Abstract
Homoharringtonine (HHT) is a plant alkaloid that inhibits the elongation phase of translation that is currently in clinical trials. Because the intrinsically short-lived antiapoptotic protein myeloid cell leukemia-1 (Mcl-1) has been reported to support the survival of chronic lymphocytic leukemia (CLL) cells, we hypothesized that inhibition of protein synthesis by HHT would decrease Mcl-1 expression and induce apoptosis in CLL. In primary CLL cells, HHT induced significant apoptosis independent of the prognostic characteristics of the patients. This was associated with inhibition of translation and decreased Mcl-1 levels in CLL cells. Mcl-1 reduction was evident as early as 2 hours and continued to decrease in the next 6-8 hours, whereas cell death started in 2 hours and continued to increase for 24 hours. Reduction of the Mcl-1 level was due to translation inhibition and proteasome degradation rather than to transcription inhibition or caspase cleavage. HHT and the transcription inhibitor SNS-032 induced synergistic cell killing. Although stromal cells induced Mcl-1 expression and protected CLL cells from the toxicity of fludarabine, this induction was reversed by HHT, which overcame stromal cell–mediated protection. Thus, these results provide a rationale for clinical development of HHT in CLL as single agent or in combinations.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by the gradual accumulation of abnormal neoplastic B cells in the bone marrow and blood. Although the early asymptomatic stage of CLL does not require treatment, the more aggressive forms of the disease cannot be cured by current treatment options. Current first-line treatment for many patients with CLL incorporates a fludarabine-based combination therapy.1 However, disease relapse invariably occurs after treatment has been discontinued, and almost all patients with CLL will ultimately develop refractory disease. Therefore, new agents targeting the molecular mechanisms of CLL disease progression are highly desired.
Antiapoptotic proteins of the B-cell lymphoma-2 (Bcl-2) family are overexpressed in most cases of CLL, and this overexpression is correlated with resistance to therapy and a poor prognosis.2 Among the Bcl-2 family proteins, myeloid cell leukemia-1 (Mcl-1) has emerged as a significant antiapoptotic protein that promotes the survival of CLL cells both in vitro and in vivo.3 Mcl-1 acts by preventing the proapoptotic proteins Bak and Bax from disrupting the mitochondrial membrane and initiating apoptosis.4 Approaches that reduce Mcl-1 levels in CLL cells by direct methods such as small interfering RNA (siRNA)5 or through indirect approaches to inhibit Mcl-1 transcription resulted in cell death.6,7
Because the inhibition of apoptosis by Bcl-2 family proteins has been recognized as a distinct oncogenic function,8,9 agents that antagonize the actions or diminish the expression of antiapoptotic proteins have been developed to induce apoptosis in CLL cells. These compounds, including oblimersen, an antisense oligonucleotide targeting Bcl-2 mRNA,10 or the BH3 mimetics that interfere with the interaction of the proapoptotic and antiapoptotic proteins of the Bcl-2 family11,12 are currently in clinical trials for treating CLL. A third strategy takes advantage of the fact that the key antiapoptotic protein in CLL, Mcl-1, is intrinsically unstable.13 Transient exposure to flavopiridol, roscovitine, or SNS-032, small molecules that block transcription by inhibiting Cdk9, diminishes Mcl-1 transcripts and protein, with the subsequent induction of apoptosis.6,7,14 These compounds are currently in clinical trials for treating CLL and other B-cell malignancies, and transient exposure schedules have generated responses in fludarabine-resistant disease.15,16
Because Mcl-1 is thought to function as an oncogene on which CLL cells depend for survival, the striking activities generated by transient exposure to these transcription inhibitors may be attributed to the diminished Mcl-1 levels. This encouraged us to explore inhibition of translation, the subsequent step in protein expression, as an additional approach to activate cell death processes.17 Earlier studies of inhibitors of translation showed that cycloheximide (CHX) was cytotoxic to CLL cells in vitro18 and that puromycin enhanced the cytotoxic activity of fludarabine in CLL cells.19 Recently, a new translation inhibitor, silvestrol, was shown to be effective against CLL, acute myelogenous leukemia (AML), and acute lymphoblastic leukemia in vitro20,21 and in an in vivo model of CLL.21 Here, we investigate the mechanism of CLL cell death induced in vitro by homoharringtonine (HHT), a potent inhibitor of translation.
HHT is a cephalotaxine ester derived from the evergreen tree Cephalotaxus harringtonia, which is native to China.22 Initial studies found that HHT inhibits protein synthesis, acting on the first cycle of the elongation phase of translation by preventing substrate binding to the acceptor site on the eukaryotic 60S ribosome subunit and therefore blocking aminoacyl-tRNA binding and peptide bond formation.23 A recent crystal structural study showed that HHT competes with the amino acid side chains of incoming aminoacyl-tRNAs for binding in the A-site cleft in the peptidyl-transferase center in the ribosome.24 Thus, the inhibition of translation by HHT occurs after the recruitment of ribosomes, suggesting that HHT may inhibit both cap-dependent and internal ribosome entry site–dependent translation.
Initial clinical studies of HHT have shown activity in AML,25 and subsequently was shown to induce remission in patients with chronic myelogenous leukemia (CML) alone or with interferon-α or cytarabine.26 Recent studies have suggested that the mechanism of action of HHT in CML may be the inhibition of protein synthesis and the consequent depletion of Bcr-Abl17 as well as short-lived proteins such as Mcl-1 and c-Myc.27,28 HHT is effective in reducing clonogenicity of imatinib-resistant CML cells with Abl kinase domain mutations.17 Currently, HHT is in phase 2/3 clinical trials in the United States in patients with CML resistant to tyrosine kinase inhibitors.29 The combination of HHT with flavopiridol killed CML cells synergistically by down-regulating Bcr-Abl transcripts as well as blocking translation of the remaining message, a strategy termed sequential blockade of gene expression.17 The combination of HHT with the BH3 mimetic ABT-737 killed the CML cells with the T315I mutation in the Abl kinase domain.30
To extend these studies to primary CLL, we investigated the hypothesis that the intrinsically rapid turnover of Mcl-1 in the context of protein synthesis inhibition by HHT would result in the reduction of the Mcl-1 protein and the initiation of apoptosis in CLL cells. As a corollary, we determined whether HHT may act synergistically with transcription inhibitors.
Methods
Patients
Samples from 78 patients with CLL were used in this study. The median age of patients was 61.5 years (range, 38-80 years), and 52 patients were men. The median white blood cell count was 61.1 × 109/L (range, 5.0-33.82 × 109/L); the median lymphocyte percentage was 90.5% (range, 23%-98%). The investigation was approved by The University of Texas M.D. Anderson Cancer Center Institutional Review Board, and all patients provided informed consent for use of their cells for in vitro studies, in accordance with the Declaration of Helsinki.
Isolation of CLL lymphocytes
Peripheral blood samples (∼ 10 mL) from the patients with CLL were collected in heparinized Vacutainer tubes and centrifuged at 1500 rpm for 10 minutes to separate the plasma. CLL cells were isolated by Ficoll density-gradient centrifugation and were cultured at 1 × 107 cells/mL in RPMI 1640 medium containing 10% autologous plasma.6
Stromal cell lines
The murine stromal cell line, KUSA-H1, and the human mesenchymal cell line StromaNKtert were provided by Dr Jan Burger from our institution and were originally purchased from the Riken Cell Bank. KUSA-H1 was maintained in RPMI 1640 medium supplemented with 2mM l-glutamine and 10% fetal bovine serum. The StromaNKtert cells were maintained in Minimum Essential Medium α supplemented with 12.5% fetal bovine serum, 12.5% human serum (Sigma-Aldrich), 1μM hydrocortisone (Sigma-Aldrich), and 100μM 2-mercaptoethanol (Sigma-Aldrich). For coculture experiments, stromal cells were seeded the day before the experiment onto 24-well plates at 5 × 104 cells/mL/well. CLL cells were added onto the stromal layers at a ratio of 100:1 (CLL/stromal).
Materials
HHT, purchased from Sigma-Aldrich, was dissolved in sterile phosphate-buffered saline (PBS) at 1mM and stored at −20°C. SNS-032 was provided by R. Hawtin, Sunesis Pharmaceuticals. Fludarabine (9-β-D-arabinofuranosyl-2-fluoroadenine) (Sigma-Aldrich) was prepared as a 2-mM stock solution in sterile water and stored at −20°C. [4,5-3H]Leucine (170 Ci/mmol [6.29 × 1012 Bq/mmol]) was purchased from ICN Pharmaceuticals. Annexin V–fluorescein isothiocyanate Apoptosis Detection Kits were obtained from BD Biosciences. Propidium iodide (PI) solution (1 mg/mL) was from Sigma-Aldrich Inc. Z-VAD-FMK (methyl ester) was purchased from MP Biomedicals. MG-132 was purchased from EMD Biosciences. Antibodies were from the following sources: Mcl-1 (S-19) and Bcl-2 (100) were from Santa Cruz Biotechnology, poly (adenosine diphosphate-ribose) polymerase (PARP) was from Biomol International Inc, X-linked inhibitor of apoptosis protein (XIAP) was from BD Biosciences Pharmingen, Alexa Fluor 680 goat anti–mouse immunoglobulin G (IgG) or IgM was from Invitrogen, and IRDye 800CW goat anti–rabbit IgG was from LI-COR Biosciences.
Cell viability
Cell death in CLL cells was evaluated by flow cytometric analysis with the use of annexin V–PI double staining. CLL cells (1 × 106 cells) were washed twice with PBS, resuspended in 100 μL of binding buffer (BD Biosciences) with 5 μL of annexin V–fluorescein isothiocyanate, and incubated for 15 minutes in the dark at room temperature before the addition of 400 μL of binding buffer and 5 μL of 50 μg/mL PI. Samples were analyzed with a Becton Dickinson FACSCalibur flow cytometer. Data acquisition and analysis were performed with the Cell Quest program (Becton Dickinson). Cells stained positive for annexin V, PI, or both were considered dead cells.
Mitochondrial membrane integrity
Mitochondrial membrane integrity was detected by DiOC6(3) staining assay. DiOC6(3) (5 μL of 10μM; Invitrogen) was added to 1 × 106 CLL cells in 1 mL of complete tissue culture medium (with 10% serum), and samples were incubated for 15 minutes at 37°C in the dark. PI solution (5 μL of 50 μg/mL stock) was added, and samples were incubated for another 5 minutes at room temperature in the dark. Samples were analyzed with a Becton Dickinson FACSCalibur flow cytometer. Cells that lost mitochondrial membrane integrity were negative for DiOC6(3) staining.
Immunoblotting
Inhibition of protein synthesis
Protein synthesis was measured by [3H]Leucine incorporation.6 Radioactivity was quantitated by a scintillation counter (Packard). All samples were tested in triplicate.
RNA isolation and real-time quantitative polymerase chain reaction
Total cellular RNA was isolated from primary CLL cells with the use of the RNeasy mini kit (QIAGEN) with DNase digestion to completely remove the genomic DNA. Total RNA (20-50 ng) was used for the one-step real-time polymerase chain reaction (PCR) in TaqMan One-Step RT-PCR Master Mix (Applied Biosystems). Each PCR was performed in a 25-μL volume on a 96-well optical reaction plate for 30 minutes at 48°C for reverse transcription reaction, followed by 10 minutes at 95°C for initial denaturing, then followed by 40 cycles of 95°C for 15 seconds and 60°C for 2 minutes in the 7900HT Sequence Detection System (Applied Biosystems). The relative gene expression was analyzed by the Comparative Ct method with the use of 18s ribosomal RNA as an endogenous control after it had been confirmed that the efficiencies of the target and the endogenous control amplifications were approximately equal. All the primers and probes and reverse transcription (RT)–PCR reaction buffers were purchased from Applied Biosystems.
Analysis of drug combinations
The combination effect of HHT and SNS-032 was assessed by annexin V–PI assays after CLL cells had been incubated for 24 hours with each individual drug and in combination. The combinations were done in a constant ratio of 1:2 (HHT:SNS-032) with the use of 5 concentrations for each drug that achieved 10%-50% inhibition alone. The effects of combinations were evaluated with the CalcuSyn software (Biosoft), which was developed on the basis of the median-effect method created by Chou and Talalay.32 A calculated combination index of < 1 indicates synergy, 1 indicates additive, and > 1 indicates antagonism.
The combination effect of HHT and MG-132 was evaluated with the fractional 2-drug analysis to compare the expected and observed cell death for the combination, measured by annexin V–P staining. After subtracting the time-matched endogenous cell death, the expected cell survival for an additive combination was calculated by multiplying the percentage of cells surviving HHT treatment (100% − percentage of HHT-induced cell death) by the percentage of cells surviving MG-132 treatment (100% − percentage of MG-132–induced cell death). Then the expected cell death for an additive combination was calculated by 100% − percentage of expected survival.
Polysome profiling analysis
CLL cells were incubated at the presence or absence of HHT for 2 hours. Three minutes before harvesting, cycloheximide was added to the cells to a final concentration of 100 μg/mL. The cells were collected and washed twice with cold PBS with 100 μg/mL cycloheximide. The pellets were then lysed with polysome lysis buffer (10mM Tris [tris(hydroxymethyl)aminomethane], pH 7.4, 1% triton X-100, 15mM MgCl2, 100 μg/mL cycloheximide, 0.3M NaCl, and 1 mg/mL heparin), loaded on top of the 10%-50% sucrose gradients, and centrifuged for 3 hours in a SW40 rotor at 35 000 rpm at 4°C. Fractions were collected, and the OD260 was measured in each fraction. Total RNA was precipitated from the fractions by guanidine HCL and isopropanol and was purified with the RNeasy mini kit. Relative amounts of Mcl-1 and β-actin mRNAs were measured by real-time RT-PCR.
Statistical analysis
Statistical analysis was performed with Student t test in GraphPad Prism software (GraphPad Software Inc). P < .05 was considered to be statistically significant.
Results
HHT induces apoptosis in CLL cells
Primary CLL cells were incubated with 50-400nM HHT for 6-24 hours, and apoptosis was quantitated by annexin V–PI staining. Although the viability of control cells was stable, HHT at concentrations as low as 50nM induced significant apoptosis in CLL cells after a 12-hour treatment (Figure 1A left). The concentration that inhibits 50% (IC50) of HHT after 24 hours of treatment was 105nM (Figure 1A right). Fifty-one patient samples were treated with 100nM HHT for 24 hours (Figure 1B). Statistical analysis showed that HHT induced significant apoptosis in these samples (P < .0001, paired t test), although variation existed among persons, indicating the heterogeneity of sensitivities of CLL samples to HHT. CLL is a heterogeneous disease with a variable clinical course that is associated with diverse responses to standard therapy. Unmutated immunoglobulin heavy-chain variable-region (IgVH) genes and expression of the ζ chain–associated protein kinase 70 (ZAP-70) are common indicators for poor prognosis for patients.33 High level of β-2-microglobulin was associated with poor responses in the regimen combining fludarabine, cyclophosphamide, and rituximab.1 Moreover, deletions of the short arm of chromosome 17 (del 17p13), which encodes the TP53 gene, or the long arm of chromosome 11 (del 11q23), which encodes the ataxia telangiectasia mutated (ATM) gene, are related to resistance to therapy and a poor prognosis.34 There was no significant difference between cells from patients with either favorable or poor prognostic features or treatment history (Figure 1C), indicating that HHT has the potential for overcoming resistance to current therapy. Although our limited samples from patients with p53 or ATM deletion did not have significant difference from the patients without these cytogenetic abnormalities (data not shown), more samples may be needed for a more accurate assessment in these groups. Only one patient was refractory to fludarabine treatment, which is insufficient for a statistical analysis.
HHT induced apoptosis in CLL cells. (A) HHT induced apoptosis in CLL cells in a time- and concentration-dependent manner. CLL cells were incubated with 0nM (○), 50nM (□), 100nM (▵), 200nM (□), or 400nM (◇) HHT for 6, 12, or 24 hours (left). Apoptosis was detected by annexin V–PI double staining and quantitated by flow cytometric analysis. Each concentration represents the mean ± SEM of 8 patient samples. HHT induced apoptosis in CLL cells with an IC50 of 105nM at 24 hours (right). CLL cells were incubated with various concentrations of HHT for 24 hours. Each data point represents the mean ± SEM of 28 patient samples. (B) HHT (100nM) induced significant apoptosis in CLL cells at 24 hours (P < .0001, paired t test). CLL cells from 51 patients were mock-incubated or with 100nM HHT for 24 hours, and apoptosis was detected by annexin V–PI staining. Bars indicate medium values of each group; 17.5% cell death for the control group and 59.9% for the HHT-treated group. (C) HHT induced similar cell death regardless of patient characteristics and treatment history. Cell death induced by 100nM HHT after 24-our incubation was compared in CLL cells from patients with poor (□) or favorable (▩) prognostic factors. A P ≤ .05 from an unpaired t test was considered significant. Induced cell death was the difference of cell death between HHT-treated samples and that of time-matched controls. Each data point represents the mean ± SEM. (D) HHT-induced apoptosis required the continuous presence of HHT. CLL cells were incubated with 200nM HHT (□) for 6, 12, or 24 hours and compared with control (○). At 6 hours, a portion of the culture was washed into drug-free medium (▵). Apoptosis was detected by annexin V–PI staining. Each data point represents the mean ± SEM of 4 patient samples. (E) HHT induced loss of mitochondrial transmembrane potential. CLL cells were incubated with 100nM HHT for 24 hours. Mitochondrial transmembrane potential and viability were measured by DiOC6(3) and PI double staining. Representative figures from 3 independent experiments were shown.
HHT induced apoptosis in CLL cells. (A) HHT induced apoptosis in CLL cells in a time- and concentration-dependent manner. CLL cells were incubated with 0nM (○), 50nM (□), 100nM (▵), 200nM (□), or 400nM (◇) HHT for 6, 12, or 24 hours (left). Apoptosis was detected by annexin V–PI double staining and quantitated by flow cytometric analysis. Each concentration represents the mean ± SEM of 8 patient samples. HHT induced apoptosis in CLL cells with an IC50 of 105nM at 24 hours (right). CLL cells were incubated with various concentrations of HHT for 24 hours. Each data point represents the mean ± SEM of 28 patient samples. (B) HHT (100nM) induced significant apoptosis in CLL cells at 24 hours (P < .0001, paired t test). CLL cells from 51 patients were mock-incubated or with 100nM HHT for 24 hours, and apoptosis was detected by annexin V–PI staining. Bars indicate medium values of each group; 17.5% cell death for the control group and 59.9% for the HHT-treated group. (C) HHT induced similar cell death regardless of patient characteristics and treatment history. Cell death induced by 100nM HHT after 24-our incubation was compared in CLL cells from patients with poor (□) or favorable (▩) prognostic factors. A P ≤ .05 from an unpaired t test was considered significant. Induced cell death was the difference of cell death between HHT-treated samples and that of time-matched controls. Each data point represents the mean ± SEM. (D) HHT-induced apoptosis required the continuous presence of HHT. CLL cells were incubated with 200nM HHT (□) for 6, 12, or 24 hours and compared with control (○). At 6 hours, a portion of the culture was washed into drug-free medium (▵). Apoptosis was detected by annexin V–PI staining. Each data point represents the mean ± SEM of 4 patient samples. (E) HHT induced loss of mitochondrial transmembrane potential. CLL cells were incubated with 100nM HHT for 24 hours. Mitochondrial transmembrane potential and viability were measured by DiOC6(3) and PI double staining. Representative figures from 3 independent experiments were shown.
To test whether the effect of HHT requires a continuous incubation, CLL cells were incubated with 200nM HHT. At 6 hours, a portion of the culture was washed into drug-free medium, and apoptosis was assayed at 6, 12, and 24 hours. The results showed that no additional apoptosis was induced after CLL cells were washed into fresh medium, whereas cell killing increased with time of continuous incubation with HHT (Figure 1D).
Loss of mitochondrial transmembrane potential occurs in early apoptosis which is associated with the release of cytochrome c and regulated by Bcl-2 family proteins.35 DiOC6(3), a membrane-permeable lipophilic cationic fluorochrome that accumulates in mitochondria in live cells, was used as a probe of mitochondrial transmembrane potential. DiOC6(3) staining showed that 100nM HHT induced loss of mitochondrial transmembrane potential at 24 hours, leading to apoptosis (Figure 1E). This indicated that HHT-induced apoptosis was associated with loss of mitochondrial membrane integrity and transmembrane potential, probably by mitochondrial translocation of proapoptotic proteins such as Bax or Bak or both.
HHT reduced Mcl-1 expression
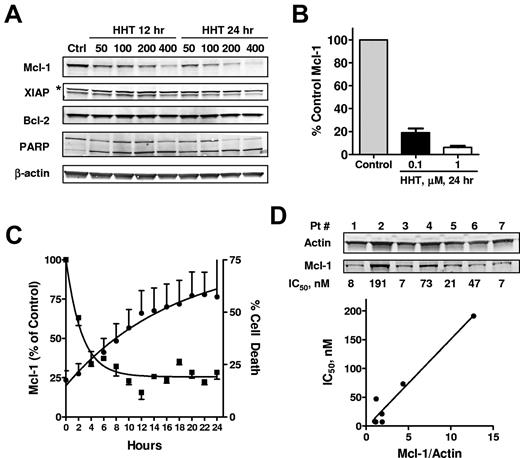
To study the effect of HHT on proapoptotic and antiapoptotic proteins, CLL cells were incubated with 50-400nM HHT for 12 and 24 hours, and PARP, Mcl-1, XIAP, and Bcl-2 were analyzed by immunoblotting. A decrease in Mcl-1 protein levels was dependent on both time and concentration (Figure 2A). Quantitation of Mcl-1 in 5 samples treated with 0.1μM HHT and 3 samples treated with 1μM HHT showed that the decrease in Mcl-1 was statistically significant compared with levels in time-matched controls (P < .01) (Figure 2B). By contrast, Bcl-2 levels did not decrease significantly. XIAP levels (lower bands) appeared to decrease but not as significantly as Mcl-1. PARP cleavage was evident in all samples, indicating the activation of apoptosis (Figure 2A). HHT did not change the levels of other Bcl-2 family proteins, including Bid, Noxa, BAK, and BAX (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). There was a slight but consistent decrease of Bim protein in all 3 CLL samples tested, probably related to its half-life of ∼ 8 hours.36 Puma was induced in 1 of 3 samples but remained unchanged in the other 2 samples. A detailed time course study showed that Mcl-1 reduction was evident as early as 2 hours and continued to decrease in the next 6-8 hours; cell death started in 2 hours and continued to increase for 24 hours (Figure 2C).
HHT induced down-regulation of Mcl-1. (A) HHT induced down-regulation of Mcl-1 protein levels. CLL cells were incubated with 50, 100, 200, or 400nM HHT for 12 or 24 hours, when the cells were collected and lysed. Proteins such as PARP, Mcl-1, XIAP, and Bcl-2 were analyzed by immunoblotting. β-Actin was used as a loading control; * indicates nonspecific binding. (B) The down-regulation of Mcl-1 by HHT was significant in multiple samples. The Mcl-1 protein levels (mean ± SEM) were normalized to β-actin and quantitated in 5 (0.1μM HHT) and 3 (1μM HHT) patient samples. (C) Time course of HHT-induced reduction of Mcl-1 protein and apoptosis. CLL cells were incubated with HHT for 24 hours. Mcl-1 level (■) was quantitated by immunoblotting (n = 2) and cell death (●) was measured by annexin/PI every 2 hours (n = 6). (D) Sensitivity of CLL cells to HHT correlated with the basal Mcl-1 expression in each patient sample. Seven CLL samples were incubated with increasing concentrations of HHT for 24 hours. Cell death was measured by annexin/PI after 24 hours and correlated to basal Mcl-1 levels, quantitated by immunoblotting.
HHT induced down-regulation of Mcl-1. (A) HHT induced down-regulation of Mcl-1 protein levels. CLL cells were incubated with 50, 100, 200, or 400nM HHT for 12 or 24 hours, when the cells were collected and lysed. Proteins such as PARP, Mcl-1, XIAP, and Bcl-2 were analyzed by immunoblotting. β-Actin was used as a loading control; * indicates nonspecific binding. (B) The down-regulation of Mcl-1 by HHT was significant in multiple samples. The Mcl-1 protein levels (mean ± SEM) were normalized to β-actin and quantitated in 5 (0.1μM HHT) and 3 (1μM HHT) patient samples. (C) Time course of HHT-induced reduction of Mcl-1 protein and apoptosis. CLL cells were incubated with HHT for 24 hours. Mcl-1 level (■) was quantitated by immunoblotting (n = 2) and cell death (●) was measured by annexin/PI every 2 hours (n = 6). (D) Sensitivity of CLL cells to HHT correlated with the basal Mcl-1 expression in each patient sample. Seven CLL samples were incubated with increasing concentrations of HHT for 24 hours. Cell death was measured by annexin/PI after 24 hours and correlated to basal Mcl-1 levels, quantitated by immunoblotting.
To investigate the relation of heterogeneous responses to HHT and basal Mcl-1 level, 7 CLL samples were treated with increasing concentrations of HHT, and cell death was measured by annexin/PI staining at 24 hours. The IC50 values for inducing cell death were calculated for each sample and correlated to the basal Mcl-1 expression. Figure 2D showed that Mcl-1 expression varied in each sample and that the IC50 of HHT correlated significantly with the basal Mcl-1 expression (R2 = 0.94, P = .0003). Further studies indicated that the IC50s are related to the HHT concentrations required to reduce Mcl-1 to a threshold level (data not shown). We are currently working on defining this threshold level of Mcl-1 for inducing CLL cell death.
HHT inhibited protein synthesis
[3H]Leucine incorporation assays showed that HHT inhibited global translation and was ∼ 100 times more potent than CHX (Figure 3A). This correlated to a more potent induction of apoptosis in CLL (Figure 3B). Both compounds down-regulated Mcl-1, but HHT was more potent than CHX in inducing PARP cleavage and apoptosis (Figure 3C). A polysome profile analysis showed that HHT shifted the distribution of Mcl-1 mRNA from the heavy polysomes to the lighter polysomes, indicating blocking of translation (Figure 3D). Similar redistribution was seen in β-actin mRNA, consistent with a global inhibition of protein synthesis by HHT. These results were similar to those reported by Robert et al28 in an Eμ-Myc mouse model. The rapid delimiting of Mcl-1 protein in contrast to the stable β-actin level (Figures 2A, 3C) probably reflects the rapid turnover of Mcl-1 protein versus that of β-actin, which has a longer half-life. Thus, these results indicated that HHT inhibited protein synthesis, caused the down-regulation of Mcl-1, and induced apoptosis in CLL cells.
HHT inhibited protein synthesis. (A) HHT was a more potent inhibitor of protein synthesis than CHX. CLL cells were incubated with 0.1, 1, 10, or 100μM HHT (□) or CHX (○) for 24 hours. Then, 1 μCi/mL (3.7 × 107 Bq/L) [3H]leucine was added to the culture for 1 hour, and radioactivity was determined by liquid scintillation counting (A). Apoptosis was detected by annexin V–PI staining (B). Each data point represents the mean ± SEM of 3 patient samples. Concentration-dependent PARP cleavage and loss of Mcl-1 were detected by immunoblotting (C). (D) Polysomal profiling analysis of CLL cells treated with HHT. CLL cells were incubated in HHT for 2 hours before lysed and resolved on a 10%-50% sucrose gradient. Twenty-two fractions were collected from each sample. The absorbance at 260 nm (OD260) was presented in the top panel. Solid line indicates control; dashed line, HHT treated. Mcl-1 and β-actin mRNA were quantitated in every other fraction and presented as percentage of total in all fractions. The analysis was repeated in 3 CLL samples with similar results. Results from one representative CLL sample were shown. Open bars indicate controls; solid bars, treatment with 200nM HHT.
HHT inhibited protein synthesis. (A) HHT was a more potent inhibitor of protein synthesis than CHX. CLL cells were incubated with 0.1, 1, 10, or 100μM HHT (□) or CHX (○) for 24 hours. Then, 1 μCi/mL (3.7 × 107 Bq/L) [3H]leucine was added to the culture for 1 hour, and radioactivity was determined by liquid scintillation counting (A). Apoptosis was detected by annexin V–PI staining (B). Each data point represents the mean ± SEM of 3 patient samples. Concentration-dependent PARP cleavage and loss of Mcl-1 were detected by immunoblotting (C). (D) Polysomal profiling analysis of CLL cells treated with HHT. CLL cells were incubated in HHT for 2 hours before lysed and resolved on a 10%-50% sucrose gradient. Twenty-two fractions were collected from each sample. The absorbance at 260 nm (OD260) was presented in the top panel. Solid line indicates control; dashed line, HHT treated. Mcl-1 and β-actin mRNA were quantitated in every other fraction and presented as percentage of total in all fractions. The analysis was repeated in 3 CLL samples with similar results. Results from one representative CLL sample were shown. Open bars indicate controls; solid bars, treatment with 200nM HHT.
HHT did not inhibit transcription and was synergistic with SNS-032
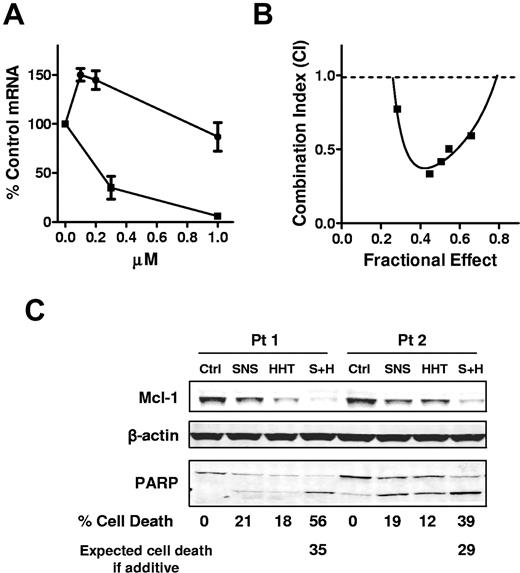
To study whether HHT affects transcription, we investigated the effect of HHT on Mcl-1 RNA levels compared with a transcription inhibitor SNS-032.7 CLL cells were incubated with 0.1, 0.2, or 1μM HHT or with 0.3 or 1μM SNS-032 for 24 hours. The mRNA levels of Mcl-1 were measured by real-time RT-PCR. HHT did not decrease the mRNA levels of Mcl-1, as did SNS-032 (Figure 4A). These results provided a rationale to sequentially inhibit the 2 steps of Mcl-1 synthesis: transcription and translation. To test whether HHT and SNS-032 are synergistic in killing CLL cells, CLL cells were incubated for 24 hours with HHT and SNS-032 alone or in a constant ratio of 1:2 (HHT/SNS) with the use of 5 concentrations, ranging from 25nM to 150nM HHT. The results showed that HHT was synergistic with SNS-032 at all concentrations tested (combination index < 1) (Figure 4B). An immunoblotting analysis showed that both compounds partially reduced Mcl-1 protei, whereas the combination depleted the Mcl-1 level more than any drug alone (Figure 4C). This was associated with a greater cleavage of PARP and induction of more cell death than the additive effect of the 2 drugs, indicating synergy.
HHT did not inhibit transcription and was synergistic with SNS-032. (A) HHT did not inhibit transcription as did SNS-032. CLL cells were incubated with 0.1, 0.2, or 1μM HHT (●) or with 0.3 or 1μM SNS-032 (■) for 24 hours. The mRNA levels of Mcl-1 were measured by real-time RT-PCR, each performed in duplicate, and compared with time-matched controls. The 18s RNA was used as normalize for loading. Each data point represents the mean ± SEM of 3 patient samples. (B) HHT was synergistic with SNS-032 in killing CLL cells. CLL cells were incubated with 25, 50, 75, 100, 125, or 150nM HHT for 24 hours with 50, 100, 150, 200, 250, or 300nM SNS-032 or with a combination of HHT and SNS-032 at a ratio of 1:2. Apoptosis was detected by annexin V–PI staining. Each data point represents the mean of 4 patient samples. The combination index was calculated by CalcuSyn with the use of the median effect method. (C) Combination of HHT and SNS-032 deplete more Mcl-1 than single drug alone. CLL cells were incubated with 100nM SNS-032 or 50nM HHT alone or in combination. PARP and Mcl-1 proteins were analyzed by immunoblotting. Cell death was measured by annexin/PI staining. Expected cell death was calculated as described in “Methods.” Two representative results from 4 CLL samples were shown. Ctrl indicates control; SNS, SNS-032; S+H, combination of SNS-032 and HHT.
HHT did not inhibit transcription and was synergistic with SNS-032. (A) HHT did not inhibit transcription as did SNS-032. CLL cells were incubated with 0.1, 0.2, or 1μM HHT (●) or with 0.3 or 1μM SNS-032 (■) for 24 hours. The mRNA levels of Mcl-1 were measured by real-time RT-PCR, each performed in duplicate, and compared with time-matched controls. The 18s RNA was used as normalize for loading. Each data point represents the mean ± SEM of 3 patient samples. (B) HHT was synergistic with SNS-032 in killing CLL cells. CLL cells were incubated with 25, 50, 75, 100, 125, or 150nM HHT for 24 hours with 50, 100, 150, 200, 250, or 300nM SNS-032 or with a combination of HHT and SNS-032 at a ratio of 1:2. Apoptosis was detected by annexin V–PI staining. Each data point represents the mean of 4 patient samples. The combination index was calculated by CalcuSyn with the use of the median effect method. (C) Combination of HHT and SNS-032 deplete more Mcl-1 than single drug alone. CLL cells were incubated with 100nM SNS-032 or 50nM HHT alone or in combination. PARP and Mcl-1 proteins were analyzed by immunoblotting. Cell death was measured by annexin/PI staining. Expected cell death was calculated as described in “Methods.” Two representative results from 4 CLL samples were shown. Ctrl indicates control; SNS, SNS-032; S+H, combination of SNS-032 and HHT.
Proteasome degradation contributes to Mcl-1 down-regulation induced by HHT
Mcl-1 is cleaved by caspase-3 in tumor necrosis factor–related apoptosis-inducing ligand–mediated apoptosis.37 Thus, we studied whether HHT-induced Mcl-1 down-regulation is also due to caspase-3 cleavage. CLL cells were incubated with 0.1 or 1μM HHT for 24 hours with or without the addition of 100μM Z-VAD-FMK, a pan-caspase inhibitor. PARP cleavage was partly blocked by Z-VAD-FMK (Figure 5A), and annexin V positivity was also reduced by Z-VAD-FMK (Figure 5B), indicating inhibition of apoptosis. However, Mcl-1 down-regulation was still observed in response to HHT (Figure 5A). These results indicated that HHT-induced Mcl-1 down-regulation was probably an early event of Mcl-1 synthesis inhibition, rather than a consequence of caspase-3 cleavage. As a secondary effect, the reduction of Mcl-1 levels would activate caspase 3, which may in turn cleave Mcl-1, leading to its further elimination.
HHT-induced Mcl-1 down-regulation was largely because of proteasome degradation. (A) HHT-induced Mcl-1 down-regulation was not a result of caspase-3 cleavage. CLL cells were incubated with 0.1 or 1μM HHT for 24 hours with or without the addition of 100μM Z-VAD-FMK. PARP and Mcl-1 proteins were analyzed by immunoblotting. (B) Apoptosis was detected by annexin V–PI staining. Each data point represents the mean ± SEM of 4 patient samples; □ indicates HHT only; ■, HHT with 100μM Z-VAD-FMK. (C) HHT-induced Mcl-1 down-regulation was largely because of proteasome degradation. CLL cells were incubated with 0.1 or 1μM HHT for 24 hours with or without the addition of 10μM MG-132. PARP and Mcl-1 proteins were analyzed by immunoblotting.
HHT-induced Mcl-1 down-regulation was largely because of proteasome degradation. (A) HHT-induced Mcl-1 down-regulation was not a result of caspase-3 cleavage. CLL cells were incubated with 0.1 or 1μM HHT for 24 hours with or without the addition of 100μM Z-VAD-FMK. PARP and Mcl-1 proteins were analyzed by immunoblotting. (B) Apoptosis was detected by annexin V–PI staining. Each data point represents the mean ± SEM of 4 patient samples; □ indicates HHT only; ■, HHT with 100μM Z-VAD-FMK. (C) HHT-induced Mcl-1 down-regulation was largely because of proteasome degradation. CLL cells were incubated with 0.1 or 1μM HHT for 24 hours with or without the addition of 10μM MG-132. PARP and Mcl-1 proteins were analyzed by immunoblotting.
Prior studies have shown that Mcl-1 is degraded by the proteasome.38 To determine whether the decrease in Mcl-1 was due to proteasome degradation when Mcl-1 synthesis was inhibited by HHT, CLL cells were incubated with 0.1 or 1μM HHT for 24 hours with or without 10μM MG-132, a proteasome inhibitor. In the presence of MG-132, the Mcl-1 level was retained to a greater extent compared with samples treated with HHT only, (Figure 5C), indicating that Mcl-1 was stabilized by the proteasome inhibitor. These data suggested that Mcl-1 was degraded by a proteasome-dependent mechanism after its synthesis was blocked by HHT.
Because the action of HHT depends on the rapid degradation of Mcl-1, we expect that MG-132, the agent that blocks Mcl-1 degradation, would antagonize the action of HHT. To determine the combination effect of HHT and MG-132, CLL cells were incubated 24 hours with HHT and MG-132 alone or in combinations, and toxicity was measured by annexin V–PI staining. HHT alone at 50 and 100nM induced 24% and 32% of cell death, respectively (Table 1). Combination of HHT and MG-132 resulted in substantially less cell death than the expected additive effect in a fractional 2-drug combination analysis. Thus, these results confirmed the antagonistic actions of HHT and MG-132, further suggesting that the depletion of short-lived antiapoptotic proteins is the main mechanism of HHT-induced cell death in CLL. These results were consistent with a previous report in a lymphoma cell model.28 Thus, we speculate that because of the potential for antagonism between their mechanisms, it would be inappropriate to combine inhibitors of translation/transcription with proteasome inhibitors in the clinic. Further experiments with bortezomib, the proteosome inhibitor that is currently used in the clinic would be useful to test this hypothesis.
Cytotoxicity of HTT and MG-132, alone and in combinations
| MG-132 alone, % . | HHT, 50nM . | HHT, 100nM . | |||
|---|---|---|---|---|---|
| Expected, % . | Observed, % . | Expected, % . | Observed, % . | ||
| MG-132, 0μM | 0 | 24 | 32 | ||
| MG-132, 1μM | 67 | 75 | 42 | 77 | 50 |
| MG-132, 10μM | 72 | 78 | 41 | 81 | 47 |
| MG-132, 100μM | 83 | 87 | 48 | 88 | 54 |
| MG-132 alone, % . | HHT, 50nM . | HHT, 100nM . | |||
|---|---|---|---|---|---|
| Expected, % . | Observed, % . | Expected, % . | Observed, % . | ||
| MG-132, 0μM | 0 | 24 | 32 | ||
| MG-132, 1μM | 67 | 75 | 42 | 77 | 50 |
| MG-132, 10μM | 72 | 78 | 41 | 81 | 47 |
| MG-132, 100μM | 83 | 87 | 48 | 88 | 54 |
CLL cells were treated with HHT or MG-132, alone or in combinations for 24 hours, cytotoxicity (percentage of cell death) was determined by annexin V–PI staining. Data represent the average of 3 independent experiments. The toxicities of the combination of HHT and MG-132 (observed) were compared with the expected cell death for an additive combination (expected).
HHT overcomes stromal cell–mediated protection
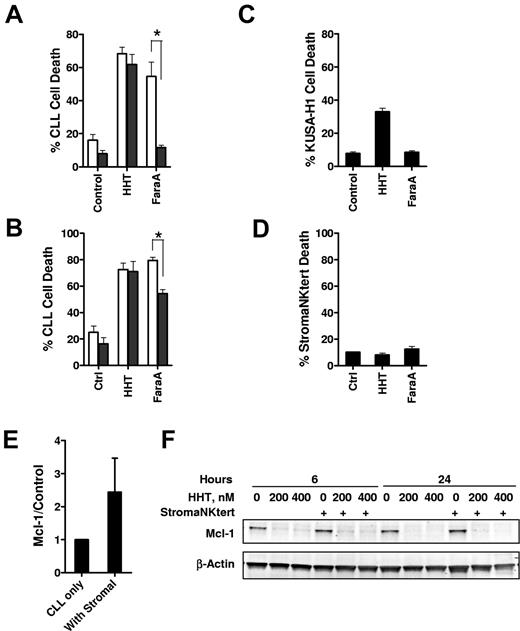
Growing evidence suggested that interaction of CLL cells with the microenvironment in vivo such as the bone marrow and secondary lymphoid organs protect CLL cells from conventional therapy.39 Thus, we compared the cytotoxicity of HHT with CLL cells in the presence or absence of marrow stromal cells. Although cell death was significantly reduced in the presence of either murine (KUSA-H1; Figure 6A) or human (StromaNKtert Figure 6B) stromal cell lines in response to fludarabine, there was no significant difference of cell death in the presence or absence of stromal cells when the CLL cells were exposed to HHT. Although HHT alone was slightly toxic to the KUSA-H1 cells (Figure 6C), there was no toxicity of HHT to the human stromal cell line StromaNKtert (Figure 6D). Both the mRNA and protein levels of Mcl-1 were induced on incubating with the StromaNKtert cells (Figure 6E; data not shown). However, this induction was reverted by HHT treatment (Figure 6F). Thus, these results indicate that HHT may overcome stromal protection of CLL cells while sparing the normal stromal cells.
The stromal cells did not protect CLL cells from HHT-induced apoptosis. (A) CLL cells were incubated with 100nM HHT or 10μM F-ara-A (fludarabine (9-β-D-arabinofuranosyl-2-fluoroadenine)) for 24 hours at the absence (□) or presence ■) of the (A) murine stromal cell line KUSA-H1 (n = 3) or the (B) human stromal cell line StromaNKtert (n = 4). Cell death was measured by annexin V–PI staining. Toxicity of HHT to the KUSA-H1 (C) or StromaNKtert (D) was measured by incubating the stromal cells with HHT or F-ara-A for 24 hours before the cells were trypsinized for cell death measurement by annexin V–PI staining. *Difference was significant, P ≤ .05. (E) Incubating of CLL cells with stromal cells induced Mcl-1 expression. CLL cells were incubated with StromaNKtert cells for 24 hours, and Mcl-1 level was measured by immunoblotting. Data (mean ± SEM) represent the average of 4 CLL samples. (F) Reducing Mcl-1 level by HHT was not affected by stromal cells. CLL cells were incubated at the presence or absence of StromaNKtert cells for 24 hours before exposure to 200 and 400nM HHT for 6 and 24 hours. Mcl-1 levels were analyzed by immunoblotting. Data showed 1 of 4 CLL samples with similar results.
The stromal cells did not protect CLL cells from HHT-induced apoptosis. (A) CLL cells were incubated with 100nM HHT or 10μM F-ara-A (fludarabine (9-β-D-arabinofuranosyl-2-fluoroadenine)) for 24 hours at the absence (□) or presence ■) of the (A) murine stromal cell line KUSA-H1 (n = 3) or the (B) human stromal cell line StromaNKtert (n = 4). Cell death was measured by annexin V–PI staining. Toxicity of HHT to the KUSA-H1 (C) or StromaNKtert (D) was measured by incubating the stromal cells with HHT or F-ara-A for 24 hours before the cells were trypsinized for cell death measurement by annexin V–PI staining. *Difference was significant, P ≤ .05. (E) Incubating of CLL cells with stromal cells induced Mcl-1 expression. CLL cells were incubated with StromaNKtert cells for 24 hours, and Mcl-1 level was measured by immunoblotting. Data (mean ± SEM) represent the average of 4 CLL samples. (F) Reducing Mcl-1 level by HHT was not affected by stromal cells. CLL cells were incubated at the presence or absence of StromaNKtert cells for 24 hours before exposure to 200 and 400nM HHT for 6 and 24 hours. Mcl-1 levels were analyzed by immunoblotting. Data showed 1 of 4 CLL samples with similar results.
Discussion
The present studies have shown that nanomolar concentrations of HHT induced significant apoptosis in CLL cells, indicating that it is a potent compound in killing CLL cells. HHT inhibited protein synthesis and caused the decrease of Mcl-1 levels, which was accompanied by the loss of mitochondrial membrane potential, PARP cleavage, and annexin V–positive staining, indicating the induction of apoptosis. The diminished level of Mcl-1 was not a consequence of caspase-3 cleavage, but instead was a result of the protein turnover through proteasome degradation. Stromal support, which greatly diminishes the cytotoxicity of fludarabine to CLL cells, had little effect on the actions of HHT. CLL cell killing by HHT was synergistic with SNS-032, an inhibitor of transcription. These results suggest that depletion of prosurvival proteins by transient exposure to a translation inhibitor, either alone or in combination with a transcription inhibitor, may be a novel therapeutic strategy for the treatment of CLL.
HHT is more potent against CLL cells compared with fludarabine; nanomolar concentrations induced significant apoptosis, with ∼ 50% cell death after 24 hours of treatment in vitro (Figure 1B), compared with 3μM fludarabine.19 In a clinical trial in hematologic malignancies the maximal tolerated dose of HHT administered subcutaneously in AML was 5 mg/m2/day for 9 days, which generated mean peak plasma concentration of 96.1 ± 20.3 ng/mL (176 ± 37nM).40 In CML clinical trials, the total dose of HHT used was 2.5 mg/m2/day, administered by subcutaneous injection twice daily for 7-14 days. This was well tolerated in each study and was subsequently shown to be active in patients with the T315I mutation of Bcr-Abl.29 Therefore, the concentrations of HHT that were active against CLL cells in our in vitro studies were consistent with those achieved in patients in current clinical trials. Maximal induction of apoptosis in CLL cells required the continuous incubation of HHT (Figure 1D), probably reflecting the time required for Mcl-1 protein levels to decline below a threshold required for viability (Figure 1D). The actions of HHT appear to be reversible because no additional death was evident in cells washed into fresh medium. Thus, consideration should be given to maintaining a plasma concentration of HHT above the therapeutic concentration when designing schedules of administration for use in the clinic.
There was a heterogeneous sensitivity to HHT among the CLL patient samples (Figure 1B). Although our samples with poor prognosis characteristics were limited, they did not show a significant difference in apoptosis compared with other samples (Figure 1C). This was also true for CLL cells incubated with the transcription inhibitors flavopiridol41 or SNS-032.7 Thus, these results indicate that HHT also induces apoptosis by a mechanism independent of these variables, raising the possibility that patients whose disease exhibit these characteristics may be sensitive to HHT. Our data showed that sensitivity to HHT correlated to the basal Mcl-1 expression in each sample (Figure 2D), provided further support for the conclusion that Mcl-1 is the main target for HHT-induced apoptosis in CLL.
Many tumors depend on the continuous activity of specific oncogenes for sustaining their malignant phenotype. This phenomenon, termed “oncogene addiction,” provides a rationale for the development of targeted therapeutics specifically directed at inhibiting the activity of the particular oncogene product.42 Previous studies that knocked down Mcl-1 with siRNA showed that CLL cells depend on Mcl-1 for survival.5 The decrease in Mcl-1 protein levels after HHT treatment was evident and was time and concentration dependent (Figures 1A, 2C). However, Bcl-2 and XIAP levels did not decrease significantly, probably because of greater intrinsic stability. For instance, Bcl-2 protein has a half-life of ∼ 10 hours in B cells.43 XIAP is a copper-binding protein with a half-life of > 12 hours in the absence of copper and 6 hours in the presence of copper.44 In contrast, Mcl-1 has a more rapid turnover rate of 0.5-1 hour,13 probably because of its 2 PEST regions, which are rich in proline (P), glutamic acid (E), serine (S), and threonine (T) and are target domains for rapid destruction by the ubiquitin-proteasome system.45 Results also showed that inhibition of the proteasome partially restored the Mcl-1 level decreased by HHT (Figure 5C). The decrease of Mcl-1 was related to the induction of apoptosis as evidenced by PARP cleavage (Figure 2A), positive annexin V staining (Figure 2C), and loss of mitochondrial transmembrane potential (Figure 1E). Studies have found that the elimination of Mcl-1 results in the release of Bax and Bak, proapoptotic proteins sequestered by Mcl-1, to translocate to the mitochondria. This results in the induction of cytochrome c release and caspase activation, leading to cell death by apoptosis.4,38 In CLL, it appears that reduction of Mcl-1 is sufficient for apoptosis, given that down-regulation of Mcl-1 by siRNA induced rapid and potent apoptosis in CLL.5 Both HHT and CHX down-regulated Mcl-1, but HHT was more potent in both inhibiting global translation and inducing apoptosis in CLL (Figure 3A-B). CHX was also reported to induce 9%-98% apoptosis in CLL cells with a concentration range of 1μM to 10mM and to inhibit protein synthesis,18 which is consistent with our results. Therefore, HHT and CHX may kill CLL cells by the same mechanism of inhibiting protein synthesis, but HHT is ∼ 100 times more potent than CHX.
Our results showed that SNS-032 was synergistic with HHT. The probable mechanism of synergy arises from the inhibition of 2 tandem steps in Mcl-1 expression: SNS-032 blocking RNA pol II activation by inhibiting Cdk9 and Cdk7 phosphorylation of the C-terminal domain of the enzyme, thereby curtailing transcription,7 and HHT by blocking translation at the level of the ribosome.24 The combination of 2 or more inhibitors acting in a single pathway has been termed a sequential blockade of gene expression.17 Under these conditions, the combination reduced Mcl-1 protein more completely than any single drug, resulting in synergistic induction of apoptosis in CLL cells (Figure 4C). Because the remaining Mcl-1 is degraded by a proteasome-dependent pathway, HHT is antagonistic with agents that block Mcl-1 degradation, such as the proteasome inhibitor MG-132 (Table 1).
The CLL microenvironment, such as the marrow and lymphoid stromal cells, have been reported to protect the CLL cells from spontaneous and drug-induced apoptosis.46 As such, the microenvironment of CLL cells may be regarded as contributing to resistance to potential therapeutic approaches, the primary example being that of fludarabine.39,47 With the use of the latter as an comparator, it is clear that the stromal layers derived from murine and human sources failed to protect CLL cells from the toxicity of HHT (Figure 5). Although stromal support stimulates Mcl-1 accumulation in CLL cells 48 (Figure 6E), the actions of HHT in blocking Mcl-1 expression cannot be overcome by such stimulation (Figure 6F). Similarly, cytotoxicity of other approaches that target the function of prosurvival Bcl-2 family members are not affected by microenvironment support.49,50 Together, our results suggested that translation inhibitors such as HHT may be a promising new class of agents for CLL therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Susan C. Smith and Susan Lerner, Department of Leukemia, for providing the patient information.
This work was supported in part by the National Cancer Institute, Department of Health and Human Services (grants CA81534, CA100632, and by Cancer Center Support grant P30 CA16672) and a grant from the CLL Global Research Foundation.
National Institutes of Health
Authorship
Contribution: R.C. and L.G. conceptualized the project, designed and performed the research, analyzed the data, and wrote the manuscript; Y.C. and Y.J. performed the research and analyzed the data; W.G.W. identified CLL patients for inclusion in the study, analyzed the data, and approved the manuscript; W.P. conceptualized the project and directed the experimental design, data analysis, and manuscript writing.
Conflict-of-interest disclosure: W.G.W. and W.P. received research funding from the Sunesis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: William Plunkett, Department of Experimental Therapeutics, Unit 71, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: wplunket@mdanderson.org.
References
Author notes
R.C. and L.G. contributed equally to this study.


![Figure 3. HHT inhibited protein synthesis. (A) HHT was a more potent inhibitor of protein synthesis than CHX. CLL cells were incubated with 0.1, 1, 10, or 100μM HHT (□) or CHX (○) for 24 hours. Then, 1 μCi/mL (3.7 × 107 Bq/L) [3H]leucine was added to the culture for 1 hour, and radioactivity was determined by liquid scintillation counting (A). Apoptosis was detected by annexin V–PI staining (B). Each data point represents the mean ± SEM of 3 patient samples. Concentration-dependent PARP cleavage and loss of Mcl-1 were detected by immunoblotting (C). (D) Polysomal profiling analysis of CLL cells treated with HHT. CLL cells were incubated in HHT for 2 hours before lysed and resolved on a 10%-50% sucrose gradient. Twenty-two fractions were collected from each sample. The absorbance at 260 nm (OD260) was presented in the top panel. Solid line indicates control; dashed line, HHT treated. Mcl-1 and β-actin mRNA were quantitated in every other fraction and presented as percentage of total in all fractions. The analysis was repeated in 3 CLL samples with similar results. Results from one representative CLL sample were shown. Open bars indicate controls; solid bars, treatment with 200nM HHT.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/1/10.1182_blood-2010-01-262808/4/m_zh89991064140003.jpeg?Expires=1769079937&Signature=y-IVoF6WeDzRXCe-Dzu7D3AorS8b4wcoSFZy~dyLsLhKTtotZBs0eIIHSKxf5eHbAWkSr9vtMHATC7a-RQRoy9k3IOasmiY9VDvkRfIBFI7F6ev7kqVc2ZcIootjMlNXXY-0hXq-7FG9k7fdCC4VEmJ2bvUnDCur4sweLaVKV5LM4NtnPwLihwuLdx2WvwmzqeFjqZ2D1aArhUh7t2gLWGfhRvAJw7gb9BXhtL3EPM3-SsJlQPUXzu~6VSRJSQfJc683tjOVT7IYJGOJDnoOx2qHHXB72zm1bUGT-XAsj1zAdkihWUPb8hm5iXWr5PPJahwkBjwVeh~LsvLZ9Qi5QQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)