with 1-μm fluorescent labeled latex beads in the absence (left panel) or presence of IL-2–activated NK cells supernatant (right panel). Shaded profiles represent uptake when PMNs were incubated on ice, and open profiles represent active uptake at 37°C. The percentage of PMNs phagocytosing 1-μm latex beads is indicated. (B) Quantitative representation of bead uptake by PMNs from 4 individual experiments. (C) Evaluation of the phagocytic capacity of PMNs when cultured with supernatants from NK cells stimulated with IL-2, IL-21, IL-15, IL-12, IL-18, or a combination of IL-12 plus IL-18 (n= 1). (D) Correlation analysis between the percentage of apoptotic PMNs and the percentage of phagocytosing PMNs. (E) Quantitative representation of the amount of superoxide produced by PMNs from 3 individual experiments. Amount of superoxide produced by PMNs was determined by measuring the electron spin of a superoxide radical. Results are expressed as mean ± SEM. ***P < .001.
To rule out the possibility of any contaminating cells, such as T cells, having an effect on PMNs, we cultured PMNs with supernatants derived from IL-2–stimulated T cells for 18 to 24 hours. We found that supernatants of T cells stimulated with IL-2 did not have any effect on PMN survival and activation (supplemental Figure 2). This indicated that the effects observed on PMNs were an effect of supernatants derived from IL-2–activated NK cells and not a result of T-cell contamination. We also cultured PMNs with supernatants derived from NK-cell clones (CNK6) stimulated with different cytokines (IL-2, IL-15, IL-21, IL-12, IL-18, and IL-12 + IL-18; supplemental Figure 3). The advantage of using NK-cell clones was that they are completely free from any contaminating cells such as monocytes or T cells, as they are derived from a single NK cell. We found that cytokine-activated CNK6 supernatants induced similar effects on PMNs as purified activated NK cells. PMNs survived longer, with elevated CD11b and reduced CD62L expression, indicating that soluble factors derived from activated human NK cells and NK-cell clones have the same effect on PMNs.
Comparison of CD56bright and CD56dim NK cell–mediated effects on PMNs
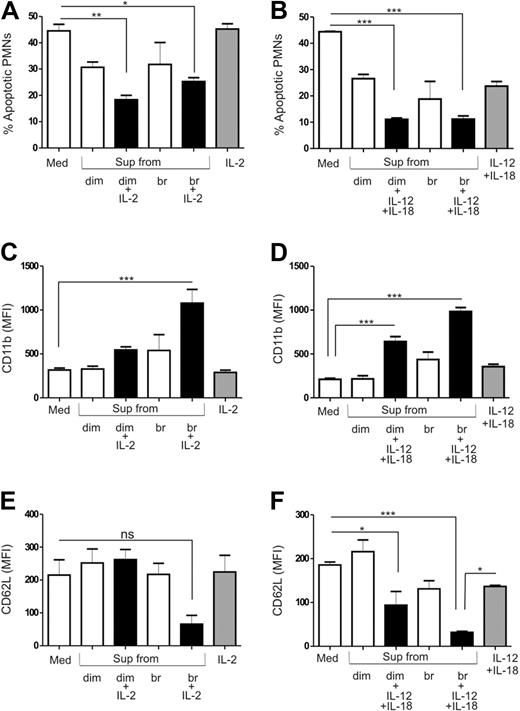
CD56bright NK cells are known to be more efficient in producing cytokines, whereas CD56dim cells are more potent killers.4-6 In order to assess differences in the effects of these subsets on PMNs, we sorted CD56dim and CD56bright NK cells and stimulated them with either IL-2 or a combination of IL-12 and IL-18. Supernatants from both cytokine-activated CD56dim and CD56bright NK cells displayed equal potential to increase the lifespan of PMNs (Figure 4A-B). We detected the highest expression of CD11b on PMNs when they were cultured with supernatants of CD56bright NK cells stimulated with either IL-2 (Figure 4C) or IL-12 plus IL-18 (Figure 4D). Supernatants from IL-12 plus IL-18–stimulated CD56dim or IL-2 or IL-12 plus IL-18–stimulated CD56bright NK cells resulted in loss of CD62L (Figure 4E-F) from the surface of PMNs. The level of CD62L expression was preserved when PMNs were cultured with unstimulated CD56dim or CD56bright cells or IL-2–stimulated CD56dim cells. Thus, supernatants from IL-2 or IL-12 plus IL-18–activated CD56bright NK cells activated PMNs more strongly than supernatants from cytokine-activated CD56dim NK cells.
Effect of cytokine-activated, sorted NK-cell subsets on PMN survival and activation. CD56dim (dim) and CD56bright (br) NK cells were sorted by FACS and stimulated with either IL-2 or a combination of IL-12 and IL-18. PMNs were then cultured in the absence or presence of supernatants from unstimulated or cytokine-activated and sorted NK-cell subsets for 18 to 24 hours and compared with control medium (Med) and PMNs treated with cytokines alone. The percentage of apoptotic PMNs cultured in the absence or presence of supernatants from (A) IL-2 (n= 4) or (B) IL-12 plus IL-18 (n= 4) activated CD56dim and CD56bright NK cells. Evaluation of CD11b surface expression on PMNs in the absence or presence of (C) IL-2 (n= 4) or (D) IL-12 plus IL-18 (n= 4) activated CD56dim and CD56bright NK-cell supernatants. Expression of CD62L on the surface of PMNs when cultured in the absence (Med) or presence of (E) IL-2 (n= 4) or (F) IL-12 plus IL-18 (n= 4) activated CD56dim and CD56bright NK-cell supernatants. Results are expressed as mean ± SEM. *P < .05; **P < .01; ***P < .001.
Effect of cytokine-activated, sorted NK-cell subsets on PMN survival and activation. CD56dim (dim) and CD56bright (br) NK cells were sorted by FACS and stimulated with either IL-2 or a combination of IL-12 and IL-18. PMNs were then cultured in the absence or presence of supernatants from unstimulated or cytokine-activated and sorted NK-cell subsets for 18 to 24 hours and compared with control medium (Med) and PMNs treated with cytokines alone. The percentage of apoptotic PMNs cultured in the absence or presence of supernatants from (A) IL-2 (n= 4) or (B) IL-12 plus IL-18 (n= 4) activated CD56dim and CD56bright NK cells. Evaluation of CD11b surface expression on PMNs in the absence or presence of (C) IL-2 (n= 4) or (D) IL-12 plus IL-18 (n= 4) activated CD56dim and CD56bright NK-cell supernatants. Expression of CD62L on the surface of PMNs when cultured in the absence (Med) or presence of (E) IL-2 (n= 4) or (F) IL-12 plus IL-18 (n= 4) activated CD56dim and CD56bright NK-cell supernatants. Results are expressed as mean ± SEM. *P < .05; **P < .01; ***P < .001.
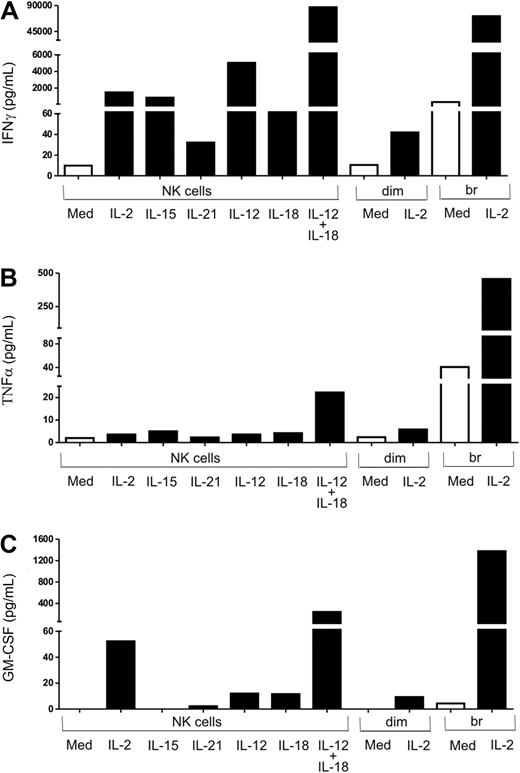
Cytokine profile of activated NK cells
The type and the amount of cytokines that are produced by cells are under specific regulation. Upon host infection, a vast variety of cytokines are produced, which in turn determine the type of inflammatory mediators produced by NK cells after activation. We observed differences in CD62L and CD11b expression patterns on PMNs when they were cultured with supernatants of NK cells stimulated with different cytokines. We also successfully demonstrated that the effects observed on PMNs were due to soluble factors produced by cytokine stimulated NK cells. We therefore performed a cytokine bead array analysis to profile the range of cytokines produced by NK cells upon cytokine stimulation.
Results of CBA analysis showed that total NK cells, when stimulated with IL-12 plus IL-18, produced IFNγ (Figure 5A), TNFα (Figure 5B), and GM-CSF (Figure 5C) in much higher amounts than when they were stimulated with either of these cytokines or with IL-2, IL-15, or IL-21. The cytokine pattern of CD56bright NK cells when stimulated with IL-2 was similar to that of total NK cells activated with IL-12 plus IL-18 in terms of IFNγ, GM-CSF, and TNFα production. The amount of IFNγ, GM-CSF, and TNFα produced by IL-2–stimulated CD56bright NK cells and IL-12 plus IL-18–stimulated total NK cells was much higher compared with either CD56dim or total NK cells stimulated with IL-2.
Cytokine-activated NK cells produce IFNγ, TNFα, and GM-CSF. Total NK cells (NK) and FACS-sorted CD56dim (dim) and CD56bright (br) NK cells were stimulated with different cytokines (IL-2, IL-15, IL-21, IL-12, IL-18, IL-12, IL-18, or IL-12 + IL-18) for 18 to 24 hours. Culture supernatants were used to measure (A) IFNγ and (B) TNFα by cytokine bead array and (C) GM-CSF by cytokine flex set.
Cytokine-activated NK cells produce IFNγ, TNFα, and GM-CSF. Total NK cells (NK) and FACS-sorted CD56dim (dim) and CD56bright (br) NK cells were stimulated with different cytokines (IL-2, IL-15, IL-21, IL-12, IL-18, IL-12, IL-18, or IL-12 + IL-18) for 18 to 24 hours. Culture supernatants were used to measure (A) IFNγ and (B) TNFα by cytokine bead array and (C) GM-CSF by cytokine flex set.
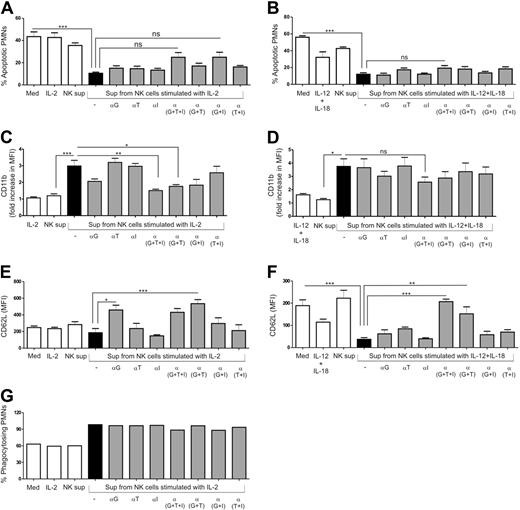
Role of GM-CSF, TNFα, and IFNγ in PMN survival, activation, and function
In our experimental setup, PMNs were cultured with supernatants from cultured NK cells. Hence, the effects observed on PMNs were a result of soluble factors released by NK cells and not dependent on cell-to-cell contact. Using CBA analysis, we found that large amounts of GM-CSF, TNFα, and IFNγ were produced by cytokine-activated total NK cells and CD56bright NK cells. Thus, to investigate whether the effects produced by supernatants of NK cells on PMNs were a result of these cytokines, we blocked their activity by using neutralizing agents. The delay in PMN apoptosis observed when cultured with IL-2–activated NK-cell supernatant was partially inhibited by blocking the activities of GM-CSF and IFNγ (Figure 6A). In contrast, the presence of neutralizing agents in the culture of PMNs with supernatant from IL-12 plus IL-18–activated NK cells had no effect on the prolonged PMN survival (Figure 6B).
Effect of neutralizing agents against IFNγ, TNFα, and GM-CSF on PMN survival, activation, and function in the presence of supernatants from cytokine-activated NK cells. PMNs were cultured in the absence or presence of supernatants from unstimulated (NK sup) or cytokine-activated NK cells for 18 to 24 hours and compared with the control medium (Med) and PMNs treated with cytokines alone. Monoclonal anti–human GM-CSF antibody (20 μg/mL, αG), purified anti–human IFNγ antibody (20 μg/mL, αI) and soluble TNF receptor (1 μg/mL, αT) were added to PMN cultures in the presence of supernatants from cytokine-activated NK cells in different combinations. Quantitative representation of (A-B) percentage of apoptotic PMNs (n = 4), (C-D) fold increase in CD11b expression (n = 4), (E-F) CD62L expression on the surface of PMNs (n = 4), and (G) percentage of phagocytosing PMNs (n = 1). Fold increase was calculated as MFI of CD11b on PMNs for different conditions divided by the MFI of PMNs when cultured alone (control medium; Med). Results are expressed as mean ± SEM. *P < .05; **P < .01; ***P < .001.
Effect of neutralizing agents against IFNγ, TNFα, and GM-CSF on PMN survival, activation, and function in the presence of supernatants from cytokine-activated NK cells. PMNs were cultured in the absence or presence of supernatants from unstimulated (NK sup) or cytokine-activated NK cells for 18 to 24 hours and compared with the control medium (Med) and PMNs treated with cytokines alone. Monoclonal anti–human GM-CSF antibody (20 μg/mL, αG), purified anti–human IFNγ antibody (20 μg/mL, αI) and soluble TNF receptor (1 μg/mL, αT) were added to PMN cultures in the presence of supernatants from cytokine-activated NK cells in different combinations. Quantitative representation of (A-B) percentage of apoptotic PMNs (n = 4), (C-D) fold increase in CD11b expression (n = 4), (E-F) CD62L expression on the surface of PMNs (n = 4), and (G) percentage of phagocytosing PMNs (n = 1). Fold increase was calculated as MFI of CD11b on PMNs for different conditions divided by the MFI of PMNs when cultured alone (control medium; Med). Results are expressed as mean ± SEM. *P < .05; **P < .01; ***P < .001.
Up-regulation of PMN CD11b expression when the cells were cultured with IL-2–activated NK-cell supernatant was abrogated in the presence of blocking agents against GM-CSF, TNFα, and IFNγ (NK + IL-2 vs NK + IL-2 + anti-[G + T + I]; P < .01; Figure 6C). Although the difference was not statistically significant, the presence of anti–GM-CSF antibody alone also partially inhibited CD11b up-regulation, indicating a critical role of GM-CSF in elevating the expression of CD11b. In contrast, there was only a partial reduction in the expression of CD11b on PMNs when cultured with IL-12 plus IL-18–stimulated NK cells supernatant in the presence of blocking agents against all 3 cytokines (Figure 6D).
Blocking the activity of GM-CSF and TNFα increased the surface expression of CD62L on PMNs when cultured in the presence of IL-2–activated NK-cell supernatant (Figure 6E). As described earlier, culture of PMNs with supernatants of IL-12 plus IL-18–stimulated NK cells resulted in CD62L shedding. This loss of CD62L expression was almost completely blocked in the presence of neutralizing agents (NK + IL-12 +IL-18 vs NK + IL-12 +IL-18 + anti-[G + T + I]; P < .001; Figure 6F). The combination of blocking agents against GM-CSF and TNFα was also sufficient to abrogate CD62L shedding from the surface of PMNs (NK + IL-12 + IL-18 vs NK + IL-12 + IL-18 + anti-[G + T]; P < .01). In contrast, blocking the activity of these cytokines did not have any effect on the preserved phagocytic capacity of PMNs when cultured with supernatants from IL-2–activated NK cells (Figure 6G). IL-15–activated NK-cell supernatants induced similar effects on PMNs as supernatants from IL-2–activated NK cells (supplemental Figure 4).
We also used recombinant IFNγ, TNFα, and GM-CSF to assess their effects on PMN survival and activation status. Consistent with other studies,28-30 we found that PMNs cultured with GM-CSF, IFNγ, and TNFα in different combinations inhibited PMN apoptosis and induced activation (supplemental Figure 5). In summary, our data show that soluble factors produced by cytokine-activated NK cells enhance PMN survival, induce activation, and preserve their functional competence. We also demonstrate that IFNγ, GM-CSF, and TNFα secreted by NK cells play a crucial role in the NK cell–mediated effects on PMNs.
Discussion
NK cells are known to have immunomodulatory effects on other immune cells, such as DCs, T cells, B cells, monocytes, and macrophages, via secretion of cytokines and chemokines.11-14,31 In this study, we report for the first time the antiapoptotic effects of NK cells on PMNs. Our results revealed that soluble factors derived from cytokine-activated NK cells have the potential to prolong PMN survival. PMNs not only survived longer, but also retained their ability to perform phagocytosis and produce ROS. The antiapoptotic effect of NK cells on PMNs was induced only upon stimulation with cytokines.
Upon host infection, NK cells, along with PMNs, are among the first cells to be recruited to the site of inflammation, suggesting bilateral crosstalk between the 2 cell types. Various murine models have successfully demonstrated the role of NK cells in bacterial infections.18,32,33 PMNs and NK cells have also been shown to play a role in certain inflammatory conditions, such as rheumatoid arthritis (RA).34,35 We therefore took synovial fluid from 2 RA patients, and detected both NK cells and PMNs via flow cytometry (supplemental Figure 6). Most of the NK cells in the synovial fluid belonged to the CD56bright subset, which is consistent with a previous study by Pridgeon et al in 2003.35 This observation thus provides evidence for a possible direct/indirect interaction between NK cells and PMNs at sites of inflammation. Most of the studies investigating the interaction between NK cells and PMNs have focused on the effects of PMNs on NK-cell functions.15-18 However, to our knowledge, the impact of NK cells on PMN biology has not been studied. We therefore investigated the effect of human NK cells on PMN survival and function. Cell death of PMNs was delayed upon incubation with supernatants from cytokine-activated NK cells. The presence of IL-2–activated NK-cell supernatant almost doubled the half-life of PMNs compared with PMNs cultured alone or with supernatant from unstimulated NK cells. The cytokine and chemokine profile of NK cells differs with the selection of cytokines used for NK-cell stimulation.21-23 Therefore, in addition to IL-2, we also used IL-15, IL-21, IL-12, IL-18, and a combination of IL-12 plus IL-18 to stimulate NK cells. IL-15, IL-12, and IL-18 are known to be produced by activated macrophages in response to infection and bacterial components,23 which in turn would trigger the NK-cell response. The switch from innate to adaptive immune responses would lead the primed T cells to produce IL-2 and IL-21, resulting in NK-cell activation. Supernatants of NK cells stimulated with different cytokines all had the potential to prolong PMN survival in culture. The fact that supernatants from unstimulated NK cells were not able to delay PMN apoptosis underscores the importance of triggering NK-cell activity to mediate this effect, as shown in our setting by cytokine stimulation. Thus, our data suggest that only under certain inflammatory conditions, that is, in the presence of cytokines, NK cells are able to prolong PMN survival.
NK cells can be categorized into 2 subsets, CD56dim CD16hi and CD56bright CD16lo/− NK cells, based on their expression of CD56 and CD16.2,3 CD56dim CD16hi cells have a higher cytotoxic potential, whereas CD56bright CD16lo/− NK cells are known as more potent cytokine producers.4-6 To assess the roles of these subsets in NK-PMN interaction, NK cells were sorted into CD56dim and CD56bright cells and stimulated with either IL-2 or IL-12 plus IL-18. Supernatants from both cytokine-activated CD56dim and CD56bright cells were able to delay PMN apoptosis compared with PMNs cultured alone. There was no difference in their potential to inhibit PMN apoptosis, indicating that the factors responsible for inhibition of PMN apoptosis were produced by both NK-cell subsets.
PMN apoptosis is apparently associated with loss of cell functions such as phagocytosis and ROS production, and reduced expression of receptors that play an important role in PMN function and migration.36 We therefore studied the expression of CD11b and CD62L on PMNs, as up-regulation of CD11b, together with CD62L shedding, are commonly used markers of in vitro neutrophil activation.24,25 CD62L is involved in migration and accumulation, whereas CD11b/CD18 molecules are known to regulate several other PMN functions such as chemotaxis, phagocytosis, and ROS production.26,27 Although CD11b/CD18 is not required for inhibition of PMN cell death in vivo, in vitro studies indicate that crosslinking of CD11b or CD11a results in the delay of PMN apoptosis.37 Thus, regulation of adhesion molecules seems to modulate PMN survival. Supernatants from total or sorted NK cells stimulated with different cytokines rescued PMNs from apoptosis but induced differential activation in terms of CD11b and CD62L expression. This suggests that production of one or several common factors by virtually all NK cells upon stimulation with different cytokines is responsible for the delay in PMN apoptosis. However, the expression levels of CD11b and CD62L seem to be regulated by soluble mediators that are secreted by NK cells only upon stimulation with particular cytokines. Costimulation with both IL-12 and IL-18 induces a stronger cytokine response by NK cells compared with individual stimulation with IL-12 or IL-18.23 This could explain the difference in the level of CD62L and CD11b expression induced in PMNs by supernatants of NK cells stimulated with a combination of IL-12 and IL-18 compared with supernatants from NK cells stimulated with IL-12 and IL-18 separately. Because CD56dim NK cells constitute 90% of the total NK cells,3 soluble factors derived from them modulated CD62L and CD11b expression on PMNs to levels comparable with activated total NK-cell supernatant. Previous literature shows that CD56bright NK cells, upon stimulation with different combinations of physiologic stimuli, produce higher amounts of cytokines and chemokines compared with CD56dim NK cells.23 This could be a plausible explanation for the supernatants from cytokine-activated CD56bright NK cells having more pronounced effects on PMN activation than CD56dim NK cells. We observed that although supernatants from unstimulated, sorted NK-cell subsets resulted in minor antiapoptotic effects and slight activation of PMNs, this difference was not statistically significant, and could possibly be a result of activation of the subsets during sorting procedures. Our findings thus indicate that the activation status of PMNs, but not survival, is dependent on the type of cytokines that activate NK cells, which in turn determines the type of inflammatory mediators released by them.
Down-regulation of CD62L and enhanced expression of CD11b promotes accumulation of surviving PMNs at the site of inflammation and supports elimination of infective pathogens. The main function of PMNs is to phagocytose microorganisms and to kill them by oxidative or nonoxidative mechanisms.9,38 We therefore evaluated the impact of NK cells on the functional competence of PMNs by measuring their ROS production and phagocytic activity. In the presence of IL-2–stimulated NK-cell supernatants, PMNs maintained their functional ability in terms of producing ROS and phagocytosis. We show that prolonged PMN survival correlated with the retention of their phagocytic capacity. Although soluble factors derived from NK cells stimulated with different cytokines were able to preserve the phagocytic capacity of PMNs, the expression levels of CD11b and CD62L were not comparable. The lack of correlation between the activation status of PMNs and their functionality could be explained by the fact that, apart from phagocytosis and ROS production, there are other functions of PMNs such as adhesion, chemotaxis, degranulation, and antibody-dependent cell-mediated cytotoxicity, which are also regulated by these molecules.39 Thus, we demonstrate that PMNs not only survived longer in the presence of soluble factors derived from cytokine-activated NK cells but also retained their functional activity to phagocytose and produce ROS.
Quantitative analysis revealed production of TNFα, GM-CSF, and IFNγ by IL-2– or IL-12 plus IL-18–activated total NK cells and IL-2–activated CD56bright NK cells in high amounts. This observation corroborates previous findings that CD56bright NK cells are more efficient in producing cytokines, and that the combination of IL-12 and IL-18 is a stronger stimulus for NK cells than others.23 These findings impelled us to investigate the involvement of TNFα, GM-CSF, and IFNγ in the NK cell–mediated effects on PMNs. We therefore blocked the activity of these cytokines when PMNs were cultured with supernatants from cytokine-activated NK cells. Although the effect on PMN apoptosis and CD11b expression was only partially inhibited, CD62L down-regulation on PMNs was completely abrogated. We observed that PMNs treated with IL-18, unlike those treated with other cytokines, also resulted in some CD62L shedding, but the effect was more pronounced when PMNs were cultured with supernatant of NK cells stimulated with both IL-12 and IL-18. The latter effect could be completely abrogated upon addition of neutralizing agents against GM-CSF and TNFα. We also observed that recombinant IFNγ enhanced CD62L expression on PMNs. Momose et al demonstrated in eosinophils that increased expression of CD62L by IFNγ is a result of increased cyclic AMP and protein synthesis, thereby providing a plausible explanation for our observation.40 This implies that although IL-18 could have a minor effect on PMNs, GM-CSF, IFNγ, and TNFα produced by NK cells play a more dominant role on CD62L expression on PMNs.
As previously mentioned, however, the delay in PMN apoptosis and CD11b up-regulation were only partially inhibited. The incomplete reversal of these effects observed in PMNs cultured with cytokine-activated NK-cell supernatants in the presence of blocking agents could have 2 possible explanations. First, it could mean that the neutralizing antibodies used in the system were not able to completely block the effects of cytokines. However, after diluting the supernatant from cytokine-activated NK cells from one of our donors, we observed that blocking the activities of GM-CSF, IFNγ, and TNFα could completely abrogate the NK cell–mediated effects on PMNs in terms of CD11b up-regulation and delay in apoptosis (supplemental Figure 7), indicating their dominant role in the NK cell–mediated effects on PMNs. Second, we do not rule out the possibility of additional unidentified NK-cell factors, which could mediate similar effects on PMNs.
Previous studies have demonstrated that several cytokines, such as TNFα, IFNγ, GM-CSF, IL-6, IL-1β, and G-CSF, some of which are produced by activated NK cells,21 are engaged in enhancing PMN survival, activation, and function.28-30 Consistent with these reports, we observed that PMNs treated with recombinant TNFα, IFNγ, and GM-CSF in different combinations survived longer and were strongly activated. The effects induced by these recombinant cytokines on PMNs were comparable with the effects observed using the cytokine-stimulated NK-cell supernatants. Thus, we demonstrated that GM-CSF, IFNγ, and TNFα produced by IL-2– or IL-12 plus IL-18–activated total NK cells and IL-2–activated CD56bright NK cells in vitro play a crucial role in rescue of PMNs from apoptosis, induction of activation, and retention of their function.
The cytokine milieu induced upon host infection determines the cytokine and chemokine profile of NK cells.23 Our study provides evidence for the role of soluble factors produced by NK cells on PMN survival and function. We demonstrate that NK cells, upon activation by cytokines, secrete inflammatory mediators that prolong PMN lifespan, promote their accumulation for extended time periods, and preserve their functional capability to destroy invading pathogens. Based on our findings, we believe our study may have implications for diseases involving impaired NK-cell and PMN function. For instance, in patients infected with HIV-1, NK cells have defective cytokine production and cytotoxic activity.41 PMNs from these patients have been shown to undergo accelerated spontaneous apoptosis, defective oxidative bursts, and phagocytosis.42-44 It is therefore reasonable to contemplate that impaired production of inflammatory mediators by NK cells could, at least in part, be the cause for defective PMN function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Tibor Horvath for his assistance in superoxide measurements and Dr Matthias Ballmaier and Christina Reimer for their assistance in cell sorting.
N.B. is supported by a fellowship of the Infection Biology PhD program of the Hannover Biomedical Research School (HBRS) at Hannover Medical School. The study was supported by the Deutsche Forschungsgemeinschaft special research field SFB738 (A5).
Authorship
Contribution: R.J. designed and supervised the project; N.B., H.S.H., and A.H. performed experiments; N.B., H.S.H., J.K.K., and R.J. analyzed the data; and N.B., H.S.H., J.K.K., G.M.B., R.E.S., and R.J. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Dr Roland Jacobs, Clinic for Immunology and Rheumatology, Hannover Medical School, Carl-Neuberg-Str 1, 30625 Hannover, Germany; e-mail: jacobs.roland@mh-hannover.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal