Abstract
Transfusion-related acute lung injury is suggested to be a “2-hit” event resulting from priming and activation of pulmonary neutrophils. Activation may result from infusion of lysophosphatidylcholines (LysoPCs), which accumulate during storage of blood products. In the present study, we developed a syngeneic in vivo transfusion model to test whether storage of platelet concentrates (PLTs) results in lung injury in healthy rats as well as in a “2-hit” model using lipopolysaccharide-pretreated rats. In addition, the effect of washing of platelets was studied. In healthy rats, transfusion of aged PLTs caused mild lung inflammation. In LPS-pretreated rats, transfusion of aged PLTs, but not fresh PLTs, augmented pulmonary systemic coagulopathy. When PLTs components were transfused separately, supernatant of aged PLTs, but not washed aged platelets, induced pulmonary injury in the “2-hit” model. Supernatants of aged PLTs contained increased concentrations of LysoPCs compared with fresh PLTs, which enhanced neutrophil priming activity in vitro. We conclude that transfusion of aged PLTs induces lung inflammation in healthy rats. In a “2-hit” model, aged PLTs contribute to pulmonary and systemic coagulopathy, which may be mediated by LysoPCs, which accumulate in the supernatant of PLTs during storage.
Introduction
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-related morbidity and mortality.1-3 TRALI is characterized by the acute onset of hypoxia within 6 hours after transfusion of a blood product.4 Although TRALI is rarely reported in the general hospital population, the incidence is high among critically ill patients, ranging between 5.3% and 8% per transfused patient.5-7
The high incidence of TRALI in this specific patient population may be explained by the pathogenesis of TRALI, which is thought to be a “2-hit” event. The “first hit” is inflicted by a proinflammatory pulmonary condition, for instance, pneumonia, sepsis, or lung contusion, causing activation of lung endothelium with subsequent sequestration and priming of polymorphonuclear neutrophils. The “second hit” is provided by transfusion of a blood product, resulting in degranulation and release of reactive oxygen species of the primed neutrophils and subsequent pulmonary edema. Besides antibodies against human leukocyte antigens, biologically active lipids (lysophosphatidylcholines [LysoPCs]) and cytokines, which are released and accumulate during storage of platelets products, have been implicated in the pathogenesis of TRALI.8-10
Transfusion of (aged) platelet concentrates (PLTs) was found to be associated with the onset of TRALI and adverse outcome.5-7 Mechanisms of the detrimental effect of PLTs on lung inflammation may include accumulation of soluble factors such as LysoPCs in the supernatant. Indeed, LysoPCs from the supernatant of aged PLTs were found to activate neutrophils in vitro. In addition, LysoPCs have been used in “2-hit” TRALI animal models.8,9,11 Alternatively to soluble factors, PLTs themselves may induce pulmonary vascular injury in TRALI. Thrombocytopenia has been observed in TRALI patients.12-14 In addition, in a mouse model of antibody-mediated TRALI, thrombocytopenia occurred, suggesting that platelets may be involved. In this model, neutrophils were found to capture platelets, resulting in vascular damage and lung injury, which did not occur after depletion of platelets.15 In a similar model depletion of platelets prevented onset of TRALI.16 Whether PLTs transfusion enhances this phenomenon is unknown. In addition, although coagulopathy is a hallmark of ALI attributable to other causes,17 knowledge on coagulation disorders in TRALI is limited.
The clinical relevance of existing TRALI models is hampered by cross species and ex vivo design. At present, no in vivo platelet transfusion model exists that confirms the “2-hit” TRALI hypothesis.18 In this work, we describe a novel syngeneic in vivo rat platelet transfusion model in which the effect of aged PLTs on lung injury was studied in healthy rats and in a “2-hit” model that used lipopolysaccharide (LPS)–pretreated rats. Besides pulmonary inflammation, markers of pulmonary coagulopathy were determined. To elucidate the mechanism of lung injury induced by aged PLTs, we performed additional experiments with specific components of the aged PLTs products.
Methods
The Institutional Animal Care and Use Committee of the Academic Medical Center and the Medical Ethical Committee of Sanquin Blood Bank Foundation approved all experiments. All animals were handled in accordance with the guidelines prescribed by the Dutch legislation and the International Guidelines on protection, care, and handling of laboratory animals.
Preparation of rat PLTs products
Male Sprague-Dawley rats (> 250 g, Harlan) were used to obtain blood. Rats were anesthetized with an intramuscular injection of ketamine 4 mg/kg (Eurovet; Bladel) and medetomidine 0.25 mg/kg (Novartis). Blood was collected from the inferior vena cava in a syringe containing 1.25 mL of citrate-phosphate-dextrose (Fresenius Kabi). Approximately 8 to 10 mL of blood could be obtained from a single rat. Blood of 5 rats was pooled for component preparation. Before pooling, cross matching was carried out to ensure compatibility.
Blood was handled and stored according to national standards for human blood (Sanquin Blood Supply Foundation), with minor changes to adapt for the smaller volumes. After overnight storage at room temperature, blood was centrifuged for 10 minutes at 1892g and 20°C. Plasma was removed, and the buffy coat was separated from the red blood cells. The buffy coat was diluted with pooled plasma to end up with a hematocrit of approximately 20% (vol/vol) and centrifuged for 10 minutes at 288g and 20°C to separate the majority of the remaining erythrocytes and leukocytes from the platelets. To enable a final platelet count in PLTs of approximately 1000 × 109/L, platelet counts were performed between the separation steps. The platelets concentrate was collected and stored at 22°C (± 2°C) horizontally shaking with 1 cycle/second (Platelet Incubator Helmer) in culture flasks (Nunclon surface 50 mL, Nunc; Thermo Fisher) 7 mL each of PLTs, under a 5% CO2/95% air mixture. The storage of aliquots of platelet concentrates in a culture bottle with 5% CO2 has been used in several studies on human platelets and results in similar storage conditions compared with storage in a standard platelet storage container.19,20
Preparation of washed aged platelets and supernatant rat PLT products
After 5 days of storage, rat PLTs products were separated into washed platelets and supernatant. The PLTs products were centrifuged for 15 minutes at 1250g and 22°C. The supernatant of the rat PLTs product was separated. The platelets were washed with the use of CompoSol (Fresenius Hemocare), which was added up to the original volume and centrifuged for 15 minutes at 1000g and 22°C. The supernatant was removed, and the CompoSol was added to the platelets up to the original volume of the rat PLT product. Platelet count in the final washed product was greater than 90% of the original count.
In vivo PLTs transfusion model
Male Sprague-Dawley rats (275 g) raised on a regular diet were randomized into 3 groups (n = 6 per group) to receive transfusion with saline (NaCl 0.9%), transfusion with fresh PLTs, or transfusion with PLTs stored for 5 days (aged PLTs). A storage time of 5 days was chosen because in pilot experiments rat PLTs stored for 5 days showed storage-related changes comparable with those found in previous studies in which the authors used aged human platelets stored for 5 days.9 For the experiments in the “2-hit” PLTs transfusion model, rats received a “first hit” of 2 mg/kg of lipopolysaccharide (from Salmonella enteritidis, Sigma-Aldrich) intraperitoneally 2 hours before transfusion. The animals in the other experiments received, instead of LPS, saline as a “first hit” (equal volume). Animals were weighed and anaesthetized with pentobarbital 50 mg/kg intraperitoneally. The tail vein was cannulated with a 24-gauge venflon (Vasofix Certo B. Braun), and blood was aspirated to verify intravascular placement and to remove 0.5 mL of blood for cross-matching and baseline measurements. A 10% circulating volume transfusion was administered during the course of 30 minutes by the use of an infusion pump (Harvard Pump 11; Harvard Apparatus). Rats were placed back in their cages to recover and were killed 6 hours after transfusion. In a separate set of experiments, LPS-pretreated and healthy animals were transfused with washed aged platelets or with supernatant of aged PLTs.
Blood and tissue sampling
After anesthesia with ketamine 45 mg/kg and medetomidine 0.25 mg/kg as described previously, blood was collected from the inferior vena cava in citrated (0.109M) Vacutainer tubes (BD Biosciences) for analysis and blood culture. The right lung was ligated, and the left lung was lavaged 3 times with 2 mL of saline. After lavage, lungs were weighed and homogenized by the use of a tissue homogenizer (Biospec Products; Bartlesville). For cytokine and chemokine measurements, lung homogenates were diluted 1:1 in lysis buffer (150 nmol/L NaCl; 15mM Tris; 1mM MgCl2-H2O; 1mM CaCl2; 1% Triton X-100; and 100 μg/mL pepstatin A, leupeptin, and aprotinin). The upper lobe of the right lung was fixed in 4% formalin and embedded in paraffin for histopathology examination.
Histology and immunohistochemistry
Sections (4 μm) were stained with hematoxylin-eosin and analyzed by 2 researchers who were blinded for group identity. A histology scoring system was used as previously described.21 In short, the following parameters were scored on a scale of 0 to 4: interstitial inflammation, endothelialitis, bronchitis, edema, thrombus, and pleuritis. The histology score was expressed as the sum of the score for all parameters. Immunohistochemistry was performed for CD40L. Positive control was a rat spleen section stained for CD40L and CD3 (T-cell marker). Deparaffinized and rehydrated lung and spleen sections were incubated with a rat antibody against CD40L or CD3 (Abcam) and were detected by the use of the DAB substrate kit (Vector Laboratories). Of each specimen, digital images were captured of 5 nonoverlapping areas (10× objective) by the use of a DFC500 digital camera mounted on a DM5000B microscope (both from Leica Microsystems). The area positive for CD40L was determined with Image Pro Plus (Media Cybernetics) and expressed as the percentage of the total surface area.
Assays
Thrombin-antithrombin complexes (TATc; Behring) and fibrin degradation products (FDP; Asserachrom D-Di, Diagnostica Stago) were measured by the use of enzyme-linked immunosorbent assay. Plasminogen activator activity (PAA), and plasminogen activator inhibitor-1 (PAI-1) activity were measured by automated amidolytic assays.22 Tumor necrosis factor, interleukin-6 (IL-6), and cytokine-induced neutrophil chemoattractant-3 (CINC-3) were measured by enzyme-linked immunosorbent assay according to instructions from the manufacturer (R&D Systems). Detection limits were 62.5, 31.25, and 125 pg/mL, respectively.
Storage-related biochemical changes in rat platelets
PLTs samples were collected at the indicated time intervals and analyzed for pH and lactate with a Rapidlab 865 blood gas analyzer (Siemens Medical Solutions Diagnostics). Cell counts for leukocytes and platelets were performed with an Advia 2120 hematology counter, with special software for counting animal blood samples (Siemens Medical Solutions Diagnostics). The Kunicki morphology score,23 as well as the aggregation response to adenosine diphosphate (ADP) and to the combination of ADP and collagen, was measured as described.24 The percentage of platelets positive for annexin V was determined as described earlier.19 Supernatants were prepared by centrifugation for 10 minutes at 12 500g to remove cells and acellular debris. Aliquots of supernatants were stored at −80°C for analysis of LysoPC, phosphatidylcholine (PC), and cytokine levels and to test for neutrophil priming.
Lipid extraction and high-performance liquid chromatography tandem mass spectrometry for LysoPC and PC measurement
The supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) provides detail. Lipid extraction of supernatant from PLTs supernatant was performed by use of the Bligh and Dyer method (chloroform/methanol/aqueous solution 1:1:1). The separated CHCl3 layers were combined and dried (N2, 30°C). Samples were dissolved in 150 μL of CHCl3/MeOH/H2O/NH3 25% (50/45/5/0.01 vol/vol/vol/vol) for further analysis.
LysoPCs and PC concentrations in supernatant of PLTs were determined by the use of high-performance liquid chromatography tandem mass spectrometry (MS/MS). A total of 10 μL was injected on the high-performance liquid chromatography-MS/MS system. MS/MS analyses were performed on a TSQ Quantum AM (Thermo Finnigan) operated in the positive ion electrospray ionization mode. The parent ion scan of mass-to-charge ratio (m/z) 184.1 (m/z 400-1000, 1 second) was used for the quantization of the following precursor ions: m/z 468.3 (LysoPC 14:0, internal standard), m/z 496.3 (LysoPC 16:0), m/z 524.3 (LysoPC 18:0/PAF 16:0), m/z 522.4 (LysoPC 18:1), m/z 482.4 (LysoPAF 16:0), m/z 51.4 (LysoPAF 18:0), m/z 508.4 (LysoPAF 18:1), m/z 678.4 (PC 28:0, internal standard), m/z 758.4 (PC 34:2), and m/z 782.4 (PC 36:2).
Neutrophil priming assay
Rat neutrophils were isolated with the use of Percoll 1.076 g/mL gradient centrifugation and NH4Cl/KHCO3 lysis of red blood cells.25 Neutrophils (1.0 × 106/mL) were incubated with supernatant for 30 minutes at 37°C and thereafter activated with formyl-methionyl-leucylphenylalanine. H2O2 release was measured with the fluorescent dye Amplex Red in the presence of horseradish peroxidase.
Storage-related biochemical changes in human PLTs
Platelet concentrates in plasma were prepared from pooled buffy coat platelets as previous described.26 The concentrates were filtered through a Compostop CS leukoreduction filter (Fresenius Kabi, type T3995) with a PVC-citrate container connected to the outlet of the filter. The PLTs were stored at 22°C (± 2°C) with horizontal shaking at 1 cycle per minute (Helmer Labs Inc), according to National Blood Bank standards. Supernatants were collected after preparation of the products and at day 5 and 7 and prepared by centrifugation for 10 minutes at 14 500g at 4°C to remove cells and acellular debris. Aliquots of supernatants were stored at −80°C for analysis of LysoPC and PC levels.
Statistical analyses
Data are expressed as mean plus or minus SEM or median with interquartile ranges, as appropriate. A paired t test was used to compare the results of platelets before and after storage. Comparisons between the rat groups were performed by the use of 1-way analysis of variance or Kruskal-Wallis test, followed by post-hoc Bonferroni or Dunnett tests, depending on data distribution. A P value less than .05 was considered statistically significant. Statistical analyses were performed with SPSS 12.0 (SPSS) and Prism 4.0 (GraphPad Software).
Results
Effect of transfusion of aged PLTs in healthy rats
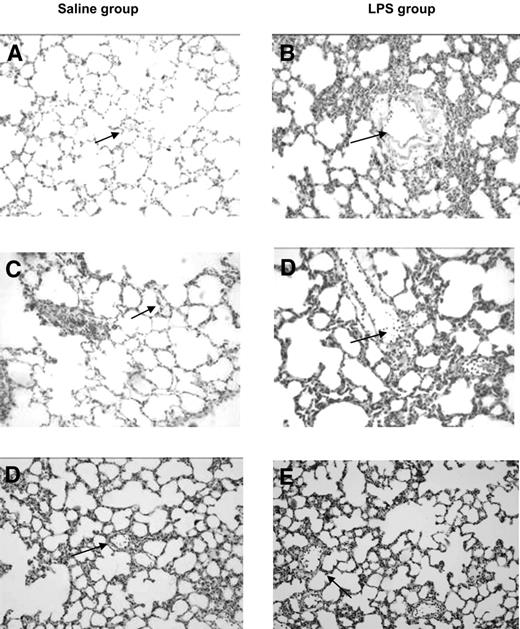
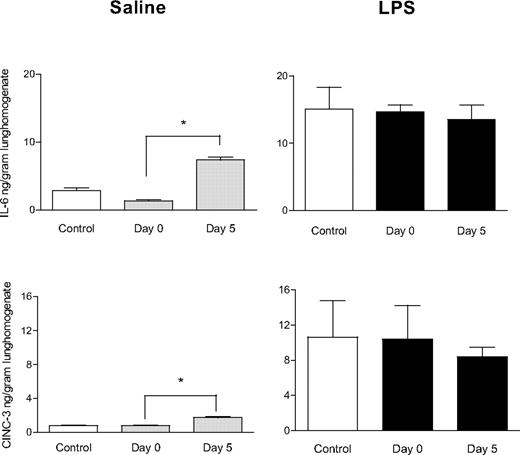
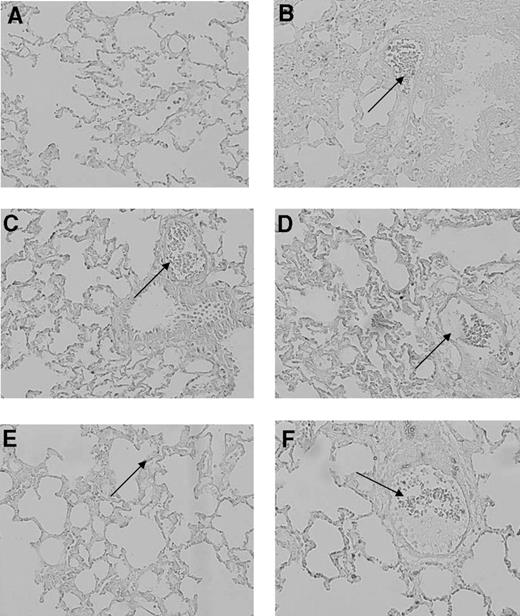
All animals completed the experimental protocol. Blood cultures from the rats collected at the end of the experimental protocol showed no outgrowth of bacteria. Transfusion of aged PLTs induced lung injury in healthy rats, characterized by neutrophil adherence on the endothelial wall and edema in lung tissue (Figure 1). The histopathology score was greater compared with transfusion of fresh PLTs and saline control groups (1.6 ± 1.1 vs 1.7 ± 1.6 and 2.2 ± 2.2, P < .05). Aged PLTs caused an increase of IL-6 and CINC-3 concentrations in the lung homogenate (P < .05 compared with control rats, Figure 2). No differences were seen between fresh or aged PLTs on markers of coagulation and fibrinolysis in neither the pulmonary (Figure 3) nor the systemic compartment (data not shown). Because the accumulation of soluble CD40 during storage of PLTs has been implicated in the pathogenesis of TRALI,27 CD40L was stained in the lung tissue by the use of immunohistochemistry. After the transfusion of PLTs, expression of CD40L was detected on pulmonary T cells and on the endovascular wall, but no differences were observed between fresh versus aged PLTs (Figure 4).
Effect of transfusion of aged platelets on lung injury in healthy and primed rats. Histologic sections of hematoxylin and eosin stained rat lungs at 20× magnification. (A) Saline control; (B) LPS control; (C) saline plus fresh PLTs day 0; (D) LPS plus PLTs day 0; (E) saline plus aged PLTs (PLTs day 5); (F) LPS plus PLTs day 5. Normal vasculature (arrows, panels A and C) neutrophils sequestrated in the vasculature (arrows, panels B,D,F).
Effect of transfusion of aged platelets on lung injury in healthy and primed rats. Histologic sections of hematoxylin and eosin stained rat lungs at 20× magnification. (A) Saline control; (B) LPS control; (C) saline plus fresh PLTs day 0; (D) LPS plus PLTs day 0; (E) saline plus aged PLTs (PLTs day 5); (F) LPS plus PLTs day 5. Normal vasculature (arrows, panels A and C) neutrophils sequestrated in the vasculature (arrows, panels B,D,F).
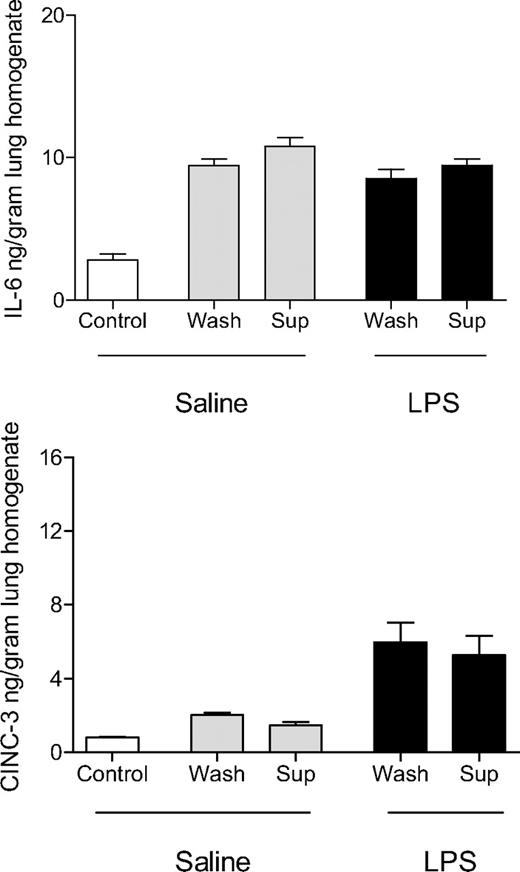
Effect of aged platelets transfusion on cytokine (IL-6) and chemokine (CINC-3) concentration in the lungs. Saline or LPS-pretreated animals were transfused with saline (control), fresh PLTs (day 0), or aged PLTs (day 5). Data are presented as mean ± SEM. *P < .05.
Effect of aged platelets transfusion on cytokine (IL-6) and chemokine (CINC-3) concentration in the lungs. Saline or LPS-pretreated animals were transfused with saline (control), fresh PLTs (day 0), or aged PLTs (day 5). Data are presented as mean ± SEM. *P < .05.
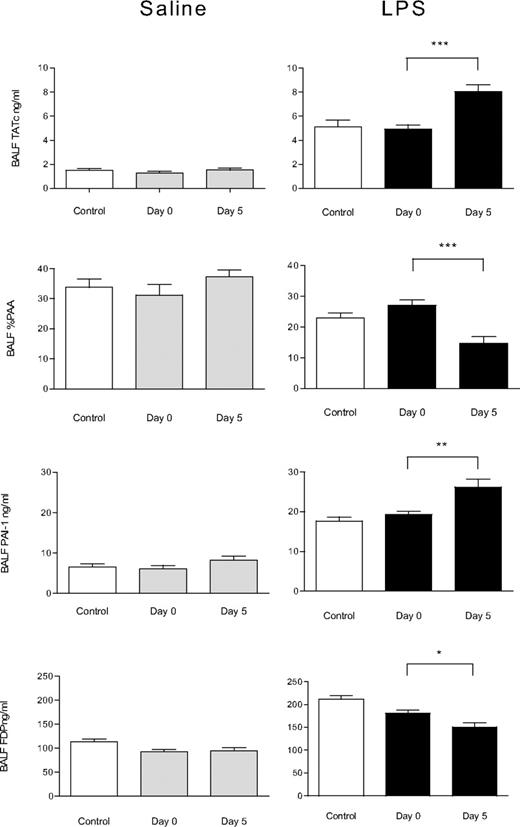
Effect of aged platelets transfusion on pulmonary coagulation and fibrinolysis. Concentrations of TATc, PAA, PAI-1, and FDP in the BALF of animals receiving saline or LPS as “first hit” and subsequently infusion of saline (control), fresh PLTs (day 0), or aged PLTs (day 5). Data are presented as mean ± SEM. Aged PLTs products activate lung coagulation and decrease fibrinolysis in LPS-primed rats as shown by an increase in TATc level in the BALF and decrease of PAA% and increase of PAI-1 levels in the BALF, respectively. *P < .05, **P < .01, ***P < .001.
Effect of aged platelets transfusion on pulmonary coagulation and fibrinolysis. Concentrations of TATc, PAA, PAI-1, and FDP in the BALF of animals receiving saline or LPS as “first hit” and subsequently infusion of saline (control), fresh PLTs (day 0), or aged PLTs (day 5). Data are presented as mean ± SEM. Aged PLTs products activate lung coagulation and decrease fibrinolysis in LPS-primed rats as shown by an increase in TATc level in the BALF and decrease of PAA% and increase of PAI-1 levels in the BALF, respectively. *P < .05, **P < .01, ***P < .001.
Effect of transfusion of aged platelets on CD40L expression in lung tissue. Histologic sections of CD40L stained rat lungs at 20× magnification. (A) saline control; (B) LPS control; (C) saline plus fresh PLTs day 0; (D) LPS plus PLTs day 0; (E) saline plus aged PLTs (PLTs day 5); (F) LPS plus PLTs day 5. CD40L is indicated by brown staining. In all sections, except for the saline-negative control, CD40L staining is present on pulmonary T cells and on the endovascular wall (arrows).
Effect of transfusion of aged platelets on CD40L expression in lung tissue. Histologic sections of CD40L stained rat lungs at 20× magnification. (A) saline control; (B) LPS control; (C) saline plus fresh PLTs day 0; (D) LPS plus PLTs day 0; (E) saline plus aged PLTs (PLTs day 5); (F) LPS plus PLTs day 5. CD40L is indicated by brown staining. In all sections, except for the saline-negative control, CD40L staining is present on pulmonary T cells and on the endovascular wall (arrows).
Effect of transfusion of aged PLTs in LPS-primed rats
LPS pretreatment resulted in neutrophil sequestration in the lung endothelium and pulmonary edema, with an elevated histopathology score compared with the saline control group (11.8 ± 1.1 vs 1.7 ± 1.6, P < .001). In addition, LPS pretreatment increased levels of IL-6 and CINC-3 in lung homogenate compared with the saline control (P < .01, Figure 2). LPS increased pulmonary coagulation as shown by increased thrombin generation (as reflected by TATc) and increased FDP levels. In addition, fibrinolysis was impaired, as evidenced by reduced PAA levels, at least in part caused by an increase in the levels of the fibrinolytic inhibitor PAI-1 compared with saline controls (P < .01, Figure 3). LPS-pretreatment also induced systemic coagulopathy, evidenced by increased plasma TATc levels compared with saline controls (1.5 ± 1.0 ng/mL vs 3.4 ± 0.3 ng/mL, P < .01).
Transfusion of aged PLTs in LPS-primed animals had a differential effect on lung injury. Histopathology score of LPS-primed rats did not worsen after transfusion of aged PLTs compared with LPS-primed controls transfused with saline or fresh PLTs (9.3 ± 2.0 vs 11.8 ± 1.1 and 1.6 ± 1.0, respectively, P = .7, Figure 1). Pulmonary wet weight was not increased (not shown). No differences were seen for CD40L staining in the lungs between LPS-pretreated animals transfused with aged PLTs compared with animals receiving fresh PLTs (Figure 4). Aged PLTs did not increase pulmonary cytokine and chemokine levels in the “2-hit” model (Figure 2). However, aged PLTs had a strong effect on pulmonary coagulopathy in LPS-primed animals, increasing bronchoalveolar lavage fluid (BALF) levels of TATc compared with fresh PLTs (P < .001, Figure 3). Aged PLTs contributed to impaired fibrinolysis in LPS-primed animals, decreasing PAA in the BALF and increasing the level of PAI-1 compared with animals receiving fresh PLTs (P < .001 and P < .01, respectively). FDP levels were reduced in LPS-primed animals receiving aged PLTs compared with animals receiving fresh PLTs (P < .05). In addition, aged PLTs caused systemic coagulation by increasing plasma TATc level compared with fresh PLTs (20.2 ± 1.3 vs 10.9 ± 0.6, P < .001).
Effect of transfusion of washed aged platelets versus the supernatant of aged PLTs in healthy and LPS-primed rats
To determine whether lung injury caused by aged PLTs was mediated by soluble factors in the storage medium or by the platelets themselves, aged PLTs were washed and separated from supernatant. By using these products, we repeated transfusion experiments in healthy and LPS-pretreated animals. In healthy rats, no difference was observed on lung inflammation or coagulation between animals transfused with washed aged PLTs or with supernatant of aged PLTs (Figures 5–6).
Effect of washing of aged platelets before transfusion on cytokine and chemokine concentrations in the lungs. Concentrations of IL-6 and CINC-3 in the lung homogenate of animals receiving saline or LPS as first hit and subsequently infusion of saline (control), supernatant (sup) of aged PLT products, or washed (wash) aged platelets. Data are presented as mean ± SEM. No differences are seen between supernatant of aged PLT products and washed aged platelets on lung inflammation in both saline and LPS-pretreated animals.
Effect of washing of aged platelets before transfusion on cytokine and chemokine concentrations in the lungs. Concentrations of IL-6 and CINC-3 in the lung homogenate of animals receiving saline or LPS as first hit and subsequently infusion of saline (control), supernatant (sup) of aged PLT products, or washed (wash) aged platelets. Data are presented as mean ± SEM. No differences are seen between supernatant of aged PLT products and washed aged platelets on lung inflammation in both saline and LPS-pretreated animals.
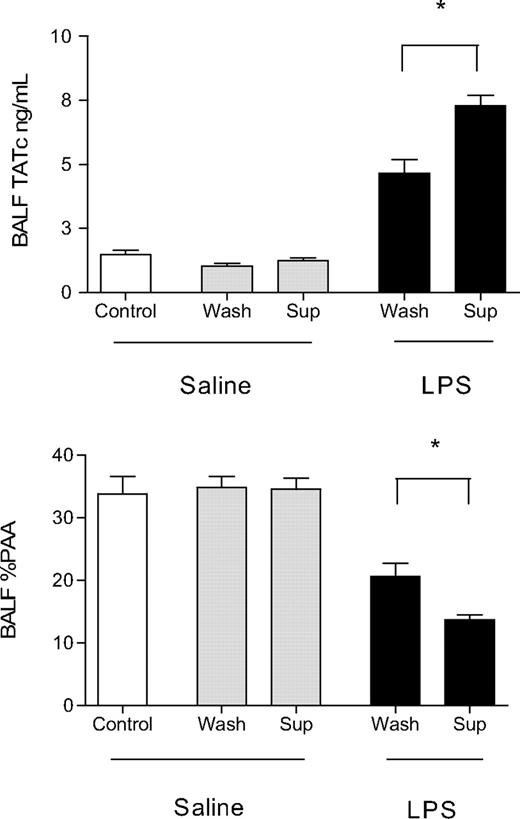
Effect of washing of aged platelets before transfusion on coagulation and fibrinolysis in the lungs. Concentrations of TATc and PAA in the BALF of animals receiving saline or LPS as first hit and subsequently infusion of saline (control), supernatant (sup) of aged PLT products, or washed (wash) aged platelets. Data are presented as mean ± SEM. Supernatant of aged PLT products and not washed aged platelets increased lung coagulation and decreased fibrinolysis in LPS-primed animals as shown by an increase in TATc and decrease in PAA% in the BALF. *P < .01.
Effect of washing of aged platelets before transfusion on coagulation and fibrinolysis in the lungs. Concentrations of TATc and PAA in the BALF of animals receiving saline or LPS as first hit and subsequently infusion of saline (control), supernatant (sup) of aged PLT products, or washed (wash) aged platelets. Data are presented as mean ± SEM. Supernatant of aged PLT products and not washed aged platelets increased lung coagulation and decreased fibrinolysis in LPS-primed animals as shown by an increase in TATc and decrease in PAA% in the BALF. *P < .01.
In contrast, in LPS-primed rats, transfusion of supernatant, but not of washed aged PLTs, worsened lung coagulopathy. Supernatant of aged PLTs caused an increase in levels of TATc and decrease in %PAA in BALF compared with those receiving washed aged PLTs (P < .01 for both, Figure 6). In addition, the increase in systemic levels of TATc caused by PLT products was reproduced after transfusion of supernatant but not after transfusion of washed aged platelets (18.2 ± 0.8 vs 11.6 ± 0.8, P < .001). No effect was seen on markers of inflammation between animals receiving transfusion of supernatant or aged washed PLTs (Figure 5).
Effect of storage time of rat PLTs on biochemical changes
Platelet count was stable during storage. At day 0, morphology and PS exposure were similar to that of human platelets. In addition, aggregation response to ADP and to the combination of ADP and collagen was normal. At day 5 of storage, morphology changed, and annexin V expression of rat platelets increased (Table 1). In addition, rat PLTs showed significant biochemical changes, exemplified by an increase in lactate levels and a decrease in pH compared with day 0 (P < .01 for all, Table 1). These storage lesions are comparable with that of human platelets stored for 7 days.24,26,28 The phenomenon of more rapid worsening of rat cells during storage was previously described for erythrocytes.29 The presence of platelet aggregates was judged by visual observation and by a hematologic cell counter. No aggregates were present until day 7 of storage for rat platelets. Concentrations of LysoPC 16:0, LysoPC 18:0/PAF 16:0, and LysoPAF 18:0 were significantly increased after 5 days of storage compared with day 0, with a concomitant decrease in PC concentrations (P < .05 for all, Table 1). The concentration of LysoPC 18:1, LysoPAF 16:0, and LysoPAF 18:1 showed a nonsignificant increase of, respectively, 5%, 8%, and 30% at day 5 storage compared with day 0 storage. IL-6 and tumor necrosis factor were not detectable in the supernatant of PLTs at either time point.
Storage-related biochemical changes in rat platelet concentrates
| . | Whole blood (fresh) . | Platelets . | |
|---|---|---|---|
| Day 0 . | Day 5 . | ||
| pH | 6.9 ± 0.1 | 7.4 ± 0.1 | 6.9 ± 0.1* |
| Lactate, mmol/L | 6.2 ± 1.2 | 8.0 ± 0.3 | 21.5 ± 1.2* |
| Leukocytes, ×109/L | 5.4 ± 0.6 | 0.03 ± 0.01 | 0.02 ± 0.01 |
| Platelets, ×109/L | 858 ± 48 | 1086 ± 65 | 1076 ± 76 |
| Morphology, Kunicki score | N/A | 270 ± 8.7 | 192 ± 20.2† |
| Percentage positive for annexin V | N/A | 4.3 ± 0.6 | 42 ± 1.5* |
| LysoPC 16:0, μM | 164 ± 13 | 183 ± 17† | |
| LysoPC 18:1, μM | 41 ± 4.3 | 43 ± 1.6 | |
| LysoPAF 16:0, μM | 4.5 ± 0.4 | 4.9 ± 0.1 | |
| LysoPAF 18:0, μM | 5.1 ± 0.5 | 6.2 ± 0.4† | |
| LysoPAF 18:1, μM | 2.0 ± 0.4 | 2.6 ± 0.4 | |
| LysoPC 18:0/PAF 16:0, μM | 5.1 ± 0.5 | 6.2 ± 0.4† | |
| PC 34:2, μM | 86 ± 11 | 46 ± 4† | |
| PC 36:0, μM | 23 ± 3.3 | 13 ± 0.9† | |
| TNF, pg/mL | < 62.5 | < 62.5 | |
| IL-6, pg/mL | < 31.25 | < 31.25 | |
| . | Whole blood (fresh) . | Platelets . | |
|---|---|---|---|
| Day 0 . | Day 5 . | ||
| pH | 6.9 ± 0.1 | 7.4 ± 0.1 | 6.9 ± 0.1* |
| Lactate, mmol/L | 6.2 ± 1.2 | 8.0 ± 0.3 | 21.5 ± 1.2* |
| Leukocytes, ×109/L | 5.4 ± 0.6 | 0.03 ± 0.01 | 0.02 ± 0.01 |
| Platelets, ×109/L | 858 ± 48 | 1086 ± 65 | 1076 ± 76 |
| Morphology, Kunicki score | N/A | 270 ± 8.7 | 192 ± 20.2† |
| Percentage positive for annexin V | N/A | 4.3 ± 0.6 | 42 ± 1.5* |
| LysoPC 16:0, μM | 164 ± 13 | 183 ± 17† | |
| LysoPC 18:1, μM | 41 ± 4.3 | 43 ± 1.6 | |
| LysoPAF 16:0, μM | 4.5 ± 0.4 | 4.9 ± 0.1 | |
| LysoPAF 18:0, μM | 5.1 ± 0.5 | 6.2 ± 0.4† | |
| LysoPAF 18:1, μM | 2.0 ± 0.4 | 2.6 ± 0.4 | |
| LysoPC 18:0/PAF 16:0, μM | 5.1 ± 0.5 | 6.2 ± 0.4† | |
| PC 34:2, μM | 86 ± 11 | 46 ± 4† | |
| PC 36:0, μM | 23 ± 3.3 | 13 ± 0.9† | |
| TNF, pg/mL | < 62.5 | < 62.5 | |
| IL-6, pg/mL | < 31.25 | < 31.25 | |
Data are presented as mean ± SD (n = 5 batches).
IL-6 indicates interleukin-6; LysoPC, lysophosphatidylcholine; N/A, not available; PAF, platelet-activating factor; PC, phosphatidylcholine; and TNF, tumor necrosis factor.
P < .01.
P < .05.
Effect of storage time of rat PLTs on neutrophil priming capacity
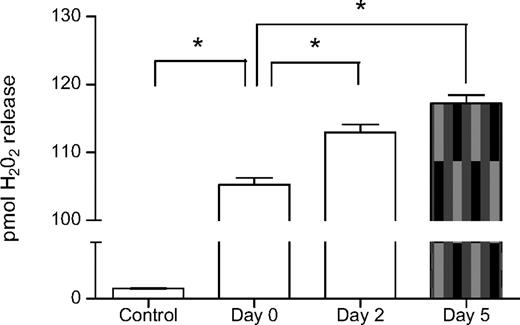
Supernatant of fresh PLTs had significant neutrophil priming capacity compared with buffer control (H2O2 release 105.2 ± 3.0 pmol/mL vs 18.0 ± 1.0 pmol/mL, P < .01, Figure 7). Storage further enhanced neutrophil priming capacity because the supernatant of PLTs stored for 2 and 5 days had greater in vitro neutrophil priming capacity compared with the supernatant of fresh PLTs (H2O2 release 117.2 ± 3.0 pmol/mL and 113.0 ± 3.0 pmol/mL vs 105.2 ± 3.0 pmol/mL, P < .01, Figure 7).
Supernatant of aged PLTs have increased neutrophil priming capacity. Neutrophil priming activity of the aged PLTs expressed in pmol H2O2 release per minute per 106 neutrophils. Supernatant of fresh PLTs (Day 0) showed an increased in vitro neutrophil priming capacity compared with buffer controls. Supernatant of aged PLTs stored for 2 days and 5 days showed an increased in vitro neutrophil priming capacity compared with supernatant of fresh PLTs. *P < .01. Data are presented as mean ± SEM.
Supernatant of aged PLTs have increased neutrophil priming capacity. Neutrophil priming activity of the aged PLTs expressed in pmol H2O2 release per minute per 106 neutrophils. Supernatant of fresh PLTs (Day 0) showed an increased in vitro neutrophil priming capacity compared with buffer controls. Supernatant of aged PLTs stored for 2 days and 5 days showed an increased in vitro neutrophil priming capacity compared with supernatant of fresh PLTs. *P < .01. Data are presented as mean ± SEM.
Effect of storage time of human PLTs on LysoPC accumulation
In contrast to previous findings,8,9 LysoPC accumulation in aged rat PLTs products was modest in this study. Therefore, additional studies in which the authors used human PLTs were performed. Comparable with our results with the rat PLTs products, concentrations of LysoPCs increased in human PLT products stored for 5 and 7 days compared with fresh PLTs (Table 2). Concentrations of LysoPC 16:0 were already increased after 5 days of storage compared with fresh concentrations, and LysoPC 18:1, LysoPAF 16:0, LysoPAF 18:1, LysoPC 18:0/PAF 16:0, and LysoPAF 18:0 were increased after 7 days of storage compared with fresh concentrations with a concomitant decrease in PC concentrations (P < .05 for all, Table 2). In line with the rat PLTs product, the maximum increase in LysoPCs was approximately 30% compared with baseline.
Storage-related changes in lysophophatidylcholines in human platelet concentrates
| . | Platelets . | ||
|---|---|---|---|
| Fresh . | Day 5 . | Day 7 . | |
| LysoPC 16:0, μM | 141 ± 10.7 | 169 ± 13.4* | 192 ± 20.8* |
| LysoPC 18:1, μM | 32 ± 4.6 | 34 ± 2.8 | 36 ± 2.8† |
| LysoPAF 16:0, μM | 3.2 ± 0.7 | 3.5 ± 0.5 | 4.1 ± 0.5† |
| LysoPAF 18:0, μM | 4.5 ± 0.7 | 5.2 ± 0.6 | 6.0 ± 1.3* |
| LysoPAF 18:1, μM | 1.7 ± 0.3 | 1.7 ± 0.3 | 2.3 ± 0.4* |
| LysoPC 18:0/PAF 16:0, μM | 4.5 ± 0.3 | 5.2 ± 0.3 | 6.0 ± 0.6* |
| PC 34:2, μM | 122 ± 13.2 | 115 ± 11.9 | 105 ± 10.0† |
| PC 36:4, μM | 42 ± 2.9 | 41 ± 2.1 | 39 ± 1.4* |
| . | Platelets . | ||
|---|---|---|---|
| Fresh . | Day 5 . | Day 7 . | |
| LysoPC 16:0, μM | 141 ± 10.7 | 169 ± 13.4* | 192 ± 20.8* |
| LysoPC 18:1, μM | 32 ± 4.6 | 34 ± 2.8 | 36 ± 2.8† |
| LysoPAF 16:0, μM | 3.2 ± 0.7 | 3.5 ± 0.5 | 4.1 ± 0.5† |
| LysoPAF 18:0, μM | 4.5 ± 0.7 | 5.2 ± 0.6 | 6.0 ± 1.3* |
| LysoPAF 18:1, μM | 1.7 ± 0.3 | 1.7 ± 0.3 | 2.3 ± 0.4* |
| LysoPC 18:0/PAF 16:0, μM | 4.5 ± 0.3 | 5.2 ± 0.3 | 6.0 ± 0.6* |
| PC 34:2, μM | 122 ± 13.2 | 115 ± 11.9 | 105 ± 10.0† |
| PC 36:4, μM | 42 ± 2.9 | 41 ± 2.1 | 39 ± 1.4* |
Data are presented as mean ± SD (n = 5 batches).
LysoPC indicates lysophosphatidylcholine; PAF, platelet-activating factor; and PC, phosphatidylcholine.
P < .01.
P < .05.
Discussion
We describe a novel in vivo syngeneic platelet transfusion model that uses clinical protocols for the preparation and storage of blood products. We demonstrate for the first time that transfusion of aged PLTs cause mild pulmonary inflammation in healthy recipients. In the “2-hit” transfusion model, aged PLTs augmented pulmonary and systemic coagulopathy, an effect that was mediated by supernatant of aged PLTs but not by washed aged PLTs. Aged platelet products had increased levels of LysoPCs, with a concomitant ability to prime neutrophils. Therefore, lysoPCs accumulating in supernatant during storage of PLTs may possibly mediate TRALI in predisposed patients.
Pulmonary injury induced by aged platelets was characterized by enhanced inflammation, as shown by extravasation of neutrophils in lung tissue, a greater total pathophysiology score, and an increase in IL-6 and CINC-3 in lung homogenate. These findings are in line with ex vivo animal studies in which the authors show pulmonary edema after transfusion of aged blood without previous priming of the animals.30-32 However, we stress that the effect of aged PLTs on lung injury was mild in this study and that pulmonary edema as a measure of pulmonary leak was absent. The results of this study need to be interpreted within the limits of the model we used. We hypothesize that aged PLTs cause inflammatory processes, which have the potential to disrupt endothelial and epithelial barriers, eventually leading to fluid accumulation within alveoli, if the pulmonary “hit” is severe enough. Because storage time and amount of PLTs transfused were found to be risk factors for the occurrence of ALI in the clinical setting,7,13 it may be hypothesized that repeated PLTs transfusion results in significant pulmonary injury. In patient populations that had experienced a “first hit” of trauma or cardiac surgery, transfusion of stored blood was associated with the occurrence of ALI. We speculate that mild lung injury caused by aged platelets may be augmented by subsequent “hits.”33-35
The authors of previous studies have pointed toward a “2-hit” TRALI hypothesis. However, TRALI models that were used were limited by ex vivo designs, use of blood products that were not manufactured according to clinical protocols, and by the use of cross species, including human blood products that were transfused in rat recipients.9,36,37 In our syngeneic in vivo transfusion model, we confirm the “2-hit” TRALI hypothesis with the use of aged PLTs.8,9,38 Our study extends previous findings, showing for the first time that aged PLTs enhance coagulation and impair fibrinolysis in the presence of primed neutrophils. Activation of coagulation and impairment of fibrinolysis have been found in ALI/acute respiratory distress syndrome as the result of many causes, including sterile inflammation as well as infection.17,21,39-41 From this point of view, TRALI may be regarded as part of the ALI/acute respiratory distress syndrome and not as a separate entity, as suggested previously.5-7 In line with this, we recently found that TRALI contributes to mortality in critically ill patients,7 suggesting that prognosis of TRALI is not as favorable as generally considered but rather may parallel ALI as the result of other causes, at least in certain patient populations.5-7
Of interest, in the “2-event” model, worsening of coagulopathy occurred in the absence of worsening of markers of inflammation, pulmonary edema, or histopathology score. In the LPS-primed animals, extravasation of neutrophils was already elicited. Transfusion of aged platelets may have resulted in activation of these neutrophils, contributing to coagulopathy. Indeed, increased expression of P-selectin (CD62p) on the cytoplasmic surface of platelets occurs during storage, which results in binding of the aged platelets to neutrophils and monocytes after transfusion.42 Subsequently, this may directly lead to activation of coagulation. Furthermore, the endothelial response to neutrophils not only includes mediation of trafficking43 but also the release of procoagulant factors. Proinflammatory cytokines stimulate expression of endothelial tissue factor, thereby eliciting the extrinsic coagulation pathway, leading to activation of coagulation.44 In accordance, a procoagulant state has been shown in patients with ALI.45 We speculate that coagulopathy may be an important mediator of injury in TRALI. The interaction between coagulopathy and neutrophil-mediated inflammation has been described in other causes of acute lung injury.46 Such interactions may also exist in TRALI. Alternatively, lung inflammation as the result of the “first hit” may have been too severe to discriminate between the 2 hits.
The effect on coagulopathy occurred after infusion with supernatant of the aged PLTs but not when washed aged PLTs were used. Of interest, plasma-stored platelets cause more transfusion complications compared with platelets stored in additive solution II.47 Further studies are required to determine whether replacement of plasma as a storage medium for PLTs or washing of aged PLTs before transfusion should be implemented.
Bioactive lipids that accumulate in the supernatant of blood products have been implicated in TRALI. We found an increase in LysoPCs and a decrease of LysoPC precursors during storage of rat platelets, as well as in aged human platelet products, which were able to prime neutrophils in vitro. These data are in line with the finding that the concentration of bioactive lipids increases during blood storage,8,9 and the finding that plasma-derived purified LysoPCs have neutrophil priming capacity.8,9,36 In addition, the authors of clinical studies show an association between the concentration of lysoPCs in the transfused products and the onset of TRALI.5,13,38 Our data suggest that bioactive lipids that have accumulated during storage of PLTs may have mediated TRALI by priming of neutrophils. Of note, fresh PLTs already showed increased neutrophil priming capacity compared with buffer control. However, fresh PLTs did not result in lung injury in our transfusion model. This finding may suggest that a threshold amount of LysoPCs may be needed before transfusion results in neutrophil priming. However, it should be noted that besides lysoPCs, other mediators that are released during platelet storage but were not measured in this study, may have contributed to the inflammatory process.
Recently, it has been suggested that sCD40L accumulating during PLT storage may be involved in the pathogenesis of TRALI.27 Interestingly, CD40L was present in pulmonary tissue after transfusion, which seemed to be expressed by both T cells, as determined by CD3 staining, and cells lining the endothelial vascular wall. In line with these findings, it has been hypothesized that sCD40L from aged PLTs ligates CD40 on primed neutrophils, resulting in endothelial damage.27 Because no differences were found between fresh and aged platelets, we could not confirm the role of CD40L in the onset of the lung injury in our model. However, we only measured cell-associated CD40L and we cannot rule out that sCD40L may have played a role. Unfortunately, we were not able to determine the concentration of sCD40L in the aged PLTs, as no assay exists to determine sCD40L in rat plasma.
Alternatively, cytokines that accumulate during storage of cell containing blood products may cause TRALI.48 High levels of IL-8 have been measured in platelet concentrates implicated in TRALI reactions.13 However, a clinical study on TRALI could not confirm an association between high IL-8 levels and the occurrence of TRALI.5 We did not find elevated levels of cytokines in aged rat platelets. Of importance, it should be noted that products used in this study were leukocyte-poor before storage by our preparation method. Finally, biochemical deterioration of the blood products, such as increase in lactate and potassium and decrease in pH, may have contributed to the observed lung injury.
An important issue is how the results from our study should be implemented in the pathogenesis of TRALI. Our results support the hypothesis that transfusion can cause lung injury in the absence of antibodies in the blood product. In accordance, the use of male-only plasma has reduced, but not prevented the occurrence of TRALI.49 Another interesting finding is that aged platelets are able to induce lung injury in the absence of a “first hit,“ thus implying that TRALI in the absence of antibodies is not necessarily a “2-hit” event. However, because PLTs also were found to augment lung injury in a “2-hit” model, it may be suggested that PLTs transfusion may contribute to morbidity in patients with primed neutrophils, ie, with an underlying inflammatory condition.5,7,50 Another issue is whether our results have implications for manufacturing processes and storage time of PLT products. It may not be feasible to avoid transfusion of platelets that do not cause neutrophil priming, as in vitro neutrophil priming occurred already after 2 days of storage. Because washing of the aged PLTs abrogated the detrimental effect, washing of the aged PLTs as a manufacturing process may reduce pulmonary complications. However, although of theoretical benefit, recommendations for washing of platelets in the clinical setting are premature for several reasons: the process is logistically complex and expensive, the platelet count (and hence the therapeutic efficacy) of a washed platelet product is reduced, the availability of a platelet product in emergent situations would be delayed, it is unknown at what age human platelets would acquire a (theoretical) incremental risk of causing TRALI, and no clinical data exist to support the efficacy of this policy in reducing TRALI.
In conclusion, we show that aged platelets cause lung injury, in which pulmonary coagulopathy is prominent, in a “2-hit” in vivo transfusion model, which may be caused by LysoPCs that have accumulated in the aged platelet products.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We thank Richard Vlaar (Sanquin Research, Department of Blood Cell Research) for assisting with the production of the rat blood components.
Authorship
Contribution: A.P.J.V., D.d.K., and N.P.J. designed the research; A.P.J.V., J.J.H., H.v.L., M.L., J.J.T.H.R., and A.T.J.T. performed the research; and A.P.J.V., J.J.H., W.K., H.v.L., R.N., M.J.S., M.L., J.J.T.H.R., A.T.J.T., D.d.K., and N.P.J. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander P. J. Vlaar, Laboratory of Experimental Intensive Care and Anesthesiology (L.E.I.C.A.), Academic Medical Center, Room M0-228, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: a.p.vlaar@amc.uva.nl.