Abstract
In view of assessing the possible contribution of dendritic cells (DCs) to HIV-related B-cell disorders, we have longitudinally measured B lymphocyte stimulator (BLyS) surface expression by myeloid DCs (mDCs) and concentrations of B-cell growth factors in the blood of subjects undergoing primary HIV infection with different rates of disease progression. We report that BLyS surface expression by mature mDCs and precursors as well as blood levels of BLyS, a proliferation-inducing ligand (APRIL), interleukin-6 (IL-6), and IL-10 increased above normal levels in both rapid and normal HIV progressors as quickly as in the acute phase of infection and persisting throughout the course of disease despite successful therapy. Consequently, hyperglobulinemia and high blood levels of circulating activated mature B cells and precursor/activated marginal zone (MZ)–like B cells were found throughout follow-up for both rapid and normal progressors. In contrast, mDC cell-surface expression of BLyS as well as blood levels of BLyS, immunoglobulin, activated mature B cells, and precursor/activated MZ-like B cells in aviremic slow progressors were similar to those observed in healthy donors. Interestingly, the levels of mature MZ B cells were significantly reduced in slow progressors. Our results suggest that DCs might modulate the outcome of the HIV-related B-cell disease progression through the expression of BLyS.
Introduction
B lymphocyte disorders are important consequences of HIV infection and can evolve toward autoimmune disorders and malignancies. Given that the status and activation requirements of the different B-cell populations vary, they are likely to be affected differentially during HIV infection, a process reflected by events such as polyclonal activation, breakage of tolerance, altered subpopulation dynamics, and exhaustion, as well as loss of the capacity to generate and maintain memory, all of which contribute to a global impairment of the humoral immune compartment in HIV-infected individuals (reviewed in Moir and Fauci1 ).
The mechanisms involved in the triggering and progression of such B-cell disorders are poorly understood and have been thought to be mainly the consequence of high viral load and the altered CD4+ T-cell compartment.1-3 However, there is a controversy regarding the persistence of these defects beyond antiretroviral treatment (ART) and in the absence of apparent disease progression. Although a previous study has shown decreased activated B-cell and increased memory B-cell frequencies upon initiation of ART,3 others have demonstrated persistence of hypergammaglobulinemia, increased apoptosis of memory B cells, and autoimmune complications in treated patients.2,4,5 This suggests that factors other than or complementary to the viral load and CD4+ T-cell levels may contribute to the alteration of the B-cell compartment.
Dendritic cells (DCs) play a pivotal role in regulating the outcome of B-cell development, activation, and survival in T-dependent and T-independent manners, mainly through production of B-cell growth factors such as B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL).6-8 Early data supporting the hypothesis that DCs are involved in the dysregulation of the B-cell compartment in the context of HIV were obtained with HIV transgenic (HIV Tg) mice expressing rev, env, and/or nef of HIV-1.9 In HIV Tg mice, myeloid DCs (mDCs) accumulated at entry points of secondary lymphoid organs, contributing to the enlargement of the marginal zone (MZ) B-cell population, polyclonal activation, and breakage of tolerance observed in these animals.10,11 This is possibly due to a process involving delivery of altered/excessive contact events and/or B-cell growth factors. In this view, BLyS Tg mice showed enlarged splenic MZ accompanied by B-cell hyperactivity, decreased T-cell numbers, and development of an autoimmune disease,12 a phenotype reminiscent of that reported for HIV Tg mice.9-11 The involvement of BLyS in the gp120–DC-SIGN–mediated polyclonal stimulation of human MZ-type B cells also supports this.13 Importantly, BLyS and factors such as interleukin-6 (IL-6) and IL-10 have been shown to be increased in the blood of HIV viremic individuals and correlate with elevated serum autoantibody titers.14-16 Finally, we and others have shown that mDC populations are affected in number, phenotype, and function in human HIV infection,17-20 suggesting that their alterations may affect the integrity of the B-cell compartment.
We aimed to investigate the potential involvement of DCs and derived growth factors in triggering and perpetuating B-cell disorders in the context of HIV. We have thus undertaken a longitudinal measurement of BLyS and other B-cell growth factors known to be expressed and/or produced by mDCs and their precursors in HIV-infected subjects with different rates of disease progression. We report that BLyS expression on blood mDCs and monocytic precursors and plasma levels of BLyS, APRIL, IL-6, and IL-10 were elevated in the early phases of infection and remained high in the chronic phase of infection, despite successful therapy in HIV rapid and normal progressors. This was concomitant with elevated levels of blood-activated B cells and hyperglobulinemia. In contrast, aviremic slow progressors had normal BLyS expression levels on mDCs and precursors as well as normal blood levels of BLyS and activated B cells. Interestingly, the levels of mature MZ B cells were significantly reduced in slow progressors. Our results suggest that DCs might affect the outcome of the HIV-related B-cell disease progression through overexpression of BLyS.
Methods
Subjects
Thirty HIV-infected subjects were selected from the Montreal Primary HIV Infection cohort: 13 were classified as rapid and 17 as normal progressors. The date of infection was estimated on the basis of clinical and laboratory results using criteria established by the Acute HIV Infection and Early Disease Research Program (NIAID, Bethesda, MD). Rapid progressors had CD4+ T-cell counts below 250 cells/mm3 within 2 years of infection. Blood samples were taken at 4 time points: acute (0-3 months) and early (5-8 months) phases of infection and 3-6 months and 9-12 months after initiation of ART. Normal progressors were ART-naive patients whose CD4+ T-cell counts remained above 500 cells/mm3 for the 2-year follow-up. Blood samples were obtained in the acute, early, and chronic phases (24 months) of infection. Blood samples were also obtained from 13 slow progressors infected for 8 years or more with CD4+ T-cell counts above 500 cells/mm3 and low (viremic, N = 7) to undetectable (aviremic, N = 6) viral loads in the absence of ART and from 20 age-matched HIV-negative volunteers. HIV viral loads were determined in the plasma using Versant HIV-1 RNA 3.0 Assay (bDNA, Siemens Medical Solutions Diagnostics, Tarrytown, NY). Blood CD4+ T-cell counts were determined as previously reported.21 None of the study subjects had syphilis, hepatitis B, or hepatitis C. Informed consent was obtained from all subjects, and the research conformed to ethical guidelines of all authors' institutions.
Evaluation of cell populations and membrane-bound BLyS by flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated after whole blood centrifugation on Ficoll gradient. Cells were resuspended in heat-inactivated fetal bovine serum (FBS) with 10% dimethyl sulfoxyde (DMSO) and stored in liquid nitrogen. Cells were thawed and washed in RPMI containing 5% heat-inactivated FBS and 1% penicillin/streptomycin. One million PBMCs per sample were used for cell surface staining. Nonspecific sites were blocked by incubation for 10 minutes on ice with fluorescence-activated cell sorting buffer (1× phosphate-buffered saline, 2% heat-inactivated FBS, and 0.1% sodium azide) supplemented with 10% heat-inactivated FBS and 10 μg of total mouse IgG per million PBMCs (Sigma-Aldrich). Cells were labeled with the following cocktails of mouse anti–human monoclonal antibodies: fluorescein isothiocyanate–conjugated anti-IgM, phycoerythrin (PE)–conjugated anti-CD21, PE-Cy7–conjugated anti-CD10 (BD Biosciences), Pacific Blue–conjugated anti-CD19, Alexa-Fluor 700–conjugated anti-CD27 (eBioscience), and allophycocyanin-conjugated anti-CD1c (Miltenyi Biotec) for identification of B cells. Membrane-bound BLyS was evaluated by using PE-conjugated anti-BLyS (eBioscience) and by costaining cells with PE-Cy7–conjugated anti–HLA-DR, allophycocyanin-conjugated anti-CD11c, PerCP-Cy5.5–conjugated anti-CD14, fluorescein isothiocyanate–conjugated anti-CD3, Alexa-Fluor 700–conjugated anti-CD16 (BD Biosciences). BLyS levels per cell were calibrated using 8-peaks Rainbow Calibration Particles (BD Biosciences). Dead cells were identified by trypan blue exclusion and 7-amino actinomycin D (7AAD) staining. Data acquisition of 100 000 events per sample was performed on a BD LSRII apparatus (Becton Dickinson), and analysis was done with FlowJo 7.5.4 software (TreeStar).
Evaluation of plasma cytokine and serum immunoglobulin concentrations
Plasma levels of IL-6 and IL-10 were determined using the Cytometric Bead Array (CBA) Human Inflammation Kit (BD Biosciences) according to the manufacturer's instructions. Data were acquired on a FACSAria apparatus and analyzed with the FCAP software (BD Biosciences). Plasma concentrations of BLyS and APRIL were measured by enzyme-linked immunospecific assay (ELISA; R&D Systems and Invitrogen, respectively). Serum immunoglobulin levels were measured using multiplex Beadlyte Human IgG, IgA, IgM Kit (Millipore) with the Luminex 200 Total System (Luminex Corporation).
Evaluation of plasma-soluble CD14, LPS, and LPS-binding protein concentrations.
Plasma levels of soluble CD14 (sCD14) were measured with a commercially available ELISA kit (R&D Systems) according to the manufacturer's protocol. Plasma lipopolysaccharide (LPS) concentrations were quantified with the Limulus Amebocyte Lysate assay (Lonza) according to the manufacturer's protocol, in plasma samples diluted 1/100 in endotoxin-free water (as determined by spike recovery test) and heated to 70°C for 10 minutes to inactivate plasma proteins. Plasma LPS-binding protein (LBP) concentrations were measured by ELISA (Hycult Biotech) according to manufacturer's protocol.
Statistical analyses.
The statistical significance of differences between groups was assessed with χ2 test for categorical variables and unpaired Student t test or one-way analysis of variance when continuous variables were normally distributed or with Mann-Whitney U test otherwise. Wilcoxon signed rank test was used for pairwise comparisons of different phases of infection within each group. Spearman rank test was used to determine correlations between continuous variables. Analyses were performed using GraphPad PRISM 5.0 for Windows (GraphPad Software Inc).
Results
Sociodemographic and clinical characteristics of HIV-infected individuals
The sociodemographic and clinical characteristics of the HIV-infected subjects are shown in Table 1, and the longitudinal assessment of blood CD4+ T-cell counts and viral loads during the course of HIV infection is depicted in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Slow progressors were divided into 2 groups: viremic and aviremic. All study groups were similar with respect to sex, race, and modes of HIV acquisition. Mean age of rapid progressors was lower than that in both normal and viremic slow progressors. Rapid progressors had lower CD4+ T-cell counts during the acute and early phases of infection compared with those observed in normal progressors and lower nadir CD4 counts than those found in both normal and slow progressors. Chronically infected normal progressors had a higher viral load than that observed in both ART-treated rapid progressors and slow progressors, but rapid progressors had the highest peak viremia. There was no significant correlation between CD4+ T-cell counts or viral loads and the monocyte, DC, B-cell populations, and cytokine levels studied here either within groups or among all patients during acute or chronic infection (data not shown).
Sociodemographic and clinical characteristics of HIV-infected individuals
| Characteristic . | Rapid progressors (n = 13) . | Normal progressors (n = 17) . | Viremic slow progressors (n = 7) . | Aviremic slow progressors (n = 6) . | P . |
|---|---|---|---|---|---|
| Age at first visit, y | 33 ± 7 | 39 ± 7 | 43 ± 4 | 41 ± 8 | .02* |
| Sex (male/female) | 12/1 | 17/0 | 7/0 | 3/3 | NS |
| Race (white/other) | 12/1 | 16/1 | 6/1 | 5/1 | NS |
| Route of transmission | 8 MSM, 3 HS, 2 IDU | 16 MSM, 1 IDU | 6 MSM, 1 IDU | 3 MSM, 3 HS | NS |
| CD4+ T cells/mm3 | |||||
| Acute phase | 544 ± 143 | 797 ± 210 | na | na | .002 |
| Early phase | 428 ± 148 | 685 ± 185 | na | na | .001 |
| Chronic phase | 394 ± 231 | 601 ± 271 | 532 ± 130 | 887 ± 122 | NS |
| Nadir | 254 ± 118 | 432 ± 140 | 506 ± 129 | 506 ± 175 | .0005† |
| Viremia, ×103 copies/mL | |||||
| Acute phase | 369 ± 703 | 85.6 ± 132 | na | na | NS |
| Early phase | 127 ± 184 | 80.1 ± 100 | na | na | NS |
| Chronic phase | 8.3 ± 17 | 101 ± 225 | 3.3 ± 1.5 | < 0.005‡ | .0009§ |
| Peak | 570 ± 808 | 202 ± 236 | 8.5 ± 6.5 | 0.065 ± 0.027 | < .0001‖ |
| Characteristic . | Rapid progressors (n = 13) . | Normal progressors (n = 17) . | Viremic slow progressors (n = 7) . | Aviremic slow progressors (n = 6) . | P . |
|---|---|---|---|---|---|
| Age at first visit, y | 33 ± 7 | 39 ± 7 | 43 ± 4 | 41 ± 8 | .02* |
| Sex (male/female) | 12/1 | 17/0 | 7/0 | 3/3 | NS |
| Race (white/other) | 12/1 | 16/1 | 6/1 | 5/1 | NS |
| Route of transmission | 8 MSM, 3 HS, 2 IDU | 16 MSM, 1 IDU | 6 MSM, 1 IDU | 3 MSM, 3 HS | NS |
| CD4+ T cells/mm3 | |||||
| Acute phase | 544 ± 143 | 797 ± 210 | na | na | .002 |
| Early phase | 428 ± 148 | 685 ± 185 | na | na | .001 |
| Chronic phase | 394 ± 231 | 601 ± 271 | 532 ± 130 | 887 ± 122 | NS |
| Nadir | 254 ± 118 | 432 ± 140 | 506 ± 129 | 506 ± 175 | .0005† |
| Viremia, ×103 copies/mL | |||||
| Acute phase | 369 ± 703 | 85.6 ± 132 | na | na | NS |
| Early phase | 127 ± 184 | 80.1 ± 100 | na | na | NS |
| Chronic phase | 8.3 ± 17 | 101 ± 225 | 3.3 ± 1.5 | < 0.005‡ | .0009§ |
| Peak | 570 ± 808 | 202 ± 236 | 8.5 ± 6.5 | 0.065 ± 0.027 | < .0001‖ |
Age, CD4, and viremia are expressed as mean ± SD. Sex, race, and route of transmission were compared using χ2 test. Pairwise comparisons of CD4 for acute and early phases were performed using unpaired t tests. Pairwise comparisons of viremia for acute and early phases were performed using Mann-Whitney tests. Comparisons among all groups (age at first visit, CD4, and viremia in the chronic phase and nadir CD4) were performed with the one-way analysis of variance test. HS, heterosexuals; IDU, intravenous drug users; MSM, men who have sex with men; NS, not significant; and na, not available.
P = .04 and .003 for the comparisons of age between rapid and normal progressors and rapid progressors and viremic slow progressors, respectively, as determined by the Mann-Whitney test.
P = .0008 and .001 and .02 nadir CD4 for the comparison between rapid and normal progressors, rapid and viremic slow progressors, and rapid and aviremic slow progressors, respectively, as determined by the Mann-Whitney test.
Fifty copies/mL corresponds to the detection threshold of the viral load test.
P = .04, .02, .002, and .002 for the comparison of viremia in chronic phase between rapid and normal progressors, normal progressors and viremic slow progressors, normal progressors and aviremic slow progressors, and viremic and aviremic slow progressors, respectively, as determined by the Mann-Whitney test.
P = .006, .0007, .0005, .0004, and .001 for the comparison of peak viremia between rapid progressors and viremic slow progressors, rapid progressors and aviremic slow progressors, normal progressors and viremic slow progressors, normal progressors and aviremic slow progressors, and viremic and aviremic slow progressors, respectively, as determined by the Mann-Whitney test.
Longitudinal monitoring of membrane-bound BLyS expression on mDCs and monocytic precursors in HIV-infected individuals with different rates of disease progression
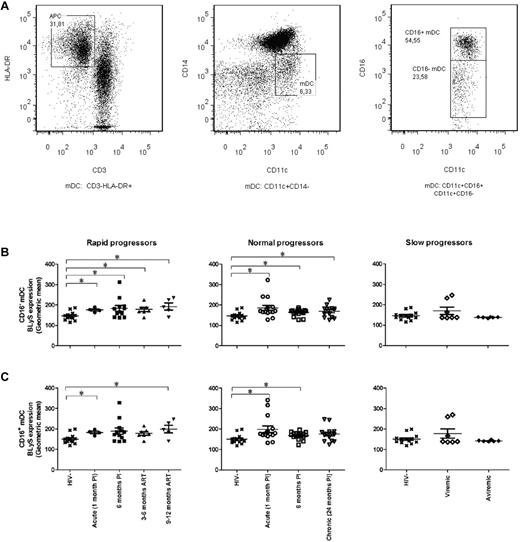
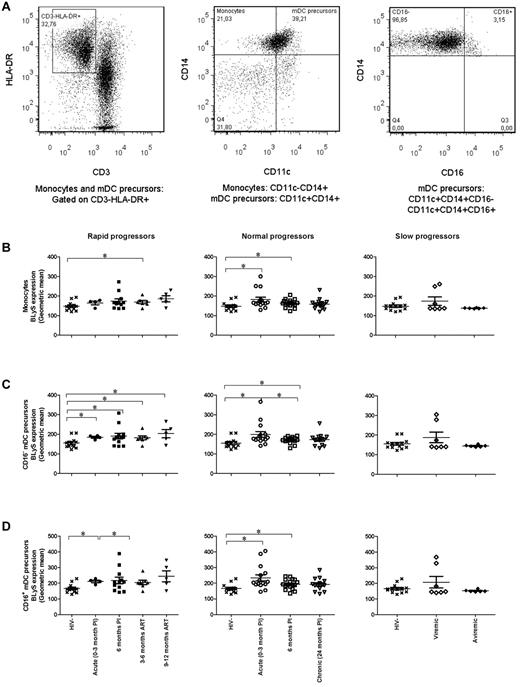
The cell gating experiments shown in Figures 1A and 2A are described in the legends. The levels of BLyS cell-surface expression on blood CD16− mDCs and CD14+CD11c+CD16− mDC precursors were significantly higher in the rapid and normal progressor groups compared with those observed in the HIV-negative donor group (Figures 1B and 2C left and middle panels). The relative levels of BLyS expression on the CD16− mDC population remained high throughout the course of infection, regardless of ART. BLyS membrane expression on CD16+ mDCs, monocytes, and CD14+CD11c+CD16+ mDC precursors in both rapid and normal progressors was also above normal levels; however, this was less sustained than for the CD16− mDC subsets (Figures 1C, 2B, and 2D left and middle panels). On the other hand, the expression levels of membrane BLyS in both viremic and aviremic slow progressors were similar to those observed in the HIV-negative donors (Figures 1–2 right panels).
BLyS expression by mDCs. (A) Cells were gated on total live PBMC and then on CD3−HLA-DR+ cells (mDCs; left panel). MDCs are CD11c+CD14−CD16− or CD11c+CD14−CD16+ (middle and right panels). (B) Membrane BLyS expression in CD16− mDCs of rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (C) Membrane BLyS expression in CD16+ mDCs of rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). The same values are used for HIV-negative donors in the left, middle, and right graphs to show this group as a control. Membrane BLyS expression is defined as the calibrated geometric and compared with the Wilcoxon signed rank and Mann-Whitney U tests for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. *P < .05. PI indicates postinfection; and ART, antiretroviral treatment.
BLyS expression by mDCs. (A) Cells were gated on total live PBMC and then on CD3−HLA-DR+ cells (mDCs; left panel). MDCs are CD11c+CD14−CD16− or CD11c+CD14−CD16+ (middle and right panels). (B) Membrane BLyS expression in CD16− mDCs of rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (C) Membrane BLyS expression in CD16+ mDCs of rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). The same values are used for HIV-negative donors in the left, middle, and right graphs to show this group as a control. Membrane BLyS expression is defined as the calibrated geometric and compared with the Wilcoxon signed rank and Mann-Whitney U tests for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. *P < .05. PI indicates postinfection; and ART, antiretroviral treatment.
BLyS expression by monocytes and mDC precursors. (A) Cells were gated on total live PBMC and then on HLA-DR+CD3− cells (left panel). Monocytes are CD11c−CD14+ (middle panel), and mDC precursors are CD11c+CD14+CD16− or CD11c+CD14+CD16+ (middle and right panels). (B) Membrane BLyS expression in monocytes of rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (C) Membrane BLyS expression in CD16− mDC precursors of rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (D) Membrane BLyS expression in CD16+ mDC precursors of rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). The same values are used for HIV-negative donors in the left, middle, and right graphs to show this group as a control. Membrane BLyS expression is defined as the calibrated geometric and compared with the Wilcoxon signed rank and Mann-Whitney U tests for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. *P < .05. PI, postinfection; ART, antiretroviral treatment.
BLyS expression by monocytes and mDC precursors. (A) Cells were gated on total live PBMC and then on HLA-DR+CD3− cells (left panel). Monocytes are CD11c−CD14+ (middle panel), and mDC precursors are CD11c+CD14+CD16− or CD11c+CD14+CD16+ (middle and right panels). (B) Membrane BLyS expression in monocytes of rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (C) Membrane BLyS expression in CD16− mDC precursors of rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (D) Membrane BLyS expression in CD16+ mDC precursors of rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). The same values are used for HIV-negative donors in the left, middle, and right graphs to show this group as a control. Membrane BLyS expression is defined as the calibrated geometric and compared with the Wilcoxon signed rank and Mann-Whitney U tests for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. *P < .05. PI, postinfection; ART, antiretroviral treatment.
The percentage of mDCs and precursors expressing BLyS in rapid progressors was elevated throughout the study follow-up and despite successful therapy (CD16+ mDC precursors, 9-12 months post-ART, P = .01). As for normal progressors, this percentage was elevated during acute infection and decreased to normal values in the subsequent phases of infection, with a significant drop between the acute and early phases of infection (CD16− mDCs, P = .02; CD16+ mDCs, P = .01; CD16+ mDC precursors, P = .006). We observed no significant difference in the percentage of monocytes expressing BLyS between the HIV-negative and HIV-positive groups at all time points analyzed.
Longitudinal monitoring of plasma concentrations of B-cell growth factors in HIV-infected individuals with different rates of disease progression
Consistent with the above observations, BLyS plasma concentrations were increased in the acute and early phases of infection in both rapid and normal progressors (Figure 3A left and middle panels, respectively). Although BLyS concentrations decreased slightly upon initiation of ART in rapid progressors, it remained elevated in both chronically infected ART-naive normal progressors and viremic slow progressors (Figure 3A middle and right panels). Thus, despite the lack of correlation between plasma BLyS concentration and viral load (data not shown), significantly higher levels of BLyS were observed in the blood of HIV-infected patients with uncontrolled viremia.
Plasma concentration comparisons. (A) BLyS, (B) APRIL, (C) IL-6, and (D) IL-10 in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). The same values are used for HIV-negative donors in the left, middle, and right graphs to show this group as a control. Plasma concentrations were compared with the Wilcoxon signed rank and Mann-Whitney U tests for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. *P < .05; **P < .001; ***P < .0001. PI, postinfection; ART, antiretroviral treatment. Values greater than the mean plus 3 times standard deviation were removed from the analysis.
Plasma concentration comparisons. (A) BLyS, (B) APRIL, (C) IL-6, and (D) IL-10 in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). The same values are used for HIV-negative donors in the left, middle, and right graphs to show this group as a control. Plasma concentrations were compared with the Wilcoxon signed rank and Mann-Whitney U tests for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. *P < .05; **P < .001; ***P < .0001. PI, postinfection; ART, antiretroviral treatment. Values greater than the mean plus 3 times standard deviation were removed from the analysis.
The blood levels of APRIL, IL-6, and IL-10 were higher throughout the course of infection in all HIV-infected individuals compared with those observed in HIV-negative donors (Figure 3B-D). In contrast to BLyS, the secretion of APRIL, IL-6, and IL-10 was also increased in aviremic subjects. Indeed, significant increases in the plasma levels of these cytokines were observed in ART-treated rapid progressors and aviremic slow progressors (Figure 3C-D right panels).
Longitudinal monitoring of blood-circulating B-cell subpopulations in HIV-infected individuals with different rates of disease progression
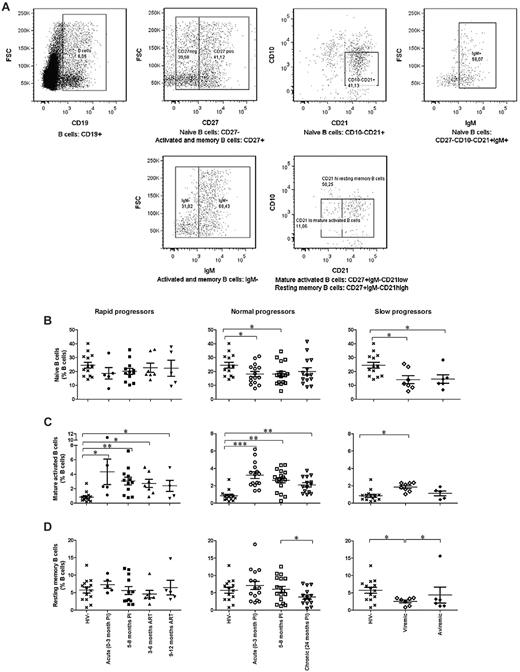
The relative frequencies of naive CD19+CD10−CD27−IgM+ CD21+, mature activated CD19+CD10−CD27+IgM−CD21low, and resting memory CD19+CD10−CD27+IgM−CD21high B cells are depicted in Figure 4. The mean cell percentages of naive B cells in all HIV-infected individuals were lower throughout the course of infection than those observed in HIV-negative donors, reaching statistical significance in both normal and slow progressors (Figure 4B). However, the situation is quite different for mature activated B cells, where the mean percentages remained significantly above normal levels in rapid, normal, and viremic slow progressors (Figure 4C). A significant decrease in memory B-cell frequencies was observed in chronically infected normal progressors and viremic slow progressors (Figure 4D middle and right panels).
Relative frequencies of circulating B-cell populations. (A) Cells were gated on total live PBMC and then on CD19+ cells (left panel). Naive B cells are CD27−CD10−CD21+IgM+ (top panels). Mature activated B cells are CD27+IgM−CD21low, and memory B cells are CD27+IgM−CD21high (bottom panels). (B) Relative frequency of naive B cells in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (C) Relative frequency of mature activated B cells in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (D) Relative frequency of resting memory B cells in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). The same values are used for HIV-negative donors in the left, middle, and right graphs to show this group as a control. Cell population percentages were compared with the Wilcoxon signed rank and Mann-Whitney U tests for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. *P < .05; **P < .001; ***P < .0001. PI, postinfection; ART, antiretroviral treatment.
Relative frequencies of circulating B-cell populations. (A) Cells were gated on total live PBMC and then on CD19+ cells (left panel). Naive B cells are CD27−CD10−CD21+IgM+ (top panels). Mature activated B cells are CD27+IgM−CD21low, and memory B cells are CD27+IgM−CD21high (bottom panels). (B) Relative frequency of naive B cells in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (C) Relative frequency of mature activated B cells in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (D) Relative frequency of resting memory B cells in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). The same values are used for HIV-negative donors in the left, middle, and right graphs to show this group as a control. Cell population percentages were compared with the Wilcoxon signed rank and Mann-Whitney U tests for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. *P < .05; **P < .001; ***P < .0001. PI, postinfection; ART, antiretroviral treatment.
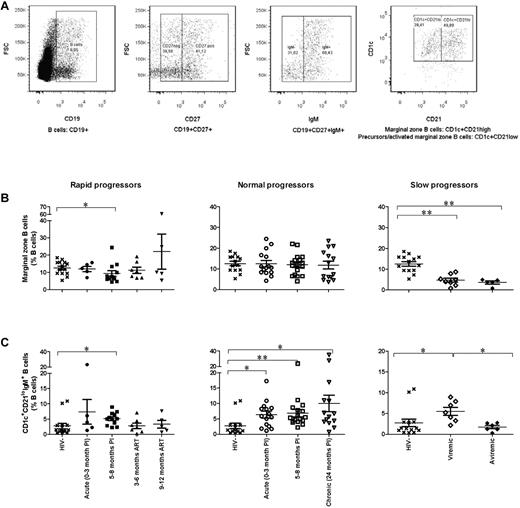
Upon analysis for variations in the relative frequencies of circulating MZ B cells, we identified CD19+CD27+CD1c+IgMhigh cells with 2 different phenotypes, namely, CD21high and CD21low (Figure 5A). CD21high cells correspond to mature MZ B cells, while CD21low cells, which also express CD10, present features of both transitional immature (TI) and MZ B cells22,23 and are thus considered here as precursor/activated MZ-like B cells. Although, the frequencies of mature MZ B cells remained unaltered in both rapid and normal progressors (Figure 5B left and middle panels), the percentages were significantly below normal levels in slow progressors (Figure 5B right panel). On the other hand, the mean cell percentages of precursor/activated MZ-like B cells were increased in the acute and early phases of infection in both rapid and normal progressors (Figure 5C left and middle panels). Although frequencies decreased upon initiation of ART in rapid progressors, they remained elevated in both chronically infected ART-naive normal progressors and viremic slow progressors (Figure 5C middle and right panels). No alterations in blood frequencies of TI B cells were found in the HIV-infected subjects at the time points studied (data not shown). The elevated levels of activated mature B cells and precursor/activated MZ-like B cells observed in the blood of rapid, normal, and viremic slow progressors are indicative of a persisting immune activation, which is further illustrated by high serum levels of IgG found in these individuals over the course of infection (supplemental Figure 2).
Relative frequency of marginal zone–type B cells. (A) Cells were gated on total live PBMC, then on CD19+CD27+IgM+ cells (first, second, and third panels, respectively). The 2 populations are defined as CD1c+CD21high for marginal zone B cells and CD1c+CD21low for precursor/activated MZ-like B cells (fourth panel). (B) Relative frequency of marginal zone B cells in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (C) Relative frequency of CD1c+CD21low B cells in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). The same values are used for HIV-negative donors in the left, middle, and right graphs to show this group as a control. Cell population percentages were compared with the Wilcoxon signed rank and Mann-Whitney U tests for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. *P < .05; **P < .001. PI, postinfection; ART, antiretroviral treatment.
Relative frequency of marginal zone–type B cells. (A) Cells were gated on total live PBMC, then on CD19+CD27+IgM+ cells (first, second, and third panels, respectively). The 2 populations are defined as CD1c+CD21high for marginal zone B cells and CD1c+CD21low for precursor/activated MZ-like B cells (fourth panel). (B) Relative frequency of marginal zone B cells in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). (C) Relative frequency of CD1c+CD21low B cells in rapid progressors (left panel), normal progressors (middle panel), and slow progressors (right panel). The same values are used for HIV-negative donors in the left, middle, and right graphs to show this group as a control. Cell population percentages were compared with the Wilcoxon signed rank and Mann-Whitney U tests for pairwise comparisons of different phases of infection within each group and between the study groups, respectively. *P < .05; **P < .001. PI, postinfection; ART, antiretroviral treatment.
Discussion
We have previously shown that blood levels of mature mDCs in rapid and normal HIV progressors were decreased in the acute phase of HIV infection and remained below normal levels in the subsequent phases of infection beyond successful therapy and despite nonprogressing clinical disease.19 These HIV-infected subjects also had elevated blood levels of CCL2 (MCP-1), CCL19 (MIP-3β), and CCL20 (MIP-3α; J.F., J.P., M.R., unpublished data, July 2010), suggesting that the relatively low levels of mDCs in circulating blood may be secondary to the active recruitment of these cells to peripheral sites. We now report that these mature mDCs are driven to excessive expression of surface BLyS, with a concomitant increase in blood levels of BLyS and B-cell growth factors. A previous study by Stohl et al has shown that BLyS expression levels by blood monocytes correlated with serum autoantibody titers in HIV-infected subjects.24 Our results have also demonstrated that blood monocytes from HIV-infected individuals express BLyS, but the level of expression was less elevated and sustained than that found on mDCs. Nevertheless, a CD11c+CD14+CD16− monocytic DC precursor population, which was increased in the blood of chronically infected untreated HIV normal progressors,19 was found herein to express high levels of BLyS. A murine analog of this precursor population has been described and reported to be mainly recruited to inflammatory sites and linked to the formation of inflammatory “Tip-DCs,” which are a source of cytokines and tumor necrosis factor, of which BLyS is a family member.25 This is suggestive of pressure on the monocytic precursors to drive DC differentiation toward an inflammatory phenotype and indicative of the major involvement of DCs as a source of BLyS. However, BLyS can be produced by T cells in situations of autoimmunity,26 and we observed that BLyS expression on total activated blood T lymphocytes was also elevated throughout the course of infection in both rapid and normal progressors (data not shown).
Consistent with the high mDC BLyS expression levels and concentrations of BLyS and other B-cell growth factors in the blood of HIV rapid and normal progressors, these subjects also presented elevated levels of blood mature activated B cells (Figure 4C) and serum hyperglobulinemia (supplemental Figure 2), as previously reported in clinically progressive HIV infection.27,28 However, the fact that we find these events to occur independently of the plasma viral load and blood CD4+ T-cell counts, and beyond successful ART, resolves previous controversies,2-5 and supports the notion that although a certain control of viral replication and lymphopenia may remediate to some abnormalities, others will carry on as a consequence of the persistent HIV infection and chronic immune activation. It is unlikely that the effects of HIV disease progression on the B-cell compartment result from their direct infection. Indeed, although HIV seems to replicate in CD40-stimulated B cells in vitro, the virus has not yet been shown to infect or replicate in B cells in vivo.29-33 Therefore, our data suggest that the altered DC status we found in rapid and normal progressors may be a major contributor to driving B-cell dysregulations throughout the course of infection, as early as in the acute phase and despite successful therapy. As to whether such events are regulated by the host response and/or modulated by direct and indirect viral effects remains to be established. The chronic inflammatory status in HIV-infected subjects resulting from factors such as HIV products, excessive apoptosis, and products from microbial translocation may be responsible for such events.34,35 In support of this, blood levels of LPS, LBP, and sCD14 were elevated in HIV rapid and normal progressors as soon as in the acute phase and despite successful therapy (supplemental Figure 3).
Interestingly, our study revealed for the first time that a population with features shared by both TI and circulating MZ B cells and presenting a CD1c+CD21lowIgMhighCD10+CD27+ precursor/activated MZ-like B-cell phenotype was increased in the blood of viremic HIV-infected subjects throughout the study follow-up (Figure 5C). This finding is consistent with the fact that TI B cells were found to be increased in the blood of HIV-infected patients with advanced disease.36 However, our staining strategy suggests that these cells maybe driven toward MZ-like B-cell differentiation. Accordingly, TI B cells were shown to give rise to MZ-type B cells in conditions associated with chronic pathology.22 Furthermore, the fact that TI B cells are hyperresponsive to BLyS,22,37 raises the possibility that mDCs overexpressing/producing BLyS may contribute to the skewing of TI B cells toward a MZ phenotype and to driving their expansion. The high level of blood precursor/activated MZ-like B cells, along with the recently described defects of IgM+ memory B cells in HIV-infected individuals1 and with the fact that DCs accumulated in particularly enlarged MZ in HIV Tg mice,10,11 suggests that the DC-mediated B-cell dysregulation process is likely to affect “first-line” B-cell populations. Indeed, given their localization within lymphoid organs and mucosal associated structures, these B cells are highly influenced by DCs and constitute first-line T-independent defense against invading pathogens.38,39 First-line B cells are found in various pathologic conditions such as infection, autoimmunity, and lymphomas,37,40,41 and mDCs may be driving such complications. In addition, the fact that MZ-type B cells play a pivotal role in establishment of germinal center reactions by shuttling blood-borne immune complexes to follicular DCs42 suggests that perturbations at the level of MZ-type B cells are likely to contribute to the defects observed in the generation of adaptive responses and maintenance of memory, which are lost with chronicity in HIV-infected individuals, as shown here and previously.43,44
This study has also raised another important issue, in that the mDC and B-cell compartments are better preserved in slow progressors. Indeed, in contrast to that observed in HIV rapid and normal progressors, blood mature mDC frequencies19 and their BLyS expression levels were unaltered in slow progressors. Interestingly, monocytic DC precursors of a CD11c+CD14+CD16+ phenotype, whose murine analogs are thought to settle in peripheral organs in steady state conditions,25 were found to be significantly increased in the blood of slow progressors19 and to bear normal BLyS levels. This suggests homeostasis and replenishment of a “noninflammatory” DC subpopulation, which appears to be beneficial to the control of disease progression. Accordingly, B-cell dysregulations were found, to a lesser extent, in slow progressors, with hardly any signs detected in the blood of aviremic individuals. Although serum IgG and activated mature B cells as well as precursor/activated MZ B cells were increased in the blood of viremic individuals, serum IgA titers were not elevated in these subjects (supplemental Figure 2), suggesting a certain level of control. Interestingly, aviremic slow progressors did not show increased BLyS serum levels or enhanced frequencies of activated mature and precursor/activated MZ B cells in their blood, supporting the notion that these individuals present a greater level of “control” over disease progression. Surprisingly, both groups of slow progressors presented lower frequencies of circulating mature MZ B cells compared with healthy donors and HIV rapid and normal progressors, suggesting that recruitment of this population to peripheral sites may also be beneficial to the control of disease progression. Although our observations with slow progressors may reflect early stages of malfunction, we favor the possibility by which these events reflect disease control, which might be an active process involving the production of effective HIV-specific antibody responses, such as IgA at mucosal sites. In this view, DCs have been shown to support T-independent IgA class switch recombination in human mucosal sites.39,45 IgA is the most abundant mucosal Ig and aids several functions, including immune-mediated exclusion of both pathogenic and commensal microorganisms.39,45,46 Thus, favoring its production at mucosal sites, which are important for HIV entry, propagation, and perpetuation, is likely beneficial to the host, allowing for a better preservation of membranes. This is consistent with the normal levels of microbial products measured in the blood of slow progressors (supplemental Figure 3). In addition, mucosal HIV-specific IgA levels and neutralizing capacity have been correlated with HIV disease progression.47 Although the issue of “protection” conferred by mucosal HIV-specific IgA remains controversial, in many studies these Igs have been found to neutralize HIV infection and inhibit viral trancytosis in vitro.47-49 Furthermore, a recent study on HIV gp41-specific mucosal IgA, produced by cervical B cells from highly exposed uninfected individuals, demonstrated evidence for hypermutation, suggesting affinity maturation.50
Thus, DCs are involved in maintaining a balance between tolerance and protective immunity at both the innate and adaptive levels, which process is pivotal at mucosal sites where immune homeostasis processes warrant peripheral integrity and where the main battle with HIV takes place. Our results support the notion that the extent to which disease progression is controlled may be linked to the capacity to orchestrate DC inflammatory conditions, as reflected by their BLyS expression levels, which may influence the outcome of B-cell disorders, and this may be involved in modulating the threshold toward disease progression.
Part of this work was presented at XVIII International AIDS Conference, July 22, 2010, Vienna, Austria.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to the participants of the Montreal Primary HIV Infection and LTNP study groups. We also thank Sylvain Gimmig and Laurence Lejeune for invaluable flow cytometry expertise and Brigitte St-Denis for technical assistance. Marie-Pierre Boisvert, Maryse Lainesse, Rebecca Bordi, Véronique Lafontaine, Bader Yassine-Diab, and Younes Chouick are thanked for processing the blood samples.
This work was supported in part by the Réseau SIDA from the Fonds de la Recherche en Santé du Québec (FRSQ). J. Fontaine and H.S. Valcke hold studentships from the Canadian Institute of Health Research (CIHR). M Roger is the recipient of Research Scholar award from the FRSQ.
Authorship
Contribution: J.F. performed the flow cytometry analysis and cytokine measurements, analyzed the data, and wrote the article; J.C.C. performed the plasma LPS, LBP, and sCD14 measurements; H.S.V. performed the serum immunoglobulin measurements; J.P. supervised the project and experiments and wrote the article; and M.R. wrote the article and coordinated all aspects of this study.
Conflict-of-interest declaration: The authors declare no competing financial interests.
A complete list of Montreal Primary HIV Infection and Long-Term Non-Progressor Study Group participants can be found in the supplemental Appendix.
Correspondence: Michel Roger, Département de microbiologie, Hôpital Notre-Dame du CHUM, 1560 Sherbrooke Est, Montréal, QC H2L 4M1, Canada; e-mail: michel.roger@ssss.gouv.qc.ca; or Johanne Poudrier, Département de microbiologie, Hôpital Notre-Dame du CHUM, 1560 Sherbrooke Est, Montréal, QC H2L 4M1, Canada; e-mail: johanne.poudrier@crchum.qc.ca.
References
Author notes
J.P. and M.R. share senior authorship.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal