Abstract
Recent in vitro studies have shown that shear stress can cause platelet activation by agonist-independent pathways. However, no studies have assessed the extent of shear-induced platelet activation within human coronary arteries. We sampled blood from the coronary arteries proximal and distal to coronary lesions and from the coronary sinus in humans with stable coronary disease who were taking both aspirin and clopidogrel. A novel, computationally based technique for estimating shear stress from 3-dimensional coronary angiographic images of these arteries was developed, and the effect of stenosis severity and calculated shear stress on in vivo platelet and related leukocyte activation pathways were determined. We provide evidence of intracoronary up-regulation of platelet P-selectin, platelet-monocyte aggregation, and monocyte CD11b without platelet glycoprotein IIb-IIIa activation or soluble P-selectin up-regulation. This correlates with intracoronary stenosis severity and calculated shear stress and occurs despite the concurrent use of aspirin and clopidogrel. Our results show for the first time shear-related platelet and monocyte activation in human coronary arteries and suggest this as a potential therapeutic target that is resistant to conventional antiplatelet agents.
Introduction
Platelet activation and aggregation are critical to the pathogenesis of atherothrombosis.1,2 Platelets are also known to play a main role in inflammation, partly by the interaction between platelets and leukocytes,2-4 and it is known that shear stress can cause platelet activation,5-12 platelet-leukocyte aggregation,8,9 and leukocyte activation.8,9 Procedures such as coronary angioplasty can alleviate focal coronary stenosis. However, many patients with diffuse, severe coronary disease are unsuitable for such treatment and may therefore be exposed to long-term platelet and leukocyte activation secondary to increased intracoronary shear stress.
Although in vitro and animal studies have shown shear stress–induced platelet activation and aggregation, a direct relationship between shear stress and in situ platelet activation has not been shown within human coronary arteries.13 Previous investigators have found evidence of transcardiac platelet activation with increased P-selectin expression as platelets travel from the aortic root to the coronary sinus (CS), the main vein draining blood from the heart in patients with coronary disease.14 However, it is not known whether platelets are indeed activated as they cross coronary lesions, and no studies to date have investigated the direct effect of stenosis severity and shear stress on in situ platelet activation within human coronary arteries.
Current antiplatelet agents such as aspirin, thienopyridines, and glycoprotein (GP) IIb-IIIa inhibitors attenuate agonist-induced platelet activation,15-17 and GPIIb-IIIa inhibitors reduce shear-induced platelet aggregation.10,18,19 However, shear stress overcomes aspirin inhibition of platelet aggregation.20 Further, GPIIb-IIIa blockade fails to inhibit shear-induced platelet-leukocyte aggregation8,21 and leukocyte activation.8
We have investigated the relationship between shear stress and in situ platelet activation, platelet-leukocyte aggregation, and leukocyte activation by sampling within coronary arteries of patients with stable coronary disease being treated with both aspirin and clopidogrel. We have calculated the flow field parameters with the use of a computational fluid dynamics (CFD) package and have used the CFD results to estimate focal coronary artery shear stress. We report for the first time direct evidence of up-regulation of platelet P-selectin (CD62P), platelet-monocyte aggregate (PM-Agg) formation, and monocyte CD11b expression in human coronaries, in proportion to shear stress and stenosis severity.
Methods
Reagents
Preconjugated fluorescent antibodies were from BD Biosciences (CD42b-phycoerythrin [PE], CD62P-PE, CD45–peridinin chlorophyll protein complex, immunoglobulin G1 [IgG1]–PE, IgM–fluorescein isothiocyanate [FITC], PAC-1–FITC, IgG1-FITC, CD42b-FITC) Becton Dickinson, and Dako (CD11b-PE). Dulbeccomodified phosphate-buffered saline (DPBS) and Hanks buffered salt solution (10×) were from Invitrogen. Paraformaldehyde 16% solution was from ProSciTech. Nonpyrogenic water and 0.9% sodium chloride were from Baxter. P-selectin blocking antibody (9E1) was from R&D Systems. Indomethacin, P2Y1 (MRS2179), and P2Y12 (2-MeSAMP) inhibitors were from Sigma-Aldrich.
Study population
The study included 20 patients with stable angina who were electively scheduled for percutaneous coronary intervention (PCI). All patients had single target lesions of 40%-70% diameter stenosis (DS) (mean ± SD = 53.3% ± 9.3%) within 1 of the 3 main epicardial coronary arteries (left anterior descending, n = 13; left circumflex, n = 4; or right coronary artery, n = 3). Patients with > 1 lesion > 20% DS within the target vessel were excluded from the study. All patients were on a regimen of aspirin 100-150 mg daily and clopidogrel 75 mg daily for ≥ 3 days before PCI. Patients were excluded if they had significant renal impairment (estimated glomerular filtration rate < 60 mL/min), were on other antiplatelet agents or anticoagulants, or had other significant inflammatory disorders such as rheumatoid arthritis necessitating steroid or immunosuppressant usage. Informed written consent was obtained from all patients in accordance with the Declaration of Helsinki for this study, which was approved by our institutional human research ethics committee.
Blood sampling procedure and sequence
Patients had an overnight fast without discontinuation of their usual cardiac medications. All patients were allowed to rest in bed for ≥ 10 minutes before insertion of a 20-gauge intravenous cannula in the cubital fossa. Blood was collected from the peripheral vein with the tourniquet removed.
A 6F femoral arterial sheath and a 6F femoral venous sheath were inserted. Patients underwent standard coronary angiography with ≥ 2 nonoverlapped orthogonal angiographic views of the target vessel obtained. Intra-arterial heparin was then administered (70 U/kg). A 6F guiding catheter was used to engage the relevant coronary artery.
A 5F Simmons catheter (Cook Inc) was used to sample blood from the coronary sinus, and a 6F Export aspiration catheter (Medtronic Inc) was used to sample blood from the coronary arteries. We have previously shown that these catheters cause minimal ex vivo platelet activation with the use of the sampling techniques we have used.22 In addition, preliminary experiments confirmed that samples from the femoral vein (FV) and peripheral vein had similar, minimal platelet activation (data not shown).
The first cardiac blood sample was taken from the CS (CS1) with the use of a Simmons catheter inserted into the FV. Next, the target lesion was crossed with a 0.014-inch guidewire. A 6F Export catheter was passed distal to the lesion, with the collection tip of the Export catheter placed approximately 1 cm distal to the lesion, and blood was obtained from the coronary artery distal to the lesion (DA). The Export catheter was then pulled back proximal to the lesion, and blood was collected from the proximal artery (PA). Blood was then obtained in the CS a second time (CS2) immediately after coronary arterial sampling to ensure that sampling in the coronary arteries did not itself cause platelet activation. In 10 patients, the Simmons catheter was also pulled back to sample blood from the FV to compare samples from the CS and FV. At the conclusion of sampling, patients then underwent planned PCI. All 20 patients had blood sampled from DA and PA sites. The CS was sampled only in patients with a single target lesion in either the left anterior descending or left circumflex coronary arteries, without a significant (> 20% DS) lesion in the other left coronary artery (n = 15), and not in patients with a right coronary stenosis. The right coronary artery has an unpredictable venous drainage, and blood crossing a severe stenosis of the nonsampled branch of the left coronary artery would drain into the CS and confound results related to the target lesion.
For all sites, blood collection and processing was performed with methods previously described.22,23 An initial 5-mL syringe was used to withdraw 3 mL of blood that was discarded. A second 10-mL syringe was then used to collect 8.5 mL of blood that was transferred carefully into one tube containing EDTA (dipotassium ethylenediaminetetraacetic acid; Becton Dickinson) and one tube containing CTAD (buffered sodium citrate theophylline adenosine dipyridamole; Becton Dickinson). All samples were collected under low suction stress and at an estimated rate of 0.5 mL/s. Tubes and samples were kept at 4°C throughout sample transport and processing.
Quantitative coronary angiography
Angiographic cine images were acquired at 15 frames per second (Axiom Artis; Siemens). Two-dimensional quantitative coronary angiography (2D-QCA) was performed offline with the use of standard commercial software on a Leonardo workstation (Quant; Siemens), which is derived from the CAAS II system (Pie Medical Imaging). Automated distance calibration was used to determine pixel size. All analyses were performed during the electrocardiographic-gated end-diastolic frame. Angiographic views with the least foreshortening and yielding the best depiction of the stenoses were used. Edge detection correction was performed if required. Minimum luminal area, percentage of area stenosis, minimum luminal diameter, percentage of diameter stenosis (%DS), and lesion length were measured. Intraobserver and interobserver error in our laboratory are 3.5% ± 3.4% and 5.0% ± 3.5% for %DS, and 0.84 ± 0.78 mm and 1.30 ± 1.03 mm for lesion length.
Three-dimensional QCA (3D-QCA) was performed with the Leonardo workstation (IC3D; Siemens), which is derived from the Cardio-op B system (Paieon Medical). The contrast filled nontapered part of the guiding catheter was used to calibrate pixel size. The 2 best orthogonal angiographic views of the target lesion in the electrocardiographic-gated end-diastolic frame were used for the reconstruction of vessel lumen geometry.
Computational fluid dynamics
The geometrical models obtained from 3D reconstructions were used to construct a grid for CFD analysis as previously proposed.24 Images of one patient were not suitable for 3D reconstruction because angiographic views obtained were suboptimal, yielding 19 views for correlation with platelet activation markers and 10 views for correlation with monocyte CD11b. Models were converted to standard neutral format, and CFD analysis was performed to determine shear stress with the use of ANSYS 11.0 (ANSYS Inc). For the purposes of accuracy and economy of computations, models had at least a minimum of 100 000 mesh elements. Further, to ensure that the velocity and shear stress are accurately calculated, finer meshes were placed near the wall boundaries (< 0.1 mm). A convergence test on the adequacy of mesh resolution was performed to ensure accurate predictions.
Steady laminar flow and constant Newtonian fluid properties were assumed as in previous studies.6,11 Mass flow rate was set at 0.9 g/s. The wall was assumed to be rigid, with no deformation and to be nonslip with zero velocity. These assumptions are quite reasonable and have been widely used in previous studies.25 Blood density was assumed to be 1060 kg/m3, blood viscosity was assumed to be 0.0035 Pa/s, and reference temperature was set at 37°C.
Quantification of platelet and leukocyte activation markers
Platelet CD62P expression, GPIIb-IIIa conformational change (PAC-1 expression), and platelet-leukocyte aggregate formation were quantified in whole blood by flow cytometry, and soluble P-selectin (sCD62P) was quantified with enzyme-linked immunosorbent assays (ELISAs) as described.22,23,26
For platelet surface markers, DPBS and antibodies (isotype control of IgG or IgM; or platelet markers CD42b, CD62P, or PAC-1) were divided into aliquots into the appropriate tubes with a final volume of 50 μL, and 5 μL of CTAD anticoagulated blood was added. These were then incubated for 20 minutes and fixed with ice-cold 0.16% paraformaldehyde solution. Platelets were identified by light scatter characteristics and confirmed by CD42b expression. The isotype control was used to establish positive events on the basis of a gate/marker of 0.5% background expression. Levels of platelet expression of CD62P and PAC-1 were then quantified.
For analysis of platelet-leukocyte aggregates, appropriate antibodies (isotype control, CD45 or CD42b) were divided into aliquots in fluorescence-activated cell sorting tubes with 25 μL of CTAD anticoagulated blood and DPBS to a final volume of 100 μL. After 10 minutes of incubation, samples were fixed with ice-cold 3% paraformaldehyde solution before erythrocyte lysis with nonpyrogenic water. Leukocytes were identified by CD45 expression, and subpopulations of monocytes, neutrophils, and lymphocytes were identified by light scatter characteristics. Levels of platelet-monocyte and platelet-neutrophil aggregation were quantified by CD42b expression. We have previously shown that this whole blood technique minimizes artifactual platelet and leukocyte activation.27,28 Because initial pilot results from the first 9 patients showed shear-related up-regulation of platelet CD62P and PM-Agg across coronary lesions, leukocyte CD11b was also quantified in the last 11 patients to examine the relationship between shear stress and monocyte activation as described.27
Acquisition was performed on a BD FACSCalibur (Becton Dickinson) that was routinely calibrated with Calibrite beads in conjunction with FACSComp Version 5.1 software (Becton Dickinson). The results were analyzed with WinMDI 2.9 flow cytometric analysis software (The Scripps Institute). Negative control antibodies were used for every sample site to ensure there was no significant interassay variability. Results are presented as mean fluorescence intensity (MFI); however, identical results were obtained with MFI ratio (data not shown).
Blood containing EDTA was centrifuged at 1000g for 15 minutes and stored at −80°C for batch ELISA analysis. Plasma sCD62P levels were quantified with commercially available ELISA kit according to the manufacturer's instructions (R&D Systems).
Intracoronary platelet and leukocyte activation in the presence of high-dose antiplatelet therapy
To investigate whether translesion platelet and leukocyte activation occurred in patients who were subjected to maximal clinical doses of conventional antiplatelet agents, 5 additional patients with single target lesions of > 50% DS were recruited. These patients, who were already taking clopidogrel 75 mg and aspirin 100 mg, were given an additional 900 mg of clopidogrel and 300 mg of aspirin ≥ 3 hours before blood sampling, and heparin before blood collection. Blood was collected in sodium citrate tubes (Becton Dickinson) from the femoral artery to quantify platelet aggregation in response to arachidonic acid and adenosine diphosphate (ADP) with optical aggregometry (Aggram Analyzer; Helena Laboratories). Blood from the proximal and distal coronary arteries was subjected to flow cytometric analysis for platelet CD62P, PM-Agg, and monocyte CD11b expression as described earlier. Residual blood from the distal coronary artery collected in CTAD was centrifuged at 1000g at 15 minutes and stored at −80°C for batch analysis of thrombin generation with the use of low and high concentrations (TGA RC Low and TGA RD) of phospholipid micelles and tissue factor agonists provided in a commercial kit (Technothrombin-TGA; Technoclone).29 Platelet function and thrombin generation in the patients were compared with that of 3 healthy controls who were not given heparin or antiplatelet agents.
Shear-induced platelet and leukocyte activation in the presence of selective pathway inhibition in vitro
To quantify shear-induced platelet activation in the presence of blockade of activation pathways in vitro, blood was collected from the cubital fossa of 3 healthy subjects. A 21-gauge needle was used to collect blood into tubes containing 250 μg/mL final concentration of hirudin (Multiplate) to maximally block thrombin generation.8 Blood was then incubated immediately with or without P-selectin blocking antibody or a combination of 50μM indomethacin, 1mM P2Y1 inhibitor, and 200μM P2Y12 inhibitor8,11 for 10 minutes, then transferred into polystyrene wells coated with 1% bovine serum albumin to minimize cell adhesion. Blood in polystyrene wells was subjected to shear rates of 1800 seconds−1 (median shear rate for our patient cohort was 1782 seconds−1) for 5 minutes with the use of the Impact-R cone-plate viscometer (DiaMed), and blood in one remaining polystyrene well was left to sit without exposure to shear stress.
Statistical analysis
Results are expressed as mean ± SD unless otherwise stated. Normality of the data were determined with the D'Agostino Pearson tests for normality and verified with histogram plots. Paired t tests were used to assess for significant differences between 2 sample sites within the same patient, and unpaired t tests were used to assess for differences between 2 groups. One-way repeated analysis of variance was used to determine differences between 3 sample sites. Spearman correlation was performed for nonparametric data to determine the relationship between platelet activation and stenosis severity or shear stress. Statistical analyses were performed with GraphPad Prism Version 5.01 (GraphPad Software Inc) and SPSS Version 15 (SPSS Inc). A 2-tailed P value of < .05 is considered significant.
Results
Patient characteristics
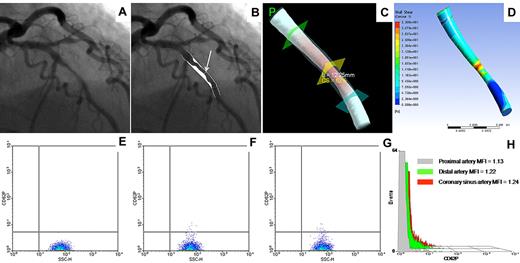
All patients had stable coronary disease. Most had a history of hypertension and hypercholesterolemia, and all were taking aspirin and clopidogrel (Table 1). The coronary angiogram, 2D-QCA, 3-dimensional reconstruction, CFD-generated shear stress map, and flow cytometric results for platelet CD62P of a representative patient are shown in Figure 1.
Baseline characteristics
| Characteristic . | Values . |
|---|---|
| Age, y, mean ± SD | 69.1 ± 10.5 |
| Male, n (%) | 13 (65) |
| Clinical history | |
| Hypertension, n (%) | 15 (75) |
| Hypercholesterolemia, n (%) | 17 (85) |
| Diabetes, n (%) | 7 (35) |
| Current smoker, n (%) | 2 (10) |
| Medications | |
| Aspirin, n (%) | 20 (100) |
| Clopidogrel, n (%) | 20 (100) |
| β-blocker, n (%) | 9 (45) |
| ACE-I/ARB, n (%) | 14 (70) |
| Statin, n (%) | 18 (90) |
| Nitrates, n (%) | 7 (35) |
| Characteristic . | Values . |
|---|---|
| Age, y, mean ± SD | 69.1 ± 10.5 |
| Male, n (%) | 13 (65) |
| Clinical history | |
| Hypertension, n (%) | 15 (75) |
| Hypercholesterolemia, n (%) | 17 (85) |
| Diabetes, n (%) | 7 (35) |
| Current smoker, n (%) | 2 (10) |
| Medications | |
| Aspirin, n (%) | 20 (100) |
| Clopidogrel, n (%) | 20 (100) |
| β-blocker, n (%) | 9 (45) |
| ACE-I/ARB, n (%) | 14 (70) |
| Statin, n (%) | 18 (90) |
| Nitrates, n (%) | 7 (35) |
ACE-I indicates angiotensin-converting enzyme inhibitor; and ARB, angiotensin receptor blocker.
Representative patient. (A) Coronary angiogram showing a lesion in the mid left anterior descending artery. (B) Two-dimensional quantitative coronary angiography showing 52% DS (arrow). (C) Three-dimensional quantitative coronary angiography showing cross-sectional area stenosis (CS) of 62% and lesion length of 12.25 mm. (D) Shear stress map generated from flow field calculated by computational fluid dynamics analysis, showing peak wall shear stress of 33.1 Pa. (E-G) Flow cytometry density plot of platelet CD62P expression in the coronary artery proximal (E) and distal (F) to the lesion, and in the coronary sinus (G). (H) Histogram overlay of platelet CD62 expression in the 3 sites. MFI indicates mean fluorescence intensity.
Representative patient. (A) Coronary angiogram showing a lesion in the mid left anterior descending artery. (B) Two-dimensional quantitative coronary angiography showing 52% DS (arrow). (C) Three-dimensional quantitative coronary angiography showing cross-sectional area stenosis (CS) of 62% and lesion length of 12.25 mm. (D) Shear stress map generated from flow field calculated by computational fluid dynamics analysis, showing peak wall shear stress of 33.1 Pa. (E-G) Flow cytometry density plot of platelet CD62P expression in the coronary artery proximal (E) and distal (F) to the lesion, and in the coronary sinus (G). (H) Histogram overlay of platelet CD62 expression in the 3 sites. MFI indicates mean fluorescence intensity.
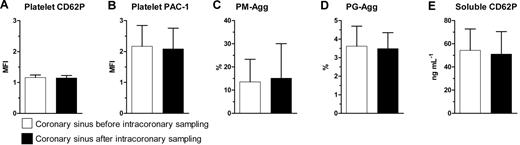
Intracoronary blood sampling does not induce detectable platelet activation in the coronary sinus
There was no evidence of platelet activation as a result of intracoronary arterial sampling, because CS1 and CS2 showed similar platelet CD62P, PAC-1, PM-Agg, platelet-granulocyte aggregate (PG-Agg), and soluble CD62P levels (Figure 2).
Comparison between coronary sinus samples taken before and after sampling in the coronary arteries. (A) Platelet CD62P, (B) platelet PAC-1, (C) platelet-monocyte aggregates (PM-Agg), (D) platelet-granulocyte aggregates (PG-Agg), and (E) soluble CD62P. MFI indicates mean fluorescence intensity. P = not significant for all comparisons shown. Solid bars and error bars represent mean ±SD.
Comparison between coronary sinus samples taken before and after sampling in the coronary arteries. (A) Platelet CD62P, (B) platelet PAC-1, (C) platelet-monocyte aggregates (PM-Agg), (D) platelet-granulocyte aggregates (PG-Agg), and (E) soluble CD62P. MFI indicates mean fluorescence intensity. P = not significant for all comparisons shown. Solid bars and error bars represent mean ±SD.
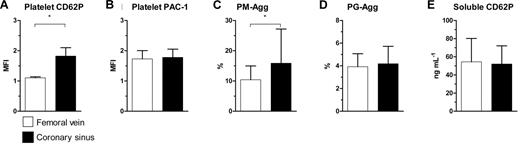
Evidence of intracardiac platelet activation
Platelet CD62P (MFI: 1.8 ± 0.27 vs 1.1 ± 0.03; P = .004) and percentage of PM-Agg (16.0% ± 11.0% vs 10.0% ± 4.5%; P = .002) were significantly greater in CS samples than in FV samples, supporting the possibility of intracardiac platelet activation. However, CS and FV samples did not differ in their levels of platelet PAC-1 (MFI: 1.8 ± 0.27 vs 1.7 ± 0.27; P = .08), PG-Agg (4.2% ± 1.5% vs 3.9% ± 1.1%; P = .63), or soluble CD62P (52.0 ± 20.0 ng/mL vs 54.0 ± 26.0 ng/mL; P = .65; Figure 3), suggesting that CD62P expression and the formation of PM-Agg were specifically up-regulated in the cardiac circulation.
Comparison between femoral vein and coronary sinus. (A) Platelet CD62P, (B) platelet PAC-1, (C) platelet-monocyte aggregates (PM-Agg), (D) platelet-granulocyte aggregates (PG-Agg), and (E) soluble CD62P. MFI indicates mean fluorescence intensity; *P < .05. Solid bars and error bars represent mean ±SD.
Comparison between femoral vein and coronary sinus. (A) Platelet CD62P, (B) platelet PAC-1, (C) platelet-monocyte aggregates (PM-Agg), (D) platelet-granulocyte aggregates (PG-Agg), and (E) soluble CD62P. MFI indicates mean fluorescence intensity; *P < .05. Solid bars and error bars represent mean ±SD.
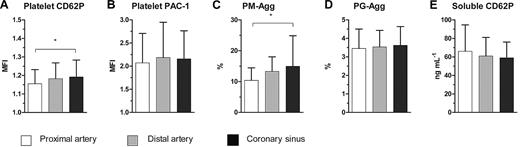
The site of platelet activation was investigated by comparing samples from PA, DA, and CS. PA, DA, and CS samples differed significantly in their levels of platelet CD62P (MFI: 1.15 ± 0.08 vs 1.18 ± 0.09 vs 1.19 ± 0.09; P = .01) and PM-Agg (10.4% ± 4.1% vs 13.3% ± 4.7% vs 14.9% ± 9.9%; P = .01), consistent with activation across the coronary circulation (Figure 4). However, platelet PAC-1, PG-Agg, and soluble CD62P did not differ significantly between the sites.
Comparison between proximal, distal, and coronary sinus. (A) Platelet CD62P, (B) platelet PAC-1, (C) platelet-monocyte aggregates (PM-Agg), (D) platelet-granulocyte aggregates (PG-Agg), and (E) soluble CD62P. MFI indicates mean fluorescence intensity; *P < .05. Solid bars and error bars represent mean ±SD.
Comparison between proximal, distal, and coronary sinus. (A) Platelet CD62P, (B) platelet PAC-1, (C) platelet-monocyte aggregates (PM-Agg), (D) platelet-granulocyte aggregates (PG-Agg), and (E) soluble CD62P. MFI indicates mean fluorescence intensity; *P < .05. Solid bars and error bars represent mean ±SD.
Translesion platelet activation correlates with stenosis severity
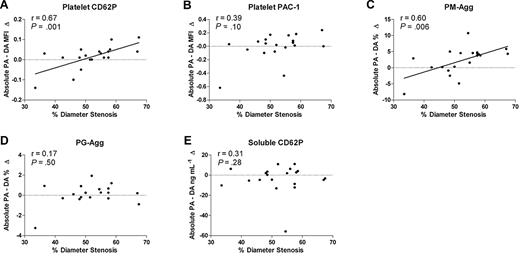
Shear stress is expected to increase with the severity of coronary stenosis, and the effects of shear stress would be expected to be most closely observed immediately after platelets cross stenotic lesions. We, therefore, investigated the relationship between %DS by 2D-QCA and the increment in platelet activation as blood flows across coronary stenoses (DA − PA). The %DS correlated with the increment in platelet CD62P (r = 0.67, P = .001) and PM-Agg (r = 0.60, P = .006), but there was no correlation with the increment of PAC-1, PG-Agg, or soluble CD62P (Figure 5A-E). Other measures of stenosis severity determined by 2D- and 3D-QCA showed similar correlations with platelet CD62P and PM-Agg (not shown). However, lesion length and atherosclerotic plaque volume, assessed by 3D-QCA, did not correlate significantly with any marker of platelet activation (eg, platelet CD62P: r = −0.39, P = .10; PM-Agg: r = −0.30, P = .25).
Correlation between %DS and absolute increase from the proximal artery to the distal artery. (A) Platelet CD62P, (B) platelet PAC-1, (C) percentage of platelet-monocyte aggregates (PM-Agg), (D) platelet-granulocyte aggregates (PG-Agg), and (E) soluble CD62P. MFI indicates mean fluorescence intensity; PA (proximal artery); DA (distal artery). Solid lines shown are derived from linear regression analyses; dotted lines intersect y-axis = 0; r and P values shown are derived from correlation analyses.
Correlation between %DS and absolute increase from the proximal artery to the distal artery. (A) Platelet CD62P, (B) platelet PAC-1, (C) percentage of platelet-monocyte aggregates (PM-Agg), (D) platelet-granulocyte aggregates (PG-Agg), and (E) soluble CD62P. MFI indicates mean fluorescence intensity; PA (proximal artery); DA (distal artery). Solid lines shown are derived from linear regression analyses; dotted lines intersect y-axis = 0; r and P values shown are derived from correlation analyses.
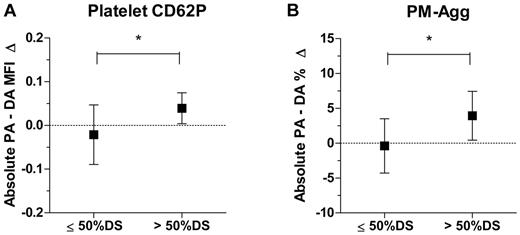
Patients with coronary stenoses > 50% are known to be at increased risk of coronary thrombotic events and commonly undergo procedures such as coronary angioplasty and stenting. We, therefore, investigated if target (sampled) lesions > 50% and ≤ 50% DS differed in their effects on apparent platelet activation. There was a significant increment in platelet CD62P and PM-Agg across lesions of > 50% stenosis, whereas there was no significant increment across lesions ≤ 50% (Figure 6). There were no significant differences in clinical characteristics or medication use between patients who had > 50% stenosis and ≤ 50%. There were no significant correlations between mean blood pressure, measured during catheterization of the ascending aorta, and translesion up-regulation of platelet CD62P (r = 0.21, P = .37), PM-Agg (r = 0.15, P = .53), or monocyte CD11b (r = −0.56, P = .07). Of the 20 patients, 16 had fasting lipid levels measured on the day of procedure. There were no significant correlations between translesion up-regulation of platelet CD62P or PM-Agg and total cholesterol, low-density lipoprotein, high-density lipoprotein, or triglyceride levels (P = ns for all) and no significant correlations between monocyte CD11b up-regulation and lipid levels (n = 8; P = ns for all).
Comparison between lesions with ≤ 50% stenosis and > 50% stenosis for absolute increase from the proximal artery to the distal artery. (A) Platelet CD62P and (B) platelet-monocyte aggregates (PM-Agg). MFI indicates mean fluorescence intensity; PA, proximal artery; DA, distal artery; *P < .05. Box and error bars represent mean ±SD. Dotted lines intersect y-axis = 0.
Comparison between lesions with ≤ 50% stenosis and > 50% stenosis for absolute increase from the proximal artery to the distal artery. (A) Platelet CD62P and (B) platelet-monocyte aggregates (PM-Agg). MFI indicates mean fluorescence intensity; PA, proximal artery; DA, distal artery; *P < .05. Box and error bars represent mean ±SD. Dotted lines intersect y-axis = 0.
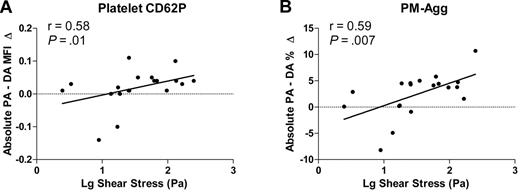
Relationship between intracoronary platelet activation and shear stress
Estimated peak wall shear stress ranged from 2.64 Pa to 281.5 Pa (mean, 87.8 Pa). Peak wall shear stress correlated with stenosis severity (r = 0.55, P < .01). Peak wall shear stress correlated with the translesion gradient of CD62P (r = 0.58, P = .01) and PM-Agg (r = 0.59, P = .007; Figure 7) but not PAC-1 (r = 0.18, P = .46) or sCD62P (r = −0.02, P = .94).
Correlation between log peak shear stress and absolute increase from the proximal artery to the distal artery. (A) Platelet CD62P, (B) percentage of platelet-monocyte aggregates (PM-Agg). PA indicates proximal artery; DA, distal artery. Solid lines shown are derived from linear regression analyses; r and P values shown are derived from correlation analyses. Dotted lines intersect y-axis = 0.
Correlation between log peak shear stress and absolute increase from the proximal artery to the distal artery. (A) Platelet CD62P, (B) percentage of platelet-monocyte aggregates (PM-Agg). PA indicates proximal artery; DA, distal artery. Solid lines shown are derived from linear regression analyses; r and P values shown are derived from correlation analyses. Dotted lines intersect y-axis = 0.
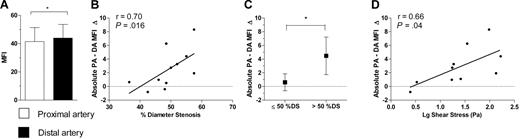
Intracoronary leukocyte activation
To investigate whether the increase in PM-Agg across coronary stenoses was associated with monocyte activation, monocyte CD11b levels were determined in 11 patients. There was a significant transcardiac increment in monocyte CD11b (Figure 8A), and the increment across coronary stenoses correlated with %DS (r = 0.70, P = .016; Figure 8B) and translesion up-regulation of PM-Agg (r = 0.72, P = .001). As observed for CD62P, there was a much greater increment in monocyte CD11b levels from PA to DA in patients with lesions of > 50% stenosis than in patients with ≤ 50% stenosis (CD11b MFI increment: 4.47 ± 2.74 vs 0.60 ± 1.24; P = .02; Figure 8C). Peak shear stress also correlated with translesion up-regulation of monocyte CD11b (r = 0.66, P = .04; n = 10; Figure 8D).
Monocyte CD11b results. (A) Comparison between levels in the coronary artery proximal and distal to the lesion. (B) Correlation between %DS and absolute increase in mean fluorescence intensity (MFI) from the proximal artery (PA) to the distal artery (DA). (C) Comparison between lesions with ≤ 50% stenosis and > 50% stenosis for absolute increase from PA to DA. (D) Correlation between log peak shear stress and absolute MFI increase from PA to DA. DS indicates diameter stenosis. Solid bars or boxes with error bars represent mean ±SD. r and P values were obtained from correlation analysis; solid lines were derived from linear regression analysis; dotted lines intersect y-axis = 0. *P < .05.
Monocyte CD11b results. (A) Comparison between levels in the coronary artery proximal and distal to the lesion. (B) Correlation between %DS and absolute increase in mean fluorescence intensity (MFI) from the proximal artery (PA) to the distal artery (DA). (C) Comparison between lesions with ≤ 50% stenosis and > 50% stenosis for absolute increase from PA to DA. (D) Correlation between log peak shear stress and absolute MFI increase from PA to DA. DS indicates diameter stenosis. Solid bars or boxes with error bars represent mean ±SD. r and P values were obtained from correlation analysis; solid lines were derived from linear regression analysis; dotted lines intersect y-axis = 0. *P < .05.
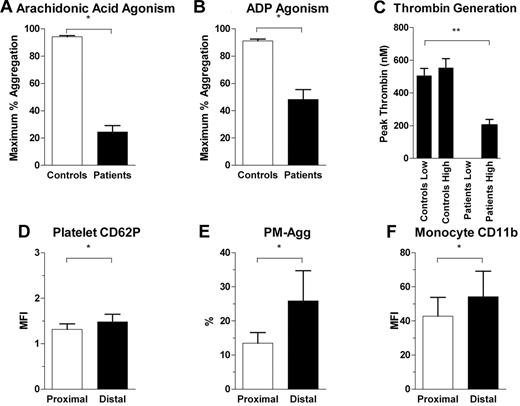
Intracoronary platelet and leukocyte activation in the presence of high-dose antiplatelet therapy
In a further 5 patients given additional high doses of aspirin (300 mg) and clopidogrel (900 mg) 3 hours before blood sampling, maximal platelet aggregation in response to arachidonic acid and ADP were significantly, but incompletely, inhibited (Figure 9A-B). In the same patients, we confirmed that weight-based heparin administration achieved substantial inhibition of thrombin generation (Figure 9C). Despite substantial inhibition of platelet aggregation and inhibition of thrombin generation, there was significant up-regulation of platelet CD62P, PM-Agg, and monocyte CD11b across stenotic coronary lesions as observed in our main cohort (Figure 9D-F). These data confirm that translesion platelet and monocyte activation occur in the presence of effective clinical doses of antiplatelet agents and heparin.
Platelet and leukocyte activation in the presence of heparin and high doses of aspirin and clopidogrel. Comparison of levels of platelet aggregation between healthy controls and subjects on antiplatelet agents for (A) arachidonic acid and (B) ADP-induced maximum percentage of platelet aggregation. (C) Thrombin generation induced by low and high concentrations of phospholipids in controls and patients on heparin. Patients on heparin had undetectable low phospholipid dose-induced thrombin generation. **P < .05 for 1-way analysis of variance between groups and post hoc Tukey test between patient and control groups and between patient groups subjected to low and high doses of phospholipid-induced thrombin generation. (D-F) Comparison between proximal and distal coronary site for levels of platelet CD62P, PM-Agg, and monocyte CD11b. *P < .05. Solid bars and error bars represent mean ±SD.
Platelet and leukocyte activation in the presence of heparin and high doses of aspirin and clopidogrel. Comparison of levels of platelet aggregation between healthy controls and subjects on antiplatelet agents for (A) arachidonic acid and (B) ADP-induced maximum percentage of platelet aggregation. (C) Thrombin generation induced by low and high concentrations of phospholipids in controls and patients on heparin. Patients on heparin had undetectable low phospholipid dose-induced thrombin generation. **P < .05 for 1-way analysis of variance between groups and post hoc Tukey test between patient and control groups and between patient groups subjected to low and high doses of phospholipid-induced thrombin generation. (D-F) Comparison between proximal and distal coronary site for levels of platelet CD62P, PM-Agg, and monocyte CD11b. *P < .05. Solid bars and error bars represent mean ±SD.
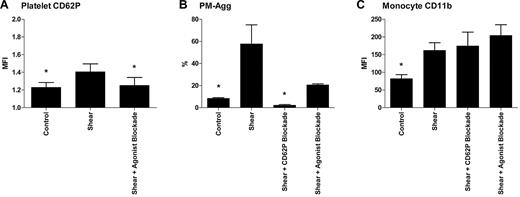
Shear-induced platelet and leukocyte activation in the presence of selective pathway inhibition in vitro
Shear-induced up-regulation of platelet CD62P, PM-Agg, and monocyte CD11b in the presence of inhibitors in vitro is shown in Figure 10. CD62P blockade completely suppressed shear-induced platelet-monocyte aggregation with no effect on shear-induced monocyte CD11b expression. Blockade of thromboxane A2, P2Y1, and P2Y12 in the presence of hirudin inhibited shear-induced platelet CD62P and platelet-monocyte aggregation but had no effect on monocyte CD11b expression. Shear-induced platelet-monocyte aggregation was incompletely inhibited by antagonism of thromboxane A2, P2Y1, and P2Y12 and was still significantly higher than baseline (21.0% ± 1.6% vs 8.5% ± 1.1%; P = .003).
Shear-induced activation in the presence of CD62P-blocking antibody and soluble platelet agonist inhibition. (A) Platelet CD62P expression, (B) PM-Agg formation, and (C) monocyte CD11b expression. Flow cytometry was performed in 3 healthy donors under control conditions, after shear stress with and without CD62P blocking antibody or soluble platelet agonist inhibition. Solid bars and error bars represent mean ±SD. P < .05 for 1-way analysis of variance and *P < .05 for difference compared with shear group by post hoc Tukey test.
Shear-induced activation in the presence of CD62P-blocking antibody and soluble platelet agonist inhibition. (A) Platelet CD62P expression, (B) PM-Agg formation, and (C) monocyte CD11b expression. Flow cytometry was performed in 3 healthy donors under control conditions, after shear stress with and without CD62P blocking antibody or soluble platelet agonist inhibition. Solid bars and error bars represent mean ±SD. P < .05 for 1-way analysis of variance and *P < .05 for difference compared with shear group by post hoc Tukey test.
Discussion
We report that, despite usage of aspirin and clopidogrel, patients with stable coronary disease exhibit in situ up-regulation of platelet CD62P, PM-Agg, and monocyte CD11b without increase in platelet GPIIb-IIIa activation and that the up-regulation of these markers correlate with stenosis severity and shear stress. These results support and extend previous reports of transcardiac up-regulation of platelet P-selectin and platelet-monocyte aggregates,14 by providing the first direct evidence of intracoronary shear-related platelet activation in humans. These results have important implications for future pharmacologic targets in patients with significant coronary stenoses.
Previous human studies showed indirect evidence of intracoronary platelet activation with increased platelet P-selectin in association with increased atheroma burden30 and increased platelet aggregation and large VWF multimer formation in the peripheral blood of patients with severe coronary stenoses.31 Our results support a direct relationship between stenosis severity, shear stress, and in situ platelet activation in human coronaries but show that atheroma burden per se, as measured by plaque volume, does not correlate with platelet activation in stable patients. This suggests that in stable coronary disease, shear stress is a more important determinant of platelet activation within coronary arteries rather than the amount of atheroma.
In contrast to significant clinical benefits shown with the use of aspirin and clopidogrel in patients with unstable coronary disease (unstable angina and myocardial infarction),32 there is only modest benefit for the use of aspirin or clopidogrel in patients with stable coronary disease.33 Recent studies indicate that shear stress can induce platelet activation and aggregation independent of classical activation pathways6 or agonist participation,11 and our data indicating that shear-induced activation is resistant to effective, clinically relevant doses of aspirin and clopidogrel are consistent with these reports. Maximal inhibition of soluble-agonist pathways in vitro inhibited shear-induced up-regulation of platelet CD62P and PM-Agg formation and had no effect on monocyte CD11b expression. Because CD62P blockade appeared to completely inhibit shear-induced PM-Agg, a mechanistic role for CD62P in shear-induced PM-Agg is probable. However, the lack of effect of either CD62P blockade or inhibition of thromboxane A2, P2Y1, and P2Y12 on monocyte CD11b up-regulation suggests the latter is up-regulated by a distinct pathway.
Previous studies have shown that stable platelet aggregation only occurs in the presence of thrombophilic surfaces in vitro6 or vessel wall injury in animals.6,11 Our results, however, indicated that up-regulation of P-selectin, PM-agg, and monocyte CD11b in human coronary arteries did not require overt lesion rupture. Although we cannot exclude the possibility of subclinical endothelial injury in these patients, our data suggest that in human coronary atherosclerosis, platelet and monocyte activation have a pathophysiologic relevance extending beyond acute coronary syndromes.
Evidence from animal studies is convincing that platelet P-selectin is involved in the progression of stable atheromatous plaques,2,3,34-37 and P-selectin–dependent adhesion of platelets to monocytes results in complexes with enhanced adhesion to endothelial cells.38 Further, blockade of P-selectin or P-selectin glycoprotein ligand-1 (its counterpart binding molecule) at the time of arterial injury significantly limits plaque macrophage content and neointima formation in a dose-dependent manner in the carotid arteries of apolipoprotein E−/− mice.38 Pathways linking shear stress–mediated up-regulation of P-selectin and PM-Agg warrant further investigation as therapeutic targets for coronary atherosclerosis.19,39
Current antiplatelet agents such as aspirin, thienopyridines, and GPIIb-IIIa inhibitors attenuate agonist-induced platelet activation,15-17 and GPIIb-IIIa inhibitors reduce shear-induced platelet aggregation.10,18,19 However, shear-induced platelet aggregation was shown to be independent of ADP and thromboxane,11 and shear stress was shown to overcome aspirin inhibition of platelet aggregation.20 Further, GPIIb-IIIa blockade fails to inhibit shear-induced platelet-leukocyte aggregation8,21 and leukocyte activation.8 Although all our patients were on aspirin and clopidogrel, which precluded our ability to directly test the effect of these agents, our results reinforce the argument that current antiplatelet agents do not inhibit platelet pathways involved in leukocyte interaction and activation, and they provide an important validation of in vitro studies. Our results contrast with a previous study that showed in vitro activation of GPIIb-IIIa by shear stress.7 This difference may be attributable to the usage of aspirin and clopidogrel by patients in our study. The distinct effects of coronary stenoses on P-selectin and GPIIb-IIIa activation under these conditions are clinically and pathologically important. They imply either that aspirin and clopidogrel are much more effective at preventing GPIIb-IIIa activation than they are at inhibiting the up-regulation of P-selectin or that shear stress preferentially up-regulates P-selectin rather than GPIIb-IIIa.
Interestingly, our results indicate a trend toward increased platelet activation in the CS compared with the distal coronary artery. We hypothesize that, as platelets move across the myocardial vascular bed, platelets could be further activated by local factors independent of coronary stenosis severity. For example, differences in microvasculature status have been shown to be associated with platelet and leukocyte activation,40 and this may affect platelet activation in samples taken from the CS.
Shear stress can cause platelet activation that then activates leukocytes.8,9 Shear stress can also cause leukocyte activation in vitro,8,9 which can in turn promote platelet activation.41 However, it is unclear whether platelet or leukocyte activation is separately or sequentially up-regulated across coronary stenoses. Results from the in vitro arm of this study show that CD62P blockade completely suppressed shear-induced platelet-monocyte aggregation with no effect on shear-induced monocyte CD11b expression (Figure 10). These results are in agreement with that of a previous study.8 The clear correlations between coronary stenosis severity and both platelet CD62P and monocyte CD11b expression implies that shear stress may directly up-regulate both parameters. Our in vitro data indicate that shear-induced platelet-monocyte aggregation critically depends on platelet CD62P expression, but that up-regulation of monocyte CD11b occurs by a distinct mechanism.
Although there was translesion and transcardiac up-regulation of PM-Agg and monocyte CD11b expression, there was no up-regulation of PG-Agg or granulocyte CD11b expression. Neutrophil activation is known to occur in unstable plaques,42 and in one study transcardiac (proximal coronary artery to CS) up-regulation of PG-Agg was only present in patients with unstable angina and not in patients with stable coronary disease.14 Our results in patients with stable coronary disease show shear-related platelet-monocyte aggregation and monocyte activation, without up-regulation of PG-Agg or neutrophil activation, suggesting that neutrophil activation may require the presence of an unstable plaque. The mechanisms underlying the interaction between plaque stability, shear stress, and neutrophil activation remain to be studied.
Although statistically significant, the difference between platelet CD62P MFI levels proximal and distal to coronary stenoses was subtle (Figures 4A and 9D). Detection of these differences was made possible by paired comparisons within patients, which diminish the confounding effect of variation between patients. That these differences are real and biologically important is supported by their correlation with stenosis severity, the increase in PM-Agg across lesions in vivo, and the marked inhibition of shear-induced aggregate formation in vitro by the use of P-selectin–blocking antibodies. Ultimately, the clinical significance of individual markers of platelet activation across coronary stenoses will only be established after evaluating the effects of their modulation in vivo.
The up-regulation of platelet CD62P, PM-Agg, and monocyte CD11b were similarly correlated to stenosis severity and to peak wall shear stress. Our model of CFD assumes steady, laminar flow with Newtonian fluid properties as have previous studies.6,11 It is probable that incorporation of direct blood flow measurements or increased complexity in the computations such as flow pulsation, more accurate boundary conditions, and variable properties will further improve the correlation between calculated shear stress and platelet activation as shown in vitro.19
Limitations
This study has some limitations. Sampling of blood within the coronary arteries can result in ex vivo activation. Although the use of whole-blood flow cytometry, the lack of apparent activation in CS samples before and after sampling, and previous validation of sampling with the use of these catheters reduces the risk of such activation,22 the possibility of ex vivo activation cannot be totally excluded. The inevitable use of radiographic contrast before sampling may attenuate platelet activation,43 but this would be expected to reduce the apparent relationship between shear stress and platelet activation and act on the whole patient population. Heparin administration, which may affect platelet activation, was required before instrumentation of the cardiac circulation. However, because one study showed no effect44 and another showed a minimal inhibitory effect of heparin on shear-induced platelet aggregation,45 we do not expect this to be an important confounder. Intravascular ultrasound scanning, which was not used in our study, is more accurate than QCA in quantifying plaque volume. However, 3D-QCA measurements have been shown to correlate well with intravascular ultrasound measurements of coronary lesions, and some correlation between plaque volume by 3D-QCA and platelet activation would have been expected if plaque volume were a main determinant of intracoronary platelet activation. Finally, our study is restricted to patients with 40%-70% DS. It is therefore probable that more severe stenoses would show more marked activation and that our results may be underestimating the effect of lesion severity on platelet activation.
Conclusions
These results provide an important validation of the role of shear-stress in causing platelet activation in humans and suggest its resistance to conventional antiplatelet therapy. The pathways regulating the up-regulation of P-selectin, platelet-monocyte aggregation, and monocyte activation by shear stress may represent novel pharmacologic targets in patients with significant coronary stenoses.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Caroline Reddel, Mr Geoff Kershaw, and Dr Jenny Curnow for help in performing and analyzing platelet aggregation studies and thrombin generation assays, and the cardiac catheterization laboratory staff at Concord Hospital for their help in sample collection.
This work was supported by the National Health and Medical Research Council of Australia (NHMRC), NHMRC (program grant 482800; L.K.) and NHMRC Postgraduate Medical Research Scholarship (A.S.C.Y.).
Authorship
Contribution: A.S.C.Y. designed and performed most of the work involved with this study and cowrote the paper with L.K.; G.J.P. collected and analyzed data and assisted in manuscript preparation; M.C., A.H., and M.B. helped with developing methods for computational fluid dynamics and analyzed data generated; T.C. helped with developing methods for platelet flow cytometry and analyzed data generated; D.B., H.C.L., and M.K.C.N. recruited patients, performed sampling procedures, and collected data; M.Q. and S.A.K. helped with designing, performing, and analyzing experiments involving the cone-plate viscometer; and L.K. designed the research, performed sampling procedures, provided overall direction, and cowrote the paper with A.S.C.Y.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leonard Kritharides, Department of Cardiology, Level 3 West, Concord Repatriation General Hospital, Concord, New South Wales 2139, Australia; e-mail: leonard.kritharides@sydney.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal