Abstract
Mantle cell lymphoma (MCL) is a B-cell non-Hodgkin lymphoma of which at least a subset arises from antigen-experienced B cells. However, what role antigen stimulation plays in its pathogenesis remains ill defined. The genetic hallmark is the chromosomal translocation t(11;14) resulting in aberrant expression of cyclin D1. Secondary genetic events increase the oncogenic potential of cyclin D1 and frequently inactivate DNA damage response pathways. In combination these changes drive cell-cycle progression and give rise to pronounced genetic instability. Several signaling pathways contribute to MCL pathogenesis, including the often constitutively activated PI3K/AKT/mTOR pathway, which promotes tumor proliferation and survival. WNT, Hedgehog, and NF-κB pathways also appear to be important. Although MCL typically responds to frontline chemotherapy, it remains incurable with standard approaches. Proteasome inhibitors (bortezomib), mTOR inhibitors (temsirolimus), and immunomodulatory drugs (lenalidomide) have recently been added to the treatment options in MCL. The molecular basis for the antitumor activity of these agents is an area of intense study that hopefully will lead to further improvements in the near future. Given its unique biology, relative rarity, and the difficulty in achieving long-lasting remissions with conventional approaches, patients with MCL should be encouraged to participate in clinical trials.
Diagnosis, clinical course, and the origin of MCL
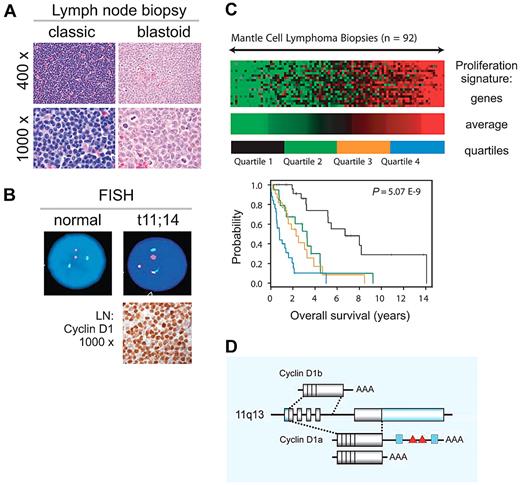
Mantle cell lymphoma (MCL), a mature B-cell neoplasm defined as a distinct entity in the early 1990s constitutes ∼ 6% of all non-Hodgkin lymphomas (NHLs).1-4 MCL is typically disseminated at presentation, with a leukemic component in 20%-30% of patients. Classic and blastoid variants are recognized, the latter associated with inferior clinical outcome (Figure 1A). The genetic hallmark of MCL is the translocation t(11;14)(q13;q32) leading to aberrant expression of cyclin D1, which is not typically expressed in normal lymphocytes (Figure 1B). However, cyclin D1–negative cases having typical morphology and gene expression profile have been described and often show overexpression of cyclin D2 or D3.5 Recently, SOX11 has been described as a diagnostic maker that is equally expressed in D1-positive and D1-negative MCL.6,7 MCL is one of the most difficult to treat B-cell lymphomas. Although conventional chemotherapy induces high-remission rates in previously untreated patients, relapse within a few years is common, contributing to a rather short median survival of 5-7 years.8,9 The best predictor of survival is tumor proliferation (Figure 1C).5 Intensification of first-line treatment has improved progression-free survival, but no curative regimen has been defined so far.2,3
Clinical and biologic characteristics of MCL. (A) MCL diagnosis is based on morphology and immunophenotyping (CD20+, CD5+, CD23−, FMC7+). Pathologic subclassification recognizes 2 main subsets: classic and blastoid. (B) Fluorescence in situ hybridization (FISH) cytogenetics showing translocation t(11;14)(q13;q32) and immunohistochemistry for detection of cyclin D1 overexpression are helpful adjuncts in the diagnosis of MCL. Immunohistochemistry images provided by Stefania Pittaluga; images of FISH testing provided by Diane C. Arthur. (C) Tumor proliferation determines outcome. Figure adapted from original5 with permission. The expression levels of 20 genes related to cell proliferation were summarized in the proliferating signature average. Lowest (green) to highest (red) expression of the proliferation signature average in lymph node biopsies from 92 patients with MCL is shown with across a 16-fold range. Kaplan-Meier analysis for patients grouped into 4 quartiles on the basis of this score is shown. (D) CCND1 locus at 11q13 and cyclin D1 mRNA isoforms. Shaded boxes represent coding sequences, open boxes represent noncoding exon sequences. The 3′UTR of full-length 4.5-kb cyclin D1a contains binding sites for miRs (blue boxes) and AU-rich elements (red triangles); cyclin D1a isoforms with a truncated 3′UTR lack these elements. The alternatively spliced 1.7-kb cyclin D1b mRNA lacks exon 5 and retains part of intron 4. Illustration by Paulette Dennis.
Clinical and biologic characteristics of MCL. (A) MCL diagnosis is based on morphology and immunophenotyping (CD20+, CD5+, CD23−, FMC7+). Pathologic subclassification recognizes 2 main subsets: classic and blastoid. (B) Fluorescence in situ hybridization (FISH) cytogenetics showing translocation t(11;14)(q13;q32) and immunohistochemistry for detection of cyclin D1 overexpression are helpful adjuncts in the diagnosis of MCL. Immunohistochemistry images provided by Stefania Pittaluga; images of FISH testing provided by Diane C. Arthur. (C) Tumor proliferation determines outcome. Figure adapted from original5 with permission. The expression levels of 20 genes related to cell proliferation were summarized in the proliferating signature average. Lowest (green) to highest (red) expression of the proliferation signature average in lymph node biopsies from 92 patients with MCL is shown with across a 16-fold range. Kaplan-Meier analysis for patients grouped into 4 quartiles on the basis of this score is shown. (D) CCND1 locus at 11q13 and cyclin D1 mRNA isoforms. Shaded boxes represent coding sequences, open boxes represent noncoding exon sequences. The 3′UTR of full-length 4.5-kb cyclin D1a contains binding sites for miRs (blue boxes) and AU-rich elements (red triangles); cyclin D1a isoforms with a truncated 3′UTR lack these elements. The alternatively spliced 1.7-kb cyclin D1b mRNA lacks exon 5 and retains part of intron 4. Illustration by Paulette Dennis.
In MCL, as in chronic lymphocytic leukemia (CLL), the variable region of the expressed immunoglobulin heavy chain gene (IGHV) displays either germline configuration (Ig-unmutated), as found in naive B cells, or contains somatic mutations (Ig-mutated), indicating a response to antigen. In CLL, the presence or absence of somatic mutations identifies 2 disease subtypes with divergent clinical outcome. In contrast, in MCL, the prognostic role of the IGHV mutation status has not been confirmed.1 However, a subset of leukemic MCL cases that are characterized by minimal nodal involvement and a relatively indolent course often belong to the Ig-mutated subtype.10,11 Absence of SOX11 expression has recently been identified as a characteristic feature of this indolent subtype.11
MCL has been thought to originate from naive, pre-germinal center (GC) lymphocytes. However, several observations suggest that this is not always the case. MCL cells show biased use of certain IGHV genes, especially IGHV3-21, IGHV3-23, IGHV4-34, and IGHV4-59, and up to 40% of cases show somatic mutations of the IgVH gene.10,12-14 Mutations are frequent in IGHV3-23 and IGHV4-59 genes and often occur in so called hotspot regions.13 IGHV3-21 is almost exclusively found in germline configuration and is commonly paired with the light chain IGLV3-19.14 Many IGHV3-21–expressing tumors show virtually identical amino acid sequences in the CDR3 region. Interestingly, the structure of the IGHV3-21 CDR3 region differs between MCL and CLL, suggesting a different antigen specificity of the B-cell receptor (BCR) in these 2 malignancies.15 Combined, these findings indicate that at least a subset of MCL, similar to CLL, is derived from antigen-experienced B cells. In keeping with this notion is the expression of genes that code for chemokine receptors involved in trafficking after initial B-cell activation.16 Furthermore, almost one-half of MCL cases express at least 1 immunohistochemical marker characteristic of a GC or post-GC phenotype, in particular IRF4.17 In rare cases plasma cell differentiation of the clonal cells has been reported.18,19 A partial plasmacytic phenotype characterized by relatively stronger CD38 and IRF4 expression is more common and can identify a biologically distinct subset of MCL.20
Cyclin D1, at the center of MCL pathogenesis
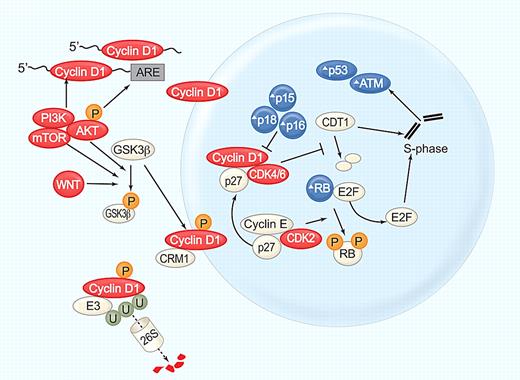
Whether the t(11;14) translocation and the resultant aberrant expression of cyclin D1 is the initiating event in the pathogenesis is difficult to ascertain, but likely given its key role in MCL biology and its continued importance for tumor proliferation and clinical outcome.21 The CCND1 gene consists of 5 exons, which can be alternatively spliced to cyclin D1a and D1b isoforms (Figure 1D). In most cases cyclin D1a and D1b mRNAs are coexpressed. However, D1b protein is not consistently detected,22 and its contribution, if any, to MCL pathogenesis remains elusive. Cyclin D1a contains a long 3′ untranslated region (UTR) harboring mRNA destabilizing elements that limit its half-life to ∼ 30 minutes.21 In addition, miR15/16 can bind the 3′ UTR and inhibit cyclin D1 translation.23,24 Abundant cyclin D1a transcripts that lack the full-length 3′ UTR are commonly found in highly proliferative tumors and predict short survival.21 Genomic deletions and point mutations in the 3′ UTR give rise to these short cyclin D1a transcripts that are more stable than the full-length mRNA. In addition, the truncation removes the miR binding sites. Combined, these additional events enhance the influence of the t(11;14) by generating higher and prolonged cyclin D1 mRNA and protein expression, resulting in higher tumor proliferation and shorter survival of patients with MCL.21
Cyclin D1a, a 30-kDa labile protein, forms a complex with the cyclin-dependent kinase CDK4 or CDK6 to promote cell-cycle entry (Figure 2). Both of these CDKs are often overexpressed in MCL. The CDK4 locus is frequently amplified,25 and decreased expression of miR-29, which targets CDK6, can lead to increased CDK6 expression and identifies patients with short survival.26 Transcription, translation, assembly into holoenzyme complexes, subcellular localization, and degradation of cyclin D1 are tightly regulated. Although overexpression of cyclin D1 is not transforming in nude mice, additional events that increase nuclear cyclin D1 levels enhance its oncogenic potential. Mutation of threonine 268 to alanine prevents phosphorylation by glycogen synthase kinase 3 β (GSK3β), thereby inhibiting cyclin D1 nuclear export. This mutant protein is potently transforming and induces a mature B-cell lymphoma in transgenic mice.27 In the cytoplasm cyclin D1 is polyubiquitinated by the E3 ligase SKp1-Cul1-F box protein (SCF; FBX4-αB Crystallin) and degraded through the proteasome.28 The T268A mutation and mutations in the E3 ubiquitin ligase complex increase nuclear cyclin D1 expression and have been detected in solid tumors.29,30 Although no such mutations have been described in MCL, phosphatidyl inositol-3 kinase (PI3K)/AKT–mediated inhibition of GSK3β could have a similar effect and contribute to cyclin D1 dysregulation in MCL.
Cyclin D1, at the center of MCL pathogenesis. Cyclin D1 mRNA stability and translation is increased by the PI3K/AKT/mTOR pathway. Cyclin D1 translocates into the nucleus and forms a holoenzyme with CDK4/6 to phosphorylate the retinoblastoma protein (RB), resulting in the release of E2F transcription factors and G1/S phase transition. In addition, cyclin D1/CDK4 complexes have kinase-independent functions, notably binding of the cell-cycle inhibitor p27kip, which is thereby titrated away from cyclinE/CDK2 complexes further promoting cell-cycle progression. Cyclin D1/CDK4 inhibits degradation of CDT1, the rate-limiting factor in DNA replication. Stabilization of CDT1 in S phase can induce the replication of already transcribed chromosomal segments, giving rise to increased numbers of double strand breaks and activation of DNA damage checkpoints. In S phase cyclin D1 is phosphorylated on threonine 286 by GSK3β, exported from the nucleus by CRM-1, polyubiquitinated by the E3 ligase SCF(FBX4-αB Crystallin), and degraded through the proteasome (reviewed in Kim and Diehl 28 ). GSK3β is phosphorylated and inactivated by AKT and WNT signaling. Several components of this cell-cycle control machinery are altered in MCL: blue symbols (Δ) indicate molecules inactivated or down-regulated; red symbols, molecules activated or overexpressed. Illustration by Paulette Dennis.
Cyclin D1, at the center of MCL pathogenesis. Cyclin D1 mRNA stability and translation is increased by the PI3K/AKT/mTOR pathway. Cyclin D1 translocates into the nucleus and forms a holoenzyme with CDK4/6 to phosphorylate the retinoblastoma protein (RB), resulting in the release of E2F transcription factors and G1/S phase transition. In addition, cyclin D1/CDK4 complexes have kinase-independent functions, notably binding of the cell-cycle inhibitor p27kip, which is thereby titrated away from cyclinE/CDK2 complexes further promoting cell-cycle progression. Cyclin D1/CDK4 inhibits degradation of CDT1, the rate-limiting factor in DNA replication. Stabilization of CDT1 in S phase can induce the replication of already transcribed chromosomal segments, giving rise to increased numbers of double strand breaks and activation of DNA damage checkpoints. In S phase cyclin D1 is phosphorylated on threonine 286 by GSK3β, exported from the nucleus by CRM-1, polyubiquitinated by the E3 ligase SCF(FBX4-αB Crystallin), and degraded through the proteasome (reviewed in Kim and Diehl 28 ). GSK3β is phosphorylated and inactivated by AKT and WNT signaling. Several components of this cell-cycle control machinery are altered in MCL: blue symbols (Δ) indicate molecules inactivated or down-regulated; red symbols, molecules activated or overexpressed. Illustration by Paulette Dennis.
Genetic instability and the role of DNA damage pathways
MCL is one of the B-cell malignancies with the highest degree of genomic instability, and a large number of secondary chromosomal alterations have been described in MCL, including losses, gains, and amplifications of chromosomal regions that contain genes involved in cell-cycle regulation, DNA damage response pathways, signal transduction, and apoptosis (Table 1).4 Recently, uniparental disomy has been described in a large number of cases and contributes to the inactivation of tumor suppressor genes. For example, one of the most commonly detected uniparental disomies involves the chromosomal band 17p and is associated with TP53 inactivation.31 The origin of the genetic instability of MCL is not clear. However, aberrant reinitiation of DNA replication during the S phase because of cyclinD1/CDK4-induced stabilization of CDT1 could be an important contributor (Figure 2).28 Reinitiation can generate segmental duplications and copy number variations, which are commonly found in MCL,31 and may give rise to break prone regions that promote additional chromosomal alterations. Acquired chromosomal aberrations in DNA damage checkpoint genes and in genes related to microtubule dynamics, such as MAP2 and MAP6, can further increase genomic instability.32
Selected recurrent genomic lesions in MCL
| Gene . | Class/product . | Function . | Locus . | Lesion . | Reference . |
|---|---|---|---|---|---|
| Cell-cycle control and DNA damage pathways | |||||
| CCND1 | Cyclin D1 | Forms holoenzyme with CDK4/CDK6 to regulate G1/S transition through Rb phosphorylation | 11q13 | + | 31 |
| CDK4 | Cyclin-dependent kinase | Forms complex with D-type cyclins to regulate early G1 phase | 12q13 | + | 25 |
| BMI1 | Oncogene | Regulates cell cycle and senescence as a transcriptional repressor of INK4a/ARF locus | 10p11 | + | 25, 125 |
| MYC | Oncogene | Induces mitogen-independent cell proliferation; often overexpressed in cancer | 8q21 | + | 25 |
| MDM2 | Negative regulator of p53 | E3 ligase for p53 targeting it for proteasomal degradation | 12q13 | + | 126 |
| RB1 | Tumor suppressor | Binds to E2F1, preventing its nuclear translocation and cell-cycle progression | 13q14.2 | − | 31, 127 |
| CDKN2C | CDK inhibitor p18 | Inhibits the activity of CDK/cyclin D complexes at G1 | 1p32 | − | 31, 99 |
| CDKN2A | CDK inhibitor encodes p14 and p16 | Inhibit the activity of CDK/cyclin D complexes at G1 | 9p21 | − | 128 |
| P53 | Tumor suppressor | Induces cell-cycle arrest, apoptosis, and senescence | 17p13 | − | 25 |
| ATM | Ser/Thr kinase of PI-3K family | After DNA damage ATM controls G1/S and G2/M cell-cycle checkpoints by phosphorylation p53, MDM2, and CHK2 | 11q22 | − | 129 |
| HIC1 | Tumor suppressor cooperates with p53 | Loss of HIC1 results in deacetylation and inactivation of p53 by reducing DNA binding | 17p13.3 | − | 130 |
| MOBKL2B | Protein kinase | Essential for spindle pole body duplication and mitotic checkpoint regulation | 9p21.2 | − | 31 |
| Signaling pathways | |||||
| SYK | Tyr kinase | Involved in BCR signaling, mediates activation of PKCβ, ΝF-κB, and PI3K-AKT pathways | 9q22 | + | 37 |
| PIK3CA | PI3K catalytic subunit α (p110α) | Sufficient to support survival of B cells with defective BCR signaling; AKT activation | 3q25 | + | 70 |
| FAF1 | Inhibitor of ΝF-κB activation | Suppresses IKK activity by binding to IKKβ, resulting in p65 retention in the cytoplasm | 1p32.3 | − | 31 |
| TNFAIP3 | A20 tumor suppressor | Inhibits ΝF-κB activation through ubiquitin editing | 6p23 | − | 88 |
| Apoptosis | |||||
| BCL2 | Key antiapoptotic regulator | Promotes cell survival by sequestering proapoptotic BAX and BAK | 18q11-23 | + | 31 |
| BCL2L11 | BIM, BH3-only protein | Binds to BCL2, BCLXL, and MCL1, acting as an apoptotic activator | 2q13 | − | 31, 99 |
| Other pathways | |||||
| PRAME | Possible tumor antigen | Expressed in solid tumors and some hematopoietic neoplasias | 22q11.22 | − | 31 |
| MAP2 | Microtubule stabilizer | Regulator of microtubule dynamics, cancer cell migration | 2q34 | − | 31 |
| SP100 | Transcription factor | Localizes to nuclear bodies; coactivator of HIPK2/p53-mediated transcription. | 2q37 | − | 31 |
| MTAP | Enzyme | Polyamine metabolism, frequently codeleted with the p16 | 9p21.3 | − | 31 |
| Gene . | Class/product . | Function . | Locus . | Lesion . | Reference . |
|---|---|---|---|---|---|
| Cell-cycle control and DNA damage pathways | |||||
| CCND1 | Cyclin D1 | Forms holoenzyme with CDK4/CDK6 to regulate G1/S transition through Rb phosphorylation | 11q13 | + | 31 |
| CDK4 | Cyclin-dependent kinase | Forms complex with D-type cyclins to regulate early G1 phase | 12q13 | + | 25 |
| BMI1 | Oncogene | Regulates cell cycle and senescence as a transcriptional repressor of INK4a/ARF locus | 10p11 | + | 25, 125 |
| MYC | Oncogene | Induces mitogen-independent cell proliferation; often overexpressed in cancer | 8q21 | + | 25 |
| MDM2 | Negative regulator of p53 | E3 ligase for p53 targeting it for proteasomal degradation | 12q13 | + | 126 |
| RB1 | Tumor suppressor | Binds to E2F1, preventing its nuclear translocation and cell-cycle progression | 13q14.2 | − | 31, 127 |
| CDKN2C | CDK inhibitor p18 | Inhibits the activity of CDK/cyclin D complexes at G1 | 1p32 | − | 31, 99 |
| CDKN2A | CDK inhibitor encodes p14 and p16 | Inhibit the activity of CDK/cyclin D complexes at G1 | 9p21 | − | 128 |
| P53 | Tumor suppressor | Induces cell-cycle arrest, apoptosis, and senescence | 17p13 | − | 25 |
| ATM | Ser/Thr kinase of PI-3K family | After DNA damage ATM controls G1/S and G2/M cell-cycle checkpoints by phosphorylation p53, MDM2, and CHK2 | 11q22 | − | 129 |
| HIC1 | Tumor suppressor cooperates with p53 | Loss of HIC1 results in deacetylation and inactivation of p53 by reducing DNA binding | 17p13.3 | − | 130 |
| MOBKL2B | Protein kinase | Essential for spindle pole body duplication and mitotic checkpoint regulation | 9p21.2 | − | 31 |
| Signaling pathways | |||||
| SYK | Tyr kinase | Involved in BCR signaling, mediates activation of PKCβ, ΝF-κB, and PI3K-AKT pathways | 9q22 | + | 37 |
| PIK3CA | PI3K catalytic subunit α (p110α) | Sufficient to support survival of B cells with defective BCR signaling; AKT activation | 3q25 | + | 70 |
| FAF1 | Inhibitor of ΝF-κB activation | Suppresses IKK activity by binding to IKKβ, resulting in p65 retention in the cytoplasm | 1p32.3 | − | 31 |
| TNFAIP3 | A20 tumor suppressor | Inhibits ΝF-κB activation through ubiquitin editing | 6p23 | − | 88 |
| Apoptosis | |||||
| BCL2 | Key antiapoptotic regulator | Promotes cell survival by sequestering proapoptotic BAX and BAK | 18q11-23 | + | 31 |
| BCL2L11 | BIM, BH3-only protein | Binds to BCL2, BCLXL, and MCL1, acting as an apoptotic activator | 2q13 | − | 31, 99 |
| Other pathways | |||||
| PRAME | Possible tumor antigen | Expressed in solid tumors and some hematopoietic neoplasias | 22q11.22 | − | 31 |
| MAP2 | Microtubule stabilizer | Regulator of microtubule dynamics, cancer cell migration | 2q34 | − | 31 |
| SP100 | Transcription factor | Localizes to nuclear bodies; coactivator of HIPK2/p53-mediated transcription. | 2q37 | − | 31 |
| MTAP | Enzyme | Polyamine metabolism, frequently codeleted with the p16 | 9p21.3 | − | 31 |
+ indicates amplification; and −, deletion/mutation.
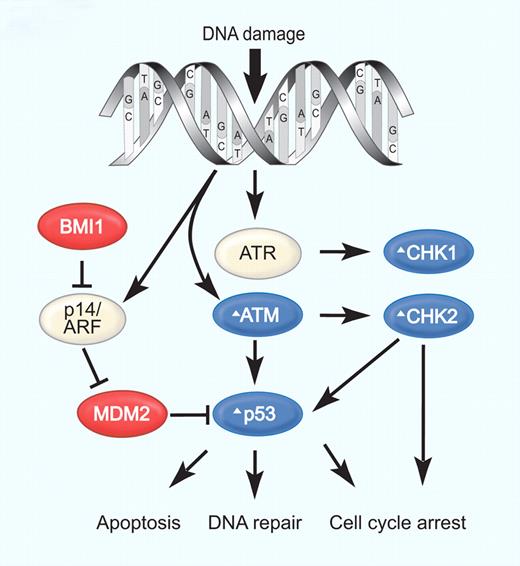
Secondary genetic alterations that affect DNA damage response pathways are of particular interest, because they may contribute to refractoriness to chemotherapy. The 11q22-23 deletions affecting the ATM gene are recurrent in MCL. The ATM kinase is critically involved in the cellular response to DNA damage and may act as a tumor suppressor gene (Figure 3). Truncating or missense mutations involving the PI3K domain of ATM are found in most MCL cases and are commonly accompanied by the loss of the other allele. The high frequency of ATM mutations in MCL is striking and has been linked to ATM expression in naive B cells in the mantle zone. Another mechanism for the strong selective pressure on ATM mutant clones may be aberrant reinitiation of DNA replication during the S phase, leading to double-strand DNA breaks and activation of the ATM pathway.28 A decrease in the expression of CHK1 and CHK2, 2 serine-threonine kinases downstream of S-phase checkpoints, has also been identified in cases of MCL with high chromosomal instability.4 The tumor suppressor gene TP53, downstream of ATM, plays an important role in DNA damage responses. Mutations of TP53 typically in conjunction with 17p13 deletions have been detected primarily in blastoid MCL cases. An alternative mechanism to disrupt the p53 pathway involves overexpression of the negative regulators MDM2 and MDM4. MDM2 overexpression due to copy number gains correlates with inferior survival. Similarly, MDM4, which is also highly expressed in MCL, decreases the expression of the CDK inhibitor p21, thereby promoting cell-cycle progression.33 Recently, high expression of the serine/threonine kinase PIM1 has been found in MCL. Consistent with the ability of PIM1 to stabilize MDM2 these tumors overexpress MDM2.34
Defective DNA damage responses in MCL. DNA damage activates the kinases ATM and ATR, together with p53 and p14/ARF, which can induce cell-cycle arrest, DNA repair, or apoptosis. The E3-ubiquitin ligase MDM2 that targets p53 for proteasomal degradation is inhibited by ARF, which in turn is inhibited by BMI1. CHK1 and CHK2, activated by ATR and ATM, respectively, phosphorylate key substrates (p53, CDC25A, CDC25B), leading to cell-cycle arrest. Several steps in this DNA damage response are altered in MCL: blue symbols (Δ) indicate molecules inactivated or down-regulated; red symbols, molecules activated or overexpressed. Illustration by Paulette Dennis.
Defective DNA damage responses in MCL. DNA damage activates the kinases ATM and ATR, together with p53 and p14/ARF, which can induce cell-cycle arrest, DNA repair, or apoptosis. The E3-ubiquitin ligase MDM2 that targets p53 for proteasomal degradation is inhibited by ARF, which in turn is inhibited by BMI1. CHK1 and CHK2, activated by ATR and ATM, respectively, phosphorylate key substrates (p53, CDC25A, CDC25B), leading to cell-cycle arrest. Several steps in this DNA damage response are altered in MCL: blue symbols (Δ) indicate molecules inactivated or down-regulated; red symbols, molecules activated or overexpressed. Illustration by Paulette Dennis.
Molecular basis of novel treatment approaches for MCL
Cyclin D1, cell-cycle control, and DNA damage pathways have been the centerpiece of research in MCL.35 More recently, signaling pathways that further enhance tumor proliferation, facilitate evasion of apoptosis, reduce immune control, and promote interactions with the cellular microenvironment have been identified. These include the BCR36,37 PI3K/AKT/mammalian target of rapamycin (mTOR),38-41 nuclear factor-κB (NF-κB),42-44 tumor necrosis factor (TNF),36,42 Hedgehog,45 and WNT pathways,5,40,46 and the BCL-2 family of apoptosis regulators.42,47 Agents targeting these pathways may provide new therapeutic options (Table 2).
Novel treatment approaches in MCL in clinical development
| Drug class . | Drugs . | Mechanism of action and effects on MCL biology . | Clinical development and results . |
|---|---|---|---|
| Proteasome inhibitors | Bortezomib (CT-L),48-50 NPI-0052 (CT-L, T-L, C-L),59 PR-171 (CT-L),60 MLN9708 (CT-L)61 | Reversible or irreversible inhibition of ≥ 1 proteasome activities; cell-cycle arrest and induction of apoptosis through oxidative and ER stress–mediated up-regulation of NOX | Bortezomib (phase 2): ORR 33%-58% in relapsed/refractory MCL; NPI-0052 and PR-171 (phase 1); MLN9708 (phase 1) |
| CDK inhibitors | Flavopiridol (pan-CDK),63 PD0332991 (CDK4/6)62 | Flavopiridol: pan-CDK inhibitor, decreased RNA stability; PD0332991: blocks cyclin D1/CDK4, leading to cell-cycle arrest | Flavopiridol (phase 1, 2): minimal responses as a single agent, modified dosing schedules may be more effective; PD0332991(phase 1) |
| Serine/threonine and tyrosine kinase inhibitors | Enzastaurin (PKC-β ΙΙ),66 fostamatinib (SYK),65 PCI-32765 (BTK)67 | Inhibition of BCR signal transduction cascade | Mostly stable disease for fostamatinib and enzastaurin; objective responses in phase 1 study of PCI-32765 |
| PI3K/AKT inhibitors | CAL-101 (PI3Kδ),73,131 ON1910.Na (multikinase /PI3Kα),72 perifosine (AKT)75 | Inactivation of AKT and mTOR; cell-cycle arrest, cyclin D1 down-regulation; activation of p53 and BAD-mediated apoptosis | CAL-101 (oral agent, phase 1): PRs in MCL and CLL; ON-01910.Na (phase 1); Perifosine (phase 1) |
| mTOR inhibitors (rapalogs) | Rapamycin, temsirolimus (CCI-779),79-81 everolimus (RAD001),83,132 deforolimus (AP23573)78 | Partial allosteric TORC1 inhibition; cell-cycle arrest; inconsistent effects on expression of cell-cycle regulators (cyclin D1, p21, p27); may induce autophagy but not apoptosis | Temsirolimus (phase 2 and 3): ORR 30%-40% in relapsed patients, mostly PRs of rapid onset (median, 1 mo), median duration of response of 6.9 mos; everolimus (phase 1 and 2): some objective responses; deferolimus (phase 2): 30% PR and 40% SD |
| BH3 mimetics | *ABT-263,103 AT-101,104 obatoclax (GX15-070)105 | Inhibition of antiapoptotic members of BCL-2 family, BCL-2, BCL-XL; GX 15-070 inhibits also MCL-1; mitochondrial depolarization through release of BH-3–only proteins and BAX/BAK activation | Obatoclax (phase 1 and 2) with or without bortezomib; AT-101 (phase 2); ABT-737 (phase 1 and 2) |
| IMIDs | Lenalidomide,113,114 thalidomide115 | Modulation of immune response; microenvironment or direct effect on tumor cells | Lenalidomide (phase 2): ORR 42%-53%, CR 20%, PFS 5.6 mos in relapsed MCL |
| HDAC inhibitor | Vorinostat (SAHA)119,120 | Cyclin D1 down-regulation, p21 and p27 up-regulation, and cell-cycle arrest; up-regulation of the BH3-only proteins BIM and BMF | SAHA (phase 1 and 2) with or without bortezomib |
| HSP90 inhibitors122 | Ansamycins: 17-AAG, 17-DMAG: IPI-504; synthetic: SNX-5422, KW-2478 | Degradation of client proteins, including cyclin D1, CDK4, IKKβ, AKT, c-MYC, and c-RAF133 ; cell-cycle arrest; activation of the mitochondrial apoptotic pathway | 17-AAG (phase 2): mostly disease stabilization, with or without bortezomib; 17-DMAG clinical development stopped; IPI-504: water-soluble 17-AAG derivative; synthetic inhibitors: entering early stage testing |
| Drug class . | Drugs . | Mechanism of action and effects on MCL biology . | Clinical development and results . |
|---|---|---|---|
| Proteasome inhibitors | Bortezomib (CT-L),48-50 NPI-0052 (CT-L, T-L, C-L),59 PR-171 (CT-L),60 MLN9708 (CT-L)61 | Reversible or irreversible inhibition of ≥ 1 proteasome activities; cell-cycle arrest and induction of apoptosis through oxidative and ER stress–mediated up-regulation of NOX | Bortezomib (phase 2): ORR 33%-58% in relapsed/refractory MCL; NPI-0052 and PR-171 (phase 1); MLN9708 (phase 1) |
| CDK inhibitors | Flavopiridol (pan-CDK),63 PD0332991 (CDK4/6)62 | Flavopiridol: pan-CDK inhibitor, decreased RNA stability; PD0332991: blocks cyclin D1/CDK4, leading to cell-cycle arrest | Flavopiridol (phase 1, 2): minimal responses as a single agent, modified dosing schedules may be more effective; PD0332991(phase 1) |
| Serine/threonine and tyrosine kinase inhibitors | Enzastaurin (PKC-β ΙΙ),66 fostamatinib (SYK),65 PCI-32765 (BTK)67 | Inhibition of BCR signal transduction cascade | Mostly stable disease for fostamatinib and enzastaurin; objective responses in phase 1 study of PCI-32765 |
| PI3K/AKT inhibitors | CAL-101 (PI3Kδ),73,131 ON1910.Na (multikinase /PI3Kα),72 perifosine (AKT)75 | Inactivation of AKT and mTOR; cell-cycle arrest, cyclin D1 down-regulation; activation of p53 and BAD-mediated apoptosis | CAL-101 (oral agent, phase 1): PRs in MCL and CLL; ON-01910.Na (phase 1); Perifosine (phase 1) |
| mTOR inhibitors (rapalogs) | Rapamycin, temsirolimus (CCI-779),79-81 everolimus (RAD001),83,132 deforolimus (AP23573)78 | Partial allosteric TORC1 inhibition; cell-cycle arrest; inconsistent effects on expression of cell-cycle regulators (cyclin D1, p21, p27); may induce autophagy but not apoptosis | Temsirolimus (phase 2 and 3): ORR 30%-40% in relapsed patients, mostly PRs of rapid onset (median, 1 mo), median duration of response of 6.9 mos; everolimus (phase 1 and 2): some objective responses; deferolimus (phase 2): 30% PR and 40% SD |
| BH3 mimetics | *ABT-263,103 AT-101,104 obatoclax (GX15-070)105 | Inhibition of antiapoptotic members of BCL-2 family, BCL-2, BCL-XL; GX 15-070 inhibits also MCL-1; mitochondrial depolarization through release of BH-3–only proteins and BAX/BAK activation | Obatoclax (phase 1 and 2) with or without bortezomib; AT-101 (phase 2); ABT-737 (phase 1 and 2) |
| IMIDs | Lenalidomide,113,114 thalidomide115 | Modulation of immune response; microenvironment or direct effect on tumor cells | Lenalidomide (phase 2): ORR 42%-53%, CR 20%, PFS 5.6 mos in relapsed MCL |
| HDAC inhibitor | Vorinostat (SAHA)119,120 | Cyclin D1 down-regulation, p21 and p27 up-regulation, and cell-cycle arrest; up-regulation of the BH3-only proteins BIM and BMF | SAHA (phase 1 and 2) with or without bortezomib |
| HSP90 inhibitors122 | Ansamycins: 17-AAG, 17-DMAG: IPI-504; synthetic: SNX-5422, KW-2478 | Degradation of client proteins, including cyclin D1, CDK4, IKKβ, AKT, c-MYC, and c-RAF133 ; cell-cycle arrest; activation of the mitochondrial apoptotic pathway | 17-AAG (phase 2): mostly disease stabilization, with or without bortezomib; 17-DMAG clinical development stopped; IPI-504: water-soluble 17-AAG derivative; synthetic inhibitors: entering early stage testing |
Information on ongoing clinical trials at is available at http://www.clinicaltrials.gov.
CT-L indicates chymotrypsin-like; T-L, trypsin-like; C-L, caspase-like; ORR, overall response rate; ER, endoplasmic reticulum; PR, partial response; SD, stable disease; CR, complete response; and PFS, progression-free survival.
ABT-263 is the oral formulation of the compound used in clinical trials; ABT-737 is the equivalent compound typically used in preclinical studies.
Proteasome inhibitors
The ubiquitin-proteasome system, the main nonlysosomal pathway through which intracellular proteins are degraded, exhibits 3 proteolytic activities: chymotrypsin-like (CT-L), trypsin-like, and caspase-like, each localized to a distinct 20S proteasome β subunit. Bortezomib, a peptide boronic acid that reversibly inhibits primarily the CT-L activity, is the first proteasome inhibitor approved by the Food and Drug Administration (FDA). Phase 2 clinical trials of single-agent bortezomib showed durable responses in 33%-58% of patients with relapsed or refractory MCL.48-50 Bortezomib is now increasingly combined with other agents. Interestingly, some studies suggest a sequence-dependent synergism with chemotherapy.51
The mechanism of how bortezomib acts as an anticancer agent has been intensely investigated and may differ between disease entities. The initial hypothesis that bortezomib exerts its cytotoxic effect through inhibition of NF-κB signaling is not supported by recent studies, at least not in multiple myeloma (MM) and MCL.52-54 The discovery that bortezomib induces apoptosis in MCL through up-regulation of the BH3-only protein NOXA showed a direct proapoptotic mechanism.55 NOXA acts as a downstream effector of an integrated cellular stress response triggered by the accumulation of undegraded, polyubiquitinated proteins that induce endoplasmic reticulum stress and the generation of reactive oxygen species. Combined, this triggers a transcriptional response that prominently involves the transcription factor NRF2, which was significantly stronger in bortezomib-sensitive tumors than in tumors of patients with inferior responses.56 Ultimately, NOXA is induced by 2 cooperating mechanisms: decreased ubiquitination of histone H2A that facilitates access to the NOXA gene (PMAIP1) promoter, and joint binding of ATF3 and ATF4 that drive its transcription.57 Finally, NOXA triggers BAK- and BAX-dependent mitochondrial apoptosis.55
Recently, gene expression analysis of bortezomib-adapted cell lines unexpectedly showed that bortezomib-resistant MCL cells showed partial plasmacytic differentiation, including increased expression of the transcription factor IRF4 and its target genes, and of the cell-surface markers CD38 and CD138.20 In contrast to plasma cells, plasmacytic MCL cells did not increase immunoglobulin secretion. Thus, plasmacytic differentiation in the absence of an increased secretory load may enable cells to withstand the stress of proteasome inhibition and lead to bortezomib resistance. This mirrors observations in MM, whereby high protein secretion sensitizes cells to proteasome inhibitors.58 Importantly, MCL tumors from patients with inferior clinical responses to bortezomib also had high IRF4 and CD38 expression, identifying possible clinical markers of bortezomib resistance.20
A second generation of proteasome inhibitors aiming for continued activity against bortezomib-resistant cells, simplified dosing schemes, and reduced toxicity is now entering the clinic. NPI-0052 (salinosporamide A), a natural product related to lactacystin, targets all 3 catalytic sites (CT-L, trypsin-like, and caspase-like), provides more potent and more durable proteasome inhibition and is active against bortezomib-resistant MM cells.59 PR-171 (carfilzomib), a modified peptide related to the natural product epoxomicin, selectively and irreversibly disables the CT-L activity, which may explain its greater cytotoxicity.60 MLN9708, a second-generation peptide boronic acid derivative, hydrolyzes to MLN2238, the biologically active form that reversibly blocks CT-L activity, and has shown preclinical efficacy in hematologic malignancies.61
Cyclin D1, cell-cycle control, and cell-cycle inhibitors
Cyclin D1 and cell-cycle control appear as natural targets in MCL. Two basic strategies have been pursued: down-regulation of cyclin D1 expression and inhibition of CDK function. PI3K and mTOR inhibitors can inhibit cyclin D1 translation and promote its degradation (discussed in “PI3K/AKT/mTOR”), whereas PD0332991 blocks cell-cycle progression through potent inhibition of CDK4/6.22,62 The pan-CDK inhibitor flavopiridol, whose mechanism of action involves inhibition of RNA synthesis, has minor clinical activity as a single agent, but a modified dosing regimen has shown considerable activity in CLL63 and is currently under investigation in MCL.64
BCR pathway
Recent studies reported constitutive activation of the BCR signal transduction components SYK and PKCβ II.36,37 SYK was amplified in both Jeko-1 cells and some primary MCL samples, but constitutive activity of SYK was only shown in the cell line.37 Jeko-1 cells were more sensitive to an SYK inhibitor than MCL cell lines without constitutive SYK activation, indicating some dependence on the pathway. In a screen for phosphoproteins, PKCβ II was found to be phosphorylated in primary MCL samples in contrast to normal B cells.36 However, it was not localized to lipid rafts as would be expected after classic BCR activation. Inhibitors targeting the signaling cascade downstream of the BCR have entered clinical testing. Fostamatinib, an inhibitor of SYK, achieved a 55% response rate in CLL, but only 1 in 9 patients with MCL responded.65 Enzastaurin, a PKCβ inhibitor, induced no objective responses in MCL.66 More recently, a phase 1 study of the BTK inhibitor PCI-32765 reported objective responses in a few patients with MCL.67 Thus, a role for BCR signaling in MCL pathogenesis and the possible clinical benefit of agents targeting this pathway deserve further investigation.
PI3K/AKT/mTOR pathway
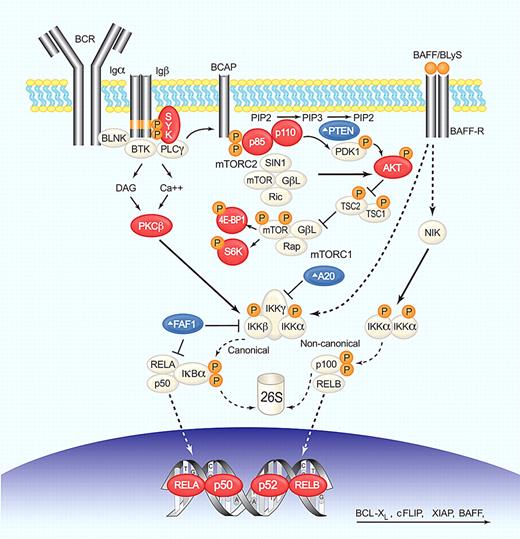
The PI3K/AKT pathway is involved in the transduction of a variety of extracellular signals and plays a prominent role in many cancers.68 In normal B cells, PI3K functions as a transducer of BCR signaling that regulates proliferation, differentiation, apoptosis, and survival (Figure 4). Gene expression profiling implicated the PI3K/AKT pathway in the pathogenesis of MCL,40 and several key components of the PI3K/AKT/mTOR pathway are activated in MCL,69 indicating a possible contribution of this pathway to MCL pathogenesis. Constitutive activation of AKT was found in most blastoid and many classic MCL tumors and was associated with the phosphorylation of downstream targets, including MDM2, Bad, and p27.39,41 Furthermore, AKT mediated activation of mTOR, and its downstream targets S6K and eukaryotic initiation factor 4E-binding protein-1 (4E-BP1) can increase translation of key proteins.
BCR, NF-κB, and PI3K/AKT/mTOR deregulation in MCL. BCR engagement induces SYK phosphorylation, which in turn activates phospholipase C-γ (PLC-γ) and protein kinase C-β (PKC-β). PI3K functions as a transducer of BCR signaling and can be activated by SYK-dependent phosphorylation of CD19 and B-cell PI3K adaptor protein (BCAP). PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) on the plasma membrane to generate the second messenger, phosphatidylinositol-3,4,5-trisphosphate (PIP3). This process is reverted by PTEN. PI3K then phosphorylates PDK1 and the serine/threonine kinase AKT (Thr308) that activates mTOR (by inactivation of the inhibitor TSC1/2) and NF-κB (by activation of IKK). Only the mTOR complex 1 (mTORC1) is under AKT control and activates cap-dependent translation through S6K and 4E-BP1. mTOR complex 2 (mTORC2) can phosphorylate AKT (Ser473), increasing its kinase activity. Canonical NF-κB activation through AKT, or PKC-β involves IKK-mediated phosphorylation of the inhibitor IκBα, resulting in its proteasomal degradation and release of bound transcription factors that can then translocate to the nucleus. A20 and FAF1 inhibit NF-κB activation. The alternative pathway is activated by phosphorylation of p100 by IKKα complexes and subsequent proteasomal generation of p52. The cytokine BAFF/BLyS by binding to BAFF-R activates both canonical and noncanonical pathways. NF-κB transcription factors form heterodimers and homodimers to activate the transcription of genes involved in survival, proliferation, and apoptosis. Several steps in these signaling pathways are altered in MCL; blue symbols (Δ) indicate molecules inactivated or down-regulated; red symbols, molecules activated or overexpressed. Illustration by Paulette Dennis.
BCR, NF-κB, and PI3K/AKT/mTOR deregulation in MCL. BCR engagement induces SYK phosphorylation, which in turn activates phospholipase C-γ (PLC-γ) and protein kinase C-β (PKC-β). PI3K functions as a transducer of BCR signaling and can be activated by SYK-dependent phosphorylation of CD19 and B-cell PI3K adaptor protein (BCAP). PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) on the plasma membrane to generate the second messenger, phosphatidylinositol-3,4,5-trisphosphate (PIP3). This process is reverted by PTEN. PI3K then phosphorylates PDK1 and the serine/threonine kinase AKT (Thr308) that activates mTOR (by inactivation of the inhibitor TSC1/2) and NF-κB (by activation of IKK). Only the mTOR complex 1 (mTORC1) is under AKT control and activates cap-dependent translation through S6K and 4E-BP1. mTOR complex 2 (mTORC2) can phosphorylate AKT (Ser473), increasing its kinase activity. Canonical NF-κB activation through AKT, or PKC-β involves IKK-mediated phosphorylation of the inhibitor IκBα, resulting in its proteasomal degradation and release of bound transcription factors that can then translocate to the nucleus. A20 and FAF1 inhibit NF-κB activation. The alternative pathway is activated by phosphorylation of p100 by IKKα complexes and subsequent proteasomal generation of p52. The cytokine BAFF/BLyS by binding to BAFF-R activates both canonical and noncanonical pathways. NF-κB transcription factors form heterodimers and homodimers to activate the transcription of genes involved in survival, proliferation, and apoptosis. Several steps in these signaling pathways are altered in MCL; blue symbols (Δ) indicate molecules inactivated or down-regulated; red symbols, molecules activated or overexpressed. Illustration by Paulette Dennis.
Several mechanisms may cause constitutive activation of AKT, including activation of upstream kinases such as SYK, and amplification of PI3KCA, the gene encoding the catalytic subunit p110α.70 In contrast to solid tumors, no activating somatic mutations of PI3KCA have been identified.41,70 Loss of PTEN, a phosphatase that turns the PI3K pathway off, is another recurrent feature in MCL and may be the result of mutations, deletions, or promoter methylation.41 PTEN can also be inactivated by phosphorylation at serine 380 and threonine 382/383, which has been found in MCL cases with constitutively active AKT.39 The mechanism of PI3K/AKT/mTOR activation can determine the therapeutic potential of small molecule inhibitors. In tumors addicted to an upstream kinase such as SYK, PI3K/AKT signaling can effectively be turned off by inhibition of the upstream kinase. In contrast, in tumors with constitutive activation of PI3K or AKT, inhibitors directed at these kinases may be required.
PI3K inhibitors.
The PI3K inhibitor Ly294002 induces p27 accumulation and cyclin D1 down-regulation in vitro, leading to G1 arrest in MCL cell lines. Eventually, apoptosis ensues probably because of activation of Bad and the down-regulation of MCL-1 and BCL-XL.39,69 Recently, several PI3K isoform-specific inhibitors have entered clinical trials in NHL. However, the relative importance of the different PI3K isoforms in MCL pathogenesis is unclear. Although PI3Kδ is primarily activated by BCR signaling, PI3Kα has been shown to be sufficient to support survival of B cells with defective BCR signaling.71 ON-01910.Na, a styryl sulfone that shows activity against PI3Kα, reduced eukaryotic initiation factor 4E–mediated cyclin D1 mRNA translation and induced apoptosis in MCL cell lines.72 CAL-101, a selective PI3Kδ inhibitor, reduced constitutive and BCR-induced AKT phosphorylation, resulting in apoptosis. It also inhibited CXCL12-induced migration of MCL cell lines.73 In a phase 1 pilot study this oral compound achieved remissions in 6 of 7 patients with MCL.125 Perifosine, a synthetic alkylphospholipid, targets the pleckstrin homology domain of AKT, thereby preventing its translocation to the plasma membrane.74 It thus inhibits AKT without affecting the activity of PI3K or phosphoinositide-dependent kinase 1 (PDK1). Perifosine has shown preclinical activity in several cancers, including MM,75 and is now undergoing clinical testing in MM, CLL, and lymphoma, including MCL.
mTOR inhibitors.
mTOR, a serine-threonine kinase downstream of AKT, forms the complexes, mTORC1 (mTOR-Raptor) and mTORC2 (mTOR-Rictor), which have distinct substrates and mechanisms of activation (Figure 4). The best-known substrates of TORC1 are ribosomal protein S6 and 4E-BP1. Recent genetic studies in a murine model showed that phosphorylation of 4E-BP1 is a key step in the oncogenic pathway downstream of AKT/mTOR, whereas phosphorylation of S6 was dispensable.76 Phosphorylation of 4E-BP1 leads to its dissociation from eIF4, resulting in increased translation of key proteins that includes cyclin D1, c-MYC, and MCL-1. The main substrate of mTORC2 is AKT.
Rapamycin and its analogs (rapalogs) are allosteric inhibitors of the mTORC1 complex that block some but not all mTORC1 functions and have no effect on mTORC2 (Table 2).77 Rapalogs do not induce apoptosis of MCL cells in vitro,78-80 but most studies report induction of G0/G1 arrest. The mechanisms of cell-cycle arrest have remained ill defined, and effects on cyclin D1 and p27 differ between different studies.39,80 One explanation for this variability could be that GSK-3β, which promotes nuclear export and proteasomal degradation of cyclin D1, is inactivated by phosphorylation through either AKT or mTOR (Figure 2). Thus, mTOR inhibitors will increase cyclin D1 proteolysis only when inactivation of GSK-3β is dependent on mTOR.39
In a multicenter trial temsirolimus was superior to investigator's choice of monochemotherapy in relapsed MCL81 and was subsequently approved for this indication by the European Medicines Agency. Clinical responses to rapalogs are mostly partial responses (PRs) in 20%-40% of patients, which are of relatively short duration.79,81-83 Analysis of MCL tumor cells from patients during treatment with temsirolimus confirmed reduced S6 phosphorylation. However, there was no correlation to clinical response.79 Explanations for the limited clinical activity of rapalogs are increasingly apparent.76,77 Rapalogs inhibit only some mTORC1 functions, and inhibition of S6 phosphorylation, while consistently detected, does not induce apoptosis and has only minimal effects on translation. Furthermore, rapalogs have no effect on mTORC2, which can activate AKT in a negative feedback loop. Thus, mTOR catalytic site inhibitors, which target mTORC1/2 equally, could be more effective.
NF-κB pathway
The NF-κB family of transcription factors, (p50/p105, p65/RELA, c-REL, RELB, and p52/p100) binds DNA as heterodimers and homodimers that activate the transcription of genes involved in survival, proliferation, and apoptosis.84 The canonical pathway is activated through phosphorylation and consequent degradation of IκBα, a cytosolic inhibitor that sequesters p50 and RELA. Constitutive activation of the canonical NF-κB pathway has been reported in MCL cell lines evidenced by the presence of pIκBα and nuclear p65, p50, and c-REL (Figure 4). Gene-expression profiling of MCL patient samples showed frequent high expression of NF-κB target genes that correlated with strong pIκBα expression on tissue microarrays.42,85 NF-κB target genes highly expressed in MCL include the antiapoptotic proteins BCL-2, BCL-XL, XIAP, and cFLIP.86,87 Activation of the noncanonical pathway (p52, RELB) has been reported in some MCL cell lines (Granta-519, JVM-2, and NCEB), but this could be due to Epstein-Barr virus transformation. BCR engagement and TNF signaling in the lymphoma microenvironment may contribute inducible NF-κB activation. NF-κB signaling can also drive transcription of the TNF family member BAFF/BLyS, a potent B-cell survival factor that binds BAFF-R, BCMA, and TACI. BAFF in turn stimulates both canonical and alternative NF-κB pathways, activating a positive feedback loop that could contribute to tumor cell survival.43 Recently, BAFF-R has been detected in the nucleus of MCL cells, where it colocalized with inhibitor of nuclear factor κB kinase β (IKKβ) and cooperated in histone H3 phosphorylation. BAFF-R associated with c-REL, leading to an increase transcription of genes that promote cell survival and proliferation.44 Constitutive activation of NF-κB signaling may also be caused by the inactivation of TNFAIP3/A20, a ubiquitin-editing enzyme that acts as a negative regulator. TNFAIP3/A20 is often inactivated in MCL through genomic deletions, mutations, and increased promoter methylation.88 Moreover, MCL cases showed mono- and bi-allelic deletions of FAF1, which inhibits p65 and IKKβ.31
IKK inhibitors.
In keeping with the role of NF-κB in MCL survival, inhibitors of this pathway, such as BAY-117082, curcumin, or the IKKβ inhibitor BMS-345541, have shown in vitro activity in MCL.86,87,89 However, despite its preclinical promise it has apparently proven difficult to translate this class of compounds into the clinic, and except for curcumin no clinical trials of these compounds are currently ongoing.90
Proteasome inhibition to block NF-κB signaling.
Inhibition of the NF-κB pathway has initially been hypothesized as mechanisms of bortezomib-induced apoptosis. However, in MM it has recently been shown that bortezomib efficiently inhibits inducible but not constitutive NF-κB activity and can even cause canonical NF-κB activation.52 Proteasome inhibitor–resistant NF-κB activity has also been described in MCL.53 However, in activated B cell–like diffuse large B-cell lymphoma, an aggressive lymphoma having constitutive activation of NF-κB, bortezomib appeared to sensitize cells to combination chemotherapy,91 suggesting that NF-κB inhibition may play a role in some tumors but not others.
WNT pathway
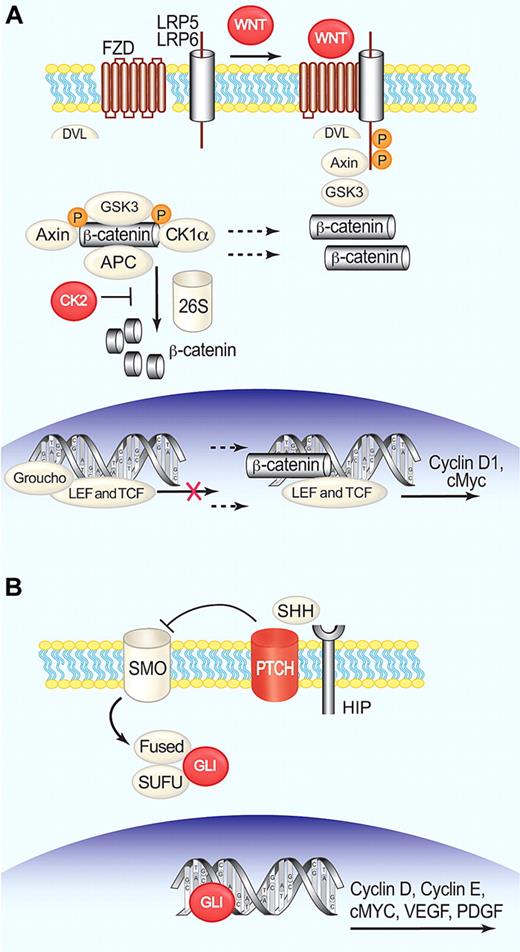
WNT signaling can occur through 2 main mechanisms, a canonical pathway through β-catenin (Figure 5A) and an alternative pathway leading to c-Jun N-terminal kinase activation. The canonical pathway is considered to be more relevant in cancer.92 Microarray analysis provided a first indication that the WNT pathway may be activated in MCL.40 Recently, nuclear expression of transcriptionally active β-catenin has been detected in MCL cell lines and tumor biopsies and correlated with GSK3β inactivation.93 WNT3 and WNT10a are consistently expressed in MCL cell lines and patient biopsies, and amplification of WNT11 on 11q13.4-q13.5 has been found in MCL cell lines. High expression of several Frizzled receptors and low density lipoprotein receptor-related protein 5 has been documented, indicating a possible autocrine loop of WNT signals. Furthermore, the serine-threonine kinase CK2, which is constitutively phosphorylated in MCL,38 phosphorylates β-catenin at Thr393, thereby impeding its proteasomal degradation. The resultant stabilization of β-catenin increases its transcriptional activity. Inhibition of the canonical pathway by small interfering RNA mediated knockdown of DVL2 and Frizzled 2 or by the pharmacologic agent quercetin reduced proliferation and induced apoptosis.46,94
Activation of WNT and hedgehog pathways in MCL. (A) In the absence of WNT ligand, β-catenin is actively degraded through a protein complex called the “destruction box,” where GSK3β phosphorylates β-catenin, targeting it for degradation by the proteasome. Binding of WNT to Frizzled (FZD)– low density lipoprotein receptor-related protein (LRP) receptor complexes at the membrane, induces the formation of Dishevelled (DVL)–FZD complexes that sequester Axin and GSK3β, inhibiting the formation of the destruction box. This allows translocation of β-catenin to the nucleus where it dimerizes with TCF and LEF.134 (B) Binding of SHH to patched (PTCH), allows smoothened (SMO) to transduce a signal into the cytoplasm that leads to the breakdown of a protein complex formed by Fused, SUFU, and the transcription factor GLI. GLI is thereby released and translocates into the nucleus.135 Red symbols indicate molecules activated or overexpressed in MCL. Illustration by Paulette Dennis.
Activation of WNT and hedgehog pathways in MCL. (A) In the absence of WNT ligand, β-catenin is actively degraded through a protein complex called the “destruction box,” where GSK3β phosphorylates β-catenin, targeting it for degradation by the proteasome. Binding of WNT to Frizzled (FZD)– low density lipoprotein receptor-related protein (LRP) receptor complexes at the membrane, induces the formation of Dishevelled (DVL)–FZD complexes that sequester Axin and GSK3β, inhibiting the formation of the destruction box. This allows translocation of β-catenin to the nucleus where it dimerizes with TCF and LEF.134 (B) Binding of SHH to patched (PTCH), allows smoothened (SMO) to transduce a signal into the cytoplasm that leads to the breakdown of a protein complex formed by Fused, SUFU, and the transcription factor GLI. GLI is thereby released and translocates into the nucleus.135 Red symbols indicate molecules activated or overexpressed in MCL. Illustration by Paulette Dennis.
Hedgehog pathway
Recent studies have suggested that stromally produced hedgehog proteins Indian hedgehog, sonic hedgehog (SHH), and desert hedgehog have a role in the proliferation of hematopoietic stem cells, lymphoid cells, and in B-cell malignancies.95 The molecules associated with SHH signaling (Figure 5B) and its target transcripts PTCH, GLI1, and GLI2 are overexpressed in MCL primary cells compared with normal B cells. Interestingly, GLI is located at 12q13 and may be coamplified with CDK4 and MDM2 in some tumors.45 Activation of PTCH by SHH may increase proliferation of MCL cells, an effect that could be inhibited by cyclopamine, a specific inhibitor of SHH-GLI signaling. Furthermore, down-regulation of GLI transcription factors with the use of antisense oligonucleotides significantly reduced cyclin D1 and BCL-2 transcript levels, decreased proliferation of MCL cells, and increased their susceptibility to chemotherapy.96 These results indicate a role for SHH-GLI signaling in MCL proliferation and identify the hedgehog pathway as a potential therapeutic target in MCL
Antiapoptotic BCL-2 family proteins
BCL-2 family proteins are key regulators of apoptosis, determining cellular fate in response to numerous insults (Figure 6).97 The BCL-2 pathway is deregulated in MCL; high-level copy number gains of the BCL2 locus at 18q21.3 are frequent,31 high MCL1 expression is common in more aggressive tumors98 and can be increased by AKT/mTOR signaling, and BCL-XL overexpression has been linked to constitutive activation of the NF-κB pathway.89 Furthermore, the proapoptotic BH3-only protein BIM is frequently inactivated through genomic deletions of the BCL2L11 gene. Homozygous deletions of the BCL2L11 locus are found in MCL cell lines (Jeko, Z138, SP53, UPN1, SP-49), and immunohistochemistry on tissue arrays showed the absence of BIM expression in 32% of primary tumors.4,99
The antiapoptotic phenotype and alterations in BCL-2 family members in MCL. The prosurvival BCL-2 family members (BCL-2, BCL-XL, MCL-1, BCL-W, and A1/BFL1) bind and sequester the apoptosis-inducing members BAX and BAK. The BH3-only proteins (BIM, PUMA, NOXA, BAD, BID, BMF, BIK, and HRK) can be activated by cytotoxic signals and selectively engage prosurvival members, which leads to release of BAX and BAK, leading to permeabilization of the mitochondrion, the release of proapoptotic factors, caspase activation, and, finally, cell death.136,137 BCL2L11/BIM is frequently deleted (blue symbol [Δ]), whereas some antiapoptotic family members are commonly overexpressed (red symbols) in MCL, including BCL-2. In addition, PI3K/AKT/mTOR signaling can inactivate BAD through phosphorylation and stabilize of MCL-1 protein. Illustration by Paulette Dennis.
The antiapoptotic phenotype and alterations in BCL-2 family members in MCL. The prosurvival BCL-2 family members (BCL-2, BCL-XL, MCL-1, BCL-W, and A1/BFL1) bind and sequester the apoptosis-inducing members BAX and BAK. The BH3-only proteins (BIM, PUMA, NOXA, BAD, BID, BMF, BIK, and HRK) can be activated by cytotoxic signals and selectively engage prosurvival members, which leads to release of BAX and BAK, leading to permeabilization of the mitochondrion, the release of proapoptotic factors, caspase activation, and, finally, cell death.136,137 BCL2L11/BIM is frequently deleted (blue symbol [Δ]), whereas some antiapoptotic family members are commonly overexpressed (red symbols) in MCL, including BCL-2. In addition, PI3K/AKT/mTOR signaling can inactivate BAD through phosphorylation and stabilize of MCL-1 protein. Illustration by Paulette Dennis.
Targeting BCL-2 family members to restore apoptosis.
The recognition of the importance of the BCL-2 family proteins in cancer has sparked efforts to target these critical survival molecules. One approach has been to reduce BCL-2 expression with compounds affecting gene or protein expression.100 A BCL-2 antisense oligonucleotide, oblimersen,101 has undergone extensive clinical testing in various malignancies, including MCL.102 However, despite encouraging preclinical data and some activity in clinical trials, the compound has repeatedly failed to gain FDA approval,100 and clinical trial activity has virtually stopped.90 In addition, oblimersen contains CpG motifs and acts as a Toll-like receptor 9 ligand, giving rise to many effects beyond BCL-2 inhibition.100 The disappointing activity of oblimersen may be due to pharmacokinetic issues or redundancy within the BCL-2 system that makes inhibition of BCL-2 alone not very effective. A different strategy that may overcome this later limitation is based on small molecules directly inhibiting the function of BCL-2 antiapoptotic proteins. Several compounds have been isolated or chemically synthesized that mimic the action of BH3-only proteins and thereby promote apoptosis. Several such BH3-mimetics (ABT-737 [oral compound in clinical trials is ABT-263], AT-101, GX15-070) have shown in vitro and in vivo activity, both as single agents and in combination with proteasome inhibitors or conventional chemotherapy.103-105 BH3-mimetic compounds differ in their specificity and affinity for various BCL-2 family members.106 GX15-070 and AT-101 are considered pan–BCL-2 inhibitors and can antagonize MCL-1, which is up-regulated by proteasome inhibition. Thus, combination of these compounds with bortezomib yields synergistic antitumor activity.105 In contrast, ABT-737 does not neutralize MCL-1 or A1/BFL-1, high expression of which may cause resistance to this BH3 mimetic.107
Microenvironment in MCL pathogenesis and immunomodulatory drugs
The microenvironment has been found to play an important role in the biology of several B-cell malignancies. There are some indications that tumor host interactions might be more important in MCL than is currently appreciated. First, MCL almost invariably involves the gastrointestinal tract, often only microscopically, but large tumors growing in the intestinal wall may cause presenting symptoms. This suggests that microenvironment factors determine disease localization or promote tumor cell expansion or both at these sites. Second, in vitro studies show that MCL cells interact with bone marrow stromal cells, and these interactions can contribute to chemoresistance.108 Third, gene expression profiling showed increased expression of factors involved in cell-cell crosstalk such as CCL4 and TNFSF9 (4-1BB-L) in MCL cells compared with normal B cells.109 An intriguing observation is that cell adhesion to bone marrow stromal cells can decrease the proteasomal destruction of the cell-cycle inhibitor p27, leading to reversible growth arrest.110 Such a “dormant” state could protect cells from cytotoxic therapy and may provide an explanation how rapidly proliferating MCL tumors can remain in remission for years before relapsing again as a highly proliferative cancer.
The immunomodulatory drugs (IMiDs), thalidomide and lenalidomide, are active in MM. Although the precise mechanism of action is not understood, effects on the microenvironment either by disruption of tumor-stroma interactions or through activation of immune effector mechanisms are hypothesized to contributed to the therapeutic effect.111 Recently, lenalidomide has been shown to improve formation of so-called “immune synapses” between CLL cells and autologous T cells, which could enhance immune-mediated antitumor effects.112 Phase 2 studies of single-agent lenalidomide in lymphoma have yielded promising results in MCL (Table 2).113 In the NHL-002 study lenalidomide induced responses in 53% of 15 heavily pretreated patients. The progression-free survival (PFS) of 5.6 months and the median diagnostic odds ratio of 13.7 months compares favorably with other treatment options for these patients.113 In the international NHL-003 study the response rate in 57 patients with MCL was 42% with a median PFS of 5.7 months.114 A study of thalidomide in combination with rituximab found a response rate of 81% and a PFS of 20 months in 16 patients with relapsed MCL.115 Despite these encouraging results no follow-up study with the use of this combination has been reported.
Epigenetic regulation of tumor biology
In contrast to the detailed profiling of genetic lesions in MCL, epigenetic changes have only recently begun to be explored. Acetylation of histones leads to an open chromatin conformation that facilitates the access of transcription factors to DNA. In contrast, methylation of gene promoters can silence gene expression. Both acetylation and methylation can be altered in tumors and can contribute to disease pathogenesis. A recent study of DNA methylation in primary MCL identified several genes that were hypermethylated and thereby silenced in tumor cells compared with normal B cells.116,117 The DNA methyltransferase inhibitor decitabine not only reversed the aberrant hypermethylation but also synergized with a histone deacetylase inhibitor (HDACi) and induced cytotoxicity.116 Several hypomethylated and thereby up-regulated genes could play a pathogenic role, including NOTCH1, CDK5, and HDAC1. Surprisingly, the frequently observed methylation of CDKN2B did not correlate with increased tumor proliferation.117
HDAC inhibitors.
Hypoacetylation of histones is found in lymphomas compared with normal lymphoid tissue, and data suggest that transformed cells are more sensitive to HDAC inhibition than normal cells.118 Studies with MCL cell lines have shown that suberoylanilide hydroxamic acid (SAHA), the only HDACi currently approved by the FDA, induces histone acetylation, up-regulates p21 and p27, causes cell-cycle arrest, and induces apoptosis. Interestingly, SAHA also reduced cyclin D1 expression at the protein but not mRNA level, through an unexpected inhibition of the PI3K/AKT/mTOR pathway.119
There is compelling evidence that HDACis also affect the handling of misfolded proteins. HDAC6 is a key factor in the aggresome pathway, a proteasome-independent pathway of protein degradation. The aggresome pathway is up-regulated in response to proteasome inhibition, suggesting that simultaneous inhibition of both degradation pathways may result in an improved therapeutic effect. In this regard, preclinical data indicated a synergistic interaction between SAHA and bortezomib, resulting in increased reactive oxygen species generation and apoptosis.120
Heat shock proteins, chaperones of tumor biology, and HSP90 inhibitors
Heat shock proteins (HSPs) are ubiquitously expressed chaperones that facilitate and guard the proteome from misfolding and aberrant aggregation. HSP90 is of particular interest because many of its clients are oncogenic signaling proteins, including cyclin D1, c-MYC, p53, CDKs, AKT, IκB kinases, and survivin. HSP90 inhibitors displace the client proteins and target them for destruction by the proteasome system.121 Resultant depletion of essential oncoproteins can induce cell-cycle arrest and apoptosis. HSP90 in tumor cells is present entirely in active, complexed form, rendering cancer cells more sensitive to HSP90 inhibitors than normal cells. HSP90 inhibitors have been a focus of drug development for more than 10 years, but no compound of this class has gained clinical approval.122 The first generation of HSP90 inhibitors 17-AAG and 17-DMAG are derivatives of the natural product geldanamycin. Most clinical trials used 17-AAG, showing moderate antitumor activity, which in part may be due to poor water solubility.122 Additional 17-AAG derivatives and several synthetic HSP90 inhibitors have been developed and could overcome some of the limitations of the older compounds. HSP90 inhibitors may be of particular value in combination with proteasome inhibitors. For example, IPI-504 synergized with bortezomib in a MM xenograft model123 and is active against bortezomib-resistant MCL cells possibly because of the down-regulation of the endoplasmic reticulum chaperone BIP/GRP78.124 An intriguing feature of this combination is that HSP90 inhibitors may reduce the neurotoxicity observed with bortezomib.122
Outlook
Progress is being made! Although median survival of MCL has typically been in the range of 3-4 years, recent series have reported 5-7 years.8,9 In addition to the introduction of dose-intensified regimens, the advent of effective salvage treatments has probably made a major contribution. To optimize the use of novel agents discussed in this review it will be imperative to incorporate comprehensive translational studies into clinical trials. Keep in mind that of the 3 classes of novel agents available for the treatment of relapsed patients, namely proteasome inhibitors, mTOR inhibitors, and lenalidomide, we still have only a limited understanding of their mechanisms of action and determinants of efficacy. In recent years MCL has been recognized as a heterogeneous disease with an extremely variable clinical course, making it essential to identify the patient subsets that are most likely to respond to distinct therapeutic approaches. Heading toward further improvement of therapy, the limiting step may be patient participation in well-designed clinical trials, which incorporate comprehensive analyses of tumor samples. This is a prerequisite to unravel disease heterogeneity, develop predictive markers, and advance therapeutic options. Thus, patients with MCL should be encouraged to enroll in clinical trials with a strong translational research program.
Acknowledgment
This work was supported by the Intramural Research Program of the National Institutes of Health, the National, Heart, Lung and Blood Institute (P.P.-G. and A.W.).
National Institutes of Health
Authorship
Contribution: P.P.-G., M.D., and A.W. wrote the paper.
Conflict-of-interest disclosure: M.D. received speakers' honoraria from Bayer, Mundiopharma/Cephalon, Pfizer, and Roche, clinical research support from Amgen, Bayer, Celgene, Janssen, Mundipharma, Pfizer, and Roche, and preclinical research support from GSK, Lilly, and Roche Glycart; and served on the scientific advisory boards of Calistoga, Celgene, Janssen, Mundipharma/Cephalon, Pfizer, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Adrian Wiestner, Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bldg 10, CRC 3-5140, 10 Center Dr, Bethesda, MD 20892-1202; e-mail: wiestnea@nhlbi.nih.gov.

![Figure 6. The antiapoptotic phenotype and alterations in BCL-2 family members in MCL. The prosurvival BCL-2 family members (BCL-2, BCL-XL, MCL-1, BCL-W, and A1/BFL1) bind and sequester the apoptosis-inducing members BAX and BAK. The BH3-only proteins (BIM, PUMA, NOXA, BAD, BID, BMF, BIK, and HRK) can be activated by cytotoxic signals and selectively engage prosurvival members, which leads to release of BAX and BAK, leading to permeabilization of the mitochondrion, the release of proapoptotic factors, caspase activation, and, finally, cell death.136,137 BCL2L11/BIM is frequently deleted (blue symbol [Δ]), whereas some antiapoptotic family members are commonly overexpressed (red symbols) in MCL, including BCL-2. In addition, PI3K/AKT/mTOR signaling can inactivate BAD through phosphorylation and stabilize of MCL-1 protein. Illustration by Paulette Dennis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/1/10.1182_blood-2010-04-189977/4/m_zh89991063490006.jpeg?Expires=1765892893&Signature=tNMNr-XosL~1H8a8qjGevT6DQx-tDx3FUqoNRXv2nEhN4jNUycjfSuiUGOU0MR4u2f-DI5xHAXU1VkdJOnORUhup5tGohHpQYcpzd8I96SqCryuPkWt0tNvIFSO8VpFAEKikwxUOuXT9kTU50P0xO5khK7jzZeXgHoSfWegzITaNrC1~Fh9~2AFgHc97WnI25JbH~7zO6Dh0bk34bLwdl5yAEhDHO45hv6Fzzh3KeDk~y-ihdW3Jap17AZjeUdL5QK9V9o4VoOz05Y6DBa1dBglRQckL420m~XT1YxcsgASi0QmfKK~wv9u~GwC2o5V8IEOfJFmT6D8YlQfW7wgKwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)