Abstract
Severe immune deficiency follows autologous stem cell transplantation for multiple myeloma and is associated with significant infectious morbidity. This study was designed to evaluate the utility of a pretransplantation vaccine and infusion of a primed autologous T-cell product in stimulating specific immunity to influenza. Twenty-one patients with multiple myeloma were enrolled from 2007 to 2009. Patients were randomly assigned to receive an influenza-primed autologous T-cell product or a nonspecifically primed autologous T-cell product. The study endpoint was the development of hemagglutination inhibition titers to the strain-specific serotypes in the influenza vaccine. Enzyme-linked immunospot assays were performed to confirm the development of influenza-specific B-cell and T-cell immunity. Patients who received the influenza-primed autologous T-cell product were significantly more likely to seroconvert in response to the influenza vaccine (P = .001). Seroconversion was accompanied by a significant B-cell response. No differences were observed in the global quantitative recovery of T-cell and B-cell subsets or in global T-cell and B-cell function. The provision of a primed autologous T-cell product significantly improved subsequent influenza vaccine responses. This trial was registered at www.clinicaltrials.gov as #NCT00499577.
Introduction
Epidemics of influenza A virus strains have been associated with hospitalization of approximately 200 000 people per year in the United States with 30 000-50 000 deaths per year. The case fatality rates have been estimated to be 0.5/100 000 in the age range from birth to 49 years and as high as 100/100 000 in the > 65-year age range.1-3 The primary method to combat influenza is the administration of a vaccine appropriate to the seasonal infecting strains.
Patients with compromised immunity are at particularly high risk of complications from influenza infection, yet, they have less protection from vaccination.4,5 Patients with malignancy have an increased attack rate of influenza, and data suggest that 10%-40% of oncology patients are infected during each seasonal epidemic.6 This is higher than in the general population in which the case rate is usually 5%-15%.
Multiple myeloma is one example of a population particularly at risk for severe influenza.7,8 Patients have severe humoral and cellular immune deficiency.9,10 This is associated with impaired responses against both tumor, microbial, and vaccine antigens.11-14 Furthermore, therapy with high-dose melphalan and autologous stem cell transplantation (ASCT) is used frequently for the treatment of relapsed or refractory disease.15,16 Although hematopoietic recovery after ASCT occurs within 3 weeks, full recovery of T- and B-cell function may take months to years, and vaccine responses are typically poor.17-21 Patients after ASCT have increased rates of morbidity associated with respiratory viruses in general.22,23 This, coupled with a higher attack rate, the potential for prolonged shedding, and the emergence of resistant viruses, mandates that improved preventive strategies be developed.24,25
The altered number, function, and dynamics of immune cell recovery after ASCT for myeloma increases patient risk of serious infections such as varicella-zoster virus, cytomegalovirus (CMV), Streptococcus pneumoniae, and influenza.26 In particular, influenza accounts for 20% of respiratory virus infections in the patients who receive a transplant.27 The ability to respond to influenza vaccination is impaired well beyond the quantitative repletion of lymphocytes.13,28 Strategies to accelerate and augment the recovery and function of autologous T cells after transplantation for myeloma may be beneficial.
In this study, we examined whether immunization with the seasonal influenza vaccine would be immunogenic in patients with myeloma undergoing ASCT and whether sustained influenza-specific protective responses could be elicited after ASCT. Our data show that vaccine-primed autologous T-cell infusions are associated with restoration of antigen-specific antibody production. Combined vaccination and adoptive transfer represents a powerful strategy to improve host defenses after ASCT.
Methods
Study design
Patients for this randomized, controlled study were recruited from the Abramson Cancer Center at the University of Pennsylvania in Philadelphia. This study was distinct from a prior study that investigated the timing of an autologous T-cell product and pneumococcal vaccine responses.29 The study protocol was approved by the University of Pennsylvania Institutional Review Board, and informed consent was obtained from patients in accordance with the Declaration of Helsinki. The analysis plan was determined before study initiation, and laboratory assays were performed in a blinded fashion.
Eligible patients were patients with symptomatic multiple myeloma scheduled to receive an ASC transplant on a companion study (UPCC 13406, ClinicalTrials.gov Identifier NCT00499577), which biologically assigned patients by human leukocyte antigen (HLA) type to peptide vaccination against human telomerase reverse transcriptase and survivin (HLA-A2 patients) or no human telomerase reverse transcriptase vaccination (all other HLA types); all patients received peptide vaccines against CMV and the pneumococcal polyvalent conjugated vaccine as well as granulocyte-macrophage colony-stimulating factor injected at the sites of peptide vaccines. Patients were required to have high-risk disease as defined by either cytogenetics or measurable disease (less than complete remission) at the time of transplantation. Patients had adequate organ function as defined by serum creatinine level < 3.0 mg/dL, left ventricular ejection fraction > 45%, and diffusing capacity of lungs for carbon monoxide > 40% predicted. Patients could not have had an influenza vaccination within 2 months before study entry.
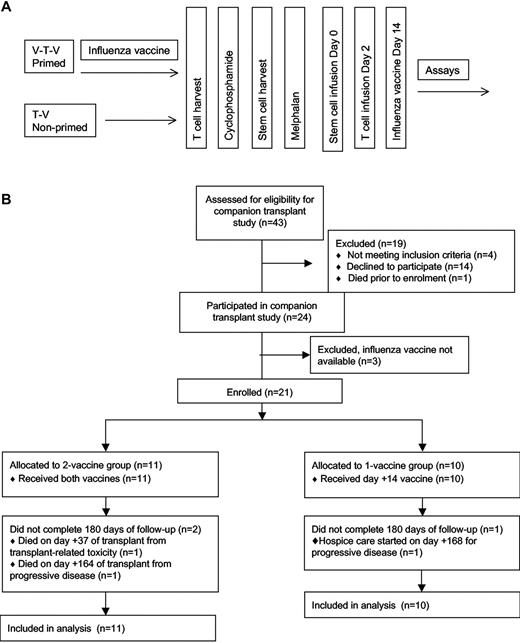
Patients were randomly assigned 1:1 to the vaccine-transfer-vaccine group or the transfer-vaccine group (Figure 1). No stratification variables were used. Patients in the vaccine-transfer-vaccine group received seasonal influenza vaccine before T-cell harvest, whereas patients in the transfer-vaccine group did not. All patients then received the seasonal influenza vaccination on day 14. Before transplantation, both groups underwent steady-state T-cell harvest (14 days after the first vaccine for the vaccine-transfer-vaccine group) by 1-hour apheresis followed by administration of cyclophosphamide 4.5 g/m2 over 12 hours and granulocyte colony-stimulating factor–stimulated blood stem cell harvest 2 weeks later. Patients then received melphalan 200 mg/m2 over 1 hour followed by infusion 24-48 hours later of ≥ 2 × 106 CD34+ cells/kg on day 0. All patients received an infusion of costimulated autologous T cells on day 2. Thus, patients in the vaccine-transfer-vaccine group received a preharvest influenza vaccine and will be referred to as the primed group, and both patient groups received an influenza vaccine after ASCT. The patients in the transfer-vaccine group will be referred to as the nonprimed group.
The study protocol. (A) The clinical protocol is shown schematically. Primed patients received a vaccine both before and after ASCT (V-T-V), whereas the nonprimed group received the vaccine only after ASCT (T-V). (B) The CONSORT diagram of the study enrollment.
The study protocol. (A) The clinical protocol is shown schematically. Primed patients received a vaccine both before and after ASCT (V-T-V), whereas the nonprimed group received the vaccine only after ASCT (T-V). (B) The CONSORT diagram of the study enrollment.
The commercially available seasonal influenza vaccine (Fluzone; Sanofi-Pasteur) was given intramuscularly. Throughout the course of the study 3 seasonal vaccines were used: 2006-2007 season used A/New Caledonia/20/99 (H1N1)–like virus, A/Wisconsin/67/2005 (H3N2)–like virus, and B/Malaysia/2506/2004-like virus; 2007-2008 season used A/Solomon Islands/3/2006 (H1N1)–like virus, A/Wisconsin/67/2005 (H3N2)–like virus, and B/Malaysia/2506/2004-like virus; and 2008-2009 season used A/Brisbane/59/2007 (H1N1)–like virus, A/Brisbane/10/2007 (H3N2)–like virus, and B/Florida/4/2006-like virus. The 7 valent-conjugated pneumococcal vaccine was given to all subjects, and responses to 4 serotypes were measured. For analytic purposes, the 4 serotype responses were summed to a single microgam per milliliter value at each time point.
Blood samples from patients undergoing ASCT were obtained at baseline; before steady-state T-cell harvest; and on days 14, 60, 100, and 180 after stem cell infusion. Patients were examined during these time points and clinical events were recorded.
T-cell infusions
Patients underwent apheresis to collect > 1 × 109 mononuclear cells. For the primed group, this procedure was scheduled 14 days after the first vaccine. The cells were depleted of monocytes and cryopreserved. The T-cell manufacturing process has been described previously30 and consisted of CD3 and CD28 bead-based stimulation. All infused T-cell products were required to meet safety and release criteria specified by the Food and Drug Administration before infusion. The target number of T cells for infusion was ≈ 5 × 1010 T cells.
Immunologic assays
The assessment of the primary endpoint, influenza vaccine response, used a standard hemagglutination inhibition (HAI) assay optimized for the vaccine administered each year.31 Flow cytometry for T-cell and B-cell subsets was performed to characterize those compartments after ASCT and T-cell infusion. Analysis of T cells used fixation with 1% paraformaldehyde and were run on an LSR II (BD Biosciences) and analyzed with FlowJo Version 7.6 software (TreeStar). Approximately 200 000-500 000 events were collected per sample. CD4-naive cells were defined as CD45RA+CD31+, CD4 central memory T cells were defined as CD27+CD45RO+CCR7+, CD4 effector memory T cells were defined as CD45RO+/CD27+/CCR7−, and CD4 reverted memory T cells were defined as CD45RA+/CD31−/CCR7+. CD8-naive cells were defined as CD45RA+CD31+, CD8 central memory T cells were defined as CD27+CD45RO+CCR7+, CD8 effector memory T cells were defined as CD45RO+/CD27+/CCR7−, and CD8 reverted memory T cells were defined as CD45RA+/CD31−/CCR7+.
For B-cell analysis, fresh venous whole blood anticoagulated with EDTA (ethylenediaminetetraacetic acid) was prepared and stained with antibodies (all from BD PharMingen), as described previously.32 B cells were defined as CD19+ lymphocytes. Analyses were performed on a FACSCalibur with CellQuest software (Version 5.2.1; Becton Dickinson). B cells were identified on the basis of CD19 expression and forward and side scatter characteristics consistent with lymphocytes. CD19+ lymphocytes were analyzed for CD27 and immunoglobulin M (IgM). A minimum of 10 000 CD19+ events were generally analyzed per tube. The absolute B-cell count was obtained by multiplying the absolute lymphocyte count by the CD19+ fraction. Five samples were excluded from the analyses in Figure 3 because of failure to meet quality control standards (primarily inadequate cell counts).
To examine functional responses to the vaccine, T-cell Enzyme-linked immunospots (ELISPOTs) and B-cell ELISPOTs were performed. A cocktail of influenza proteins (Protein Sciences), based on the described vaccines and matched to the year of inoculation, was used as specific antigen (at 5 μg/mL) in a standard γ-interferon T-cell ELISPOT assay.33 Phorbol myristate acetate and ionomycin (combined at 5 μg/mL each) were used as a positive control. This assay examined a range of epitopes and was HLA dependent. Foreign antigen sources were avoided to minimize background. The B-cell ELISPOT defined the frequency of memory B cells activated by influenza to produce antibody.34 Peripheral blood mononuclear cells (PBMCs) were stimulated for 6 days with pokeweed mitogen at 1:100 000, Staphylococcus aureus at 1:10 000, and cytosine-phosphate-guanosine 2006 at 6 mg/mL (Sigma Aldrich). After the stimulation period, cells were treated for 6 hours with the influenza protein cocktail described above in this paragraph (at 0.5 μg/mL). Finally, IgG production assessed by quantification of effector cells with the use of ImmunoSpot (CTL; Version 4) software.
Statistical analysis
The comparison of the study's endpoints measured repeatedly over time was carried out with the mixed effects models or the generalized estimating equations (GEE) method or both. The longitudinal assessments of the outcomes were statistically tested with a repeated-measures model with the following 3 main effects: the overall group differences, the overall changes over time, and the interaction effect. Baseline measurements for both groups were used as covariates to adjust for potential group differences at baseline. The independent t test or the Mann-Whitney test was used for the comparisons of responder frequency and seroconversion. The geometric mean titers were calculated with the standard formula: n-th root of (X1)(X2)… (Xn). The 95% confidence intervals of the geometric mean titers were calculated by taking the anti-log of the 95% confidence intervals of the arithmetic means of the log-transformed values. Because of the study design and the large number of needed comparisons, corrections for multiple comparisons were not performed. Significance was set at P < .05.
Results
Patients
A total of 21 patients with multiple myeloma were enrolled between December 2007 and February 2009. As shown in Figure 1, 11 patients were randomly assigned to the primed group (vaccine-transfer-vaccine, before and after transplantation influenza vaccination) and 10 were assigned to the nonprimed group (transfer-vaccine, posttransplantation influenza vaccine only). All patients received the posttransplantation influenza vaccination and were evaluable for the immunologic assessments. The patients in each group did not differ significantly in terms of age, race, sex, immunoglobulin subtype, HLA-A2 status, International Staging System, prior therapies (including dexamethasone and bortezomib), baseline organ function, CD19 count, CD3 count, or absolute lymphocyte count (Table 1).
Patient characteristics
| Characteristic . | Primed group (n = 11) . | Nonprimed group (n = 10) . |
|---|---|---|
| Median age, y (range) | 53 (48-68) | 56 (48-67) |
| Sex, male/female, n | 7/4 | 6/4 |
| Monoclonal protein, n | ||
| IgG | 7 | 4 |
| IgA | 3 | 4 |
| Light chain | 1 | 2 |
| HLA-A2+, n | 10 | 9 |
| hTERT, n | 11 | 10 |
| Race, n | ||
| White | 9 | 10 |
| African American | 2 | 0 |
| ISS, n | ||
| 1 | 9 | 7 |
| 2 | 2 | 2 |
| 3 | 0 | 1 |
| Mean B2M level, mg/L | 2.28 | 4.5 |
| Median (range) | 2.2 (1.19-3.8) | 2.07 (1.32-25.3) |
| Mean albumin level, g/L | 39 | 39.8 |
| Median (range) | 41 (34-42) | 39.5 (32-47) |
| Prior thalidomide, n/N | 2/11 | 2/10 |
| Prior lenalidomide, n/N | 7/11 | 7/10 |
| Prior bortezomib, n/N | 2/11 | 4/10 |
| Prior chemotherapy, n/N | 6/11 | 4/10 |
| Baseline creatinine level, μmol/L, median (range) | 83.1 (43.3-101.7) | 77.8 (70.7-87.5) |
| Mean baseline ALC, ×109/L | 1.4 | 1.3 |
| Median (range) | 1.5 (0.6-1.9) | 1.1 (0.9-1.7) |
| Disease status at time of SCT, n | ||
| PR | 3 | 4 |
| MR | 5 | 1 |
| SD | 3 | 5 |
| Characteristic . | Primed group (n = 11) . | Nonprimed group (n = 10) . |
|---|---|---|
| Median age, y (range) | 53 (48-68) | 56 (48-67) |
| Sex, male/female, n | 7/4 | 6/4 |
| Monoclonal protein, n | ||
| IgG | 7 | 4 |
| IgA | 3 | 4 |
| Light chain | 1 | 2 |
| HLA-A2+, n | 10 | 9 |
| hTERT, n | 11 | 10 |
| Race, n | ||
| White | 9 | 10 |
| African American | 2 | 0 |
| ISS, n | ||
| 1 | 9 | 7 |
| 2 | 2 | 2 |
| 3 | 0 | 1 |
| Mean B2M level, mg/L | 2.28 | 4.5 |
| Median (range) | 2.2 (1.19-3.8) | 2.07 (1.32-25.3) |
| Mean albumin level, g/L | 39 | 39.8 |
| Median (range) | 41 (34-42) | 39.5 (32-47) |
| Prior thalidomide, n/N | 2/11 | 2/10 |
| Prior lenalidomide, n/N | 7/11 | 7/10 |
| Prior bortezomib, n/N | 2/11 | 4/10 |
| Prior chemotherapy, n/N | 6/11 | 4/10 |
| Baseline creatinine level, μmol/L, median (range) | 83.1 (43.3-101.7) | 77.8 (70.7-87.5) |
| Mean baseline ALC, ×109/L | 1.4 | 1.3 |
| Median (range) | 1.5 (0.6-1.9) | 1.1 (0.9-1.7) |
| Disease status at time of SCT, n | ||
| PR | 3 | 4 |
| MR | 5 | 1 |
| SD | 3 | 5 |
IgG indicates immunoglobulin G, IgA, immunonoglobulin A; HLA-A2, human leukocyte antigen–A2; hTERT, human telomerase reverse transcriptase; ISS, International Staging System; B2M, β2-microglobulin; ALC, absolute lymphocyte count; SCT, stem cell transplantation; PR, partial response; MR, moderate response; and SD, stable disease.
Response to high-dose melphalan and survival were not primary endpoints of this study, which included a high-risk patient population. Nevertheless, with a median follow-up of 12 months, overall survival was 83% (18 of 21) with event-free survival of 48% (10 of 21). Response assessment at day 180 after ASCT was 6 complete response or near complete response with positive immunofixation, 3 very good partial responses (43% > very good partial response), 4 partial responses (63% > partial response), 1 stable disease, 4 progressive disease, and 3 unevaluable. There was no difference in response or survival between the primed and nonprimed groups.
HAI assay results
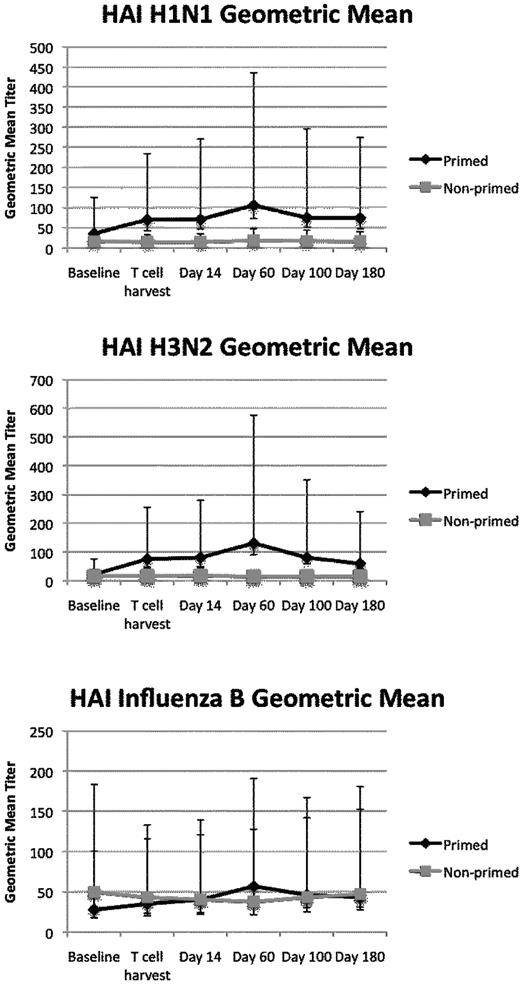
The primary endpoint of this study was antibody response to influenza as measured by the serotype-specific influenza HAI antibody responses. HAI titer is the parameter with strongest correlation to protection from wild-type infection.35,36 H3N2 and H1N1 HAI geometric mean titers were higher at all time points after vaccination in the primed patients compared with the nonprimed patients (Figure 2). Titers in the nonprimed group remained near baseline throughout all time points. A mixed effects model was used to define the differences in geometric mean titers over time between the 2 arms. A significant difference was seen comparing the primed and the nonprimed patients' responses to the H1N1 (P = .006) and H3N2 (P < .0001) components of the vaccine. Influenza B HAI geometric mean titers exhibited no difference between the 2 groups (P = NS), and this may reflect its weaker immunogenicity.37,38
Influenza titers. HAI influenza titers are induced to high levels in the primed group. Geometric mean titers and 5%-95% confidence intervals are displayed. The mixed effects model for repeated measures showed P = .006 for H1N1 and P < .0001 for H3N2.
Influenza titers. HAI influenza titers are induced to high levels in the primed group. Geometric mean titers and 5%-95% confidence intervals are displayed. The mixed effects model for repeated measures showed P = .006 for H1N1 and P < .0001 for H3N2.
We defined seroconversion as the percentage of patients with a ≥ 4-fold increase in HAI titer after immunization. Seroconversion for H1N1 occurred in 36% of the primed group and 10% of the nonprimed group (P = .31). Seroconversion for H3N2 occurred in 72% of the primed group and 0% of the nonprimed group (P < .01). Seroconversion for influenza B occurred in 45% of the primed group and 20% of the nonprimed group (P = .36). Seroconversion to any serotype occurred 73% of the time in the primed group and 30% of the time in the nonprimed group (P = .08). Therefore, by multiple criteria, influenza vaccine responses were superior in the vaccine-primed recipients compared with those with adequate T-cell recovery but without priming.
B-cell and T-cell recovery kinetics
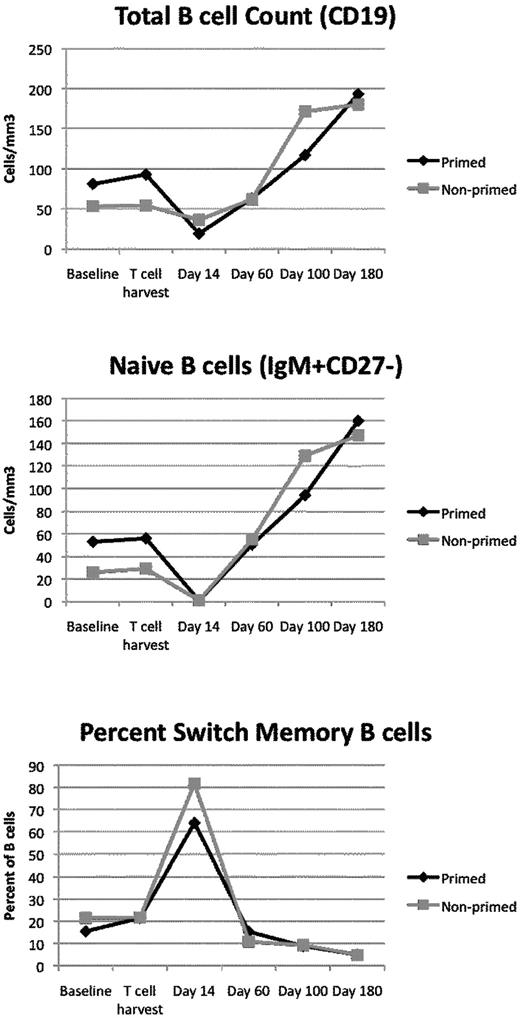
ASCT usually leads to a sustained immune deficiency.17-21,39-41 We examined B-cell and T-cell recovery kinetics to understand the landscape of the immune system after ASCT. It is clear that the provision of the autologous T-cell product led to rapid quantitative reconstitution of both the B-cell and T-cell compartments (Figures 3 and 4). The kinetics of recovery were similar in both groups. At baseline, most of the B cells had a naive phenotype (IgM+, CD27−). At day 14 after ASCT, B cells reached a nadir, and most had a switched memory phenotype (IgM−, CD27+). The GEE method was used to define group and time effects in this repeated-measures study design. There was a significant change in total B-cell counts (P = .01) and naive B-cell counts over time (P = .001). For switch memory B cells (CD27+IgM−), there was no significant change in the absolute counts over time.
B cells are depleted after melphalan conditioning. Plotted are the average absolute B-cell counts (top), absolute numbers of naive B cells (IgM+, CD27−; middle), or percentage of B cells with a switched memory phenotype (IgM−, CD27+; bottom) as a function of time. At most time points, most of the B cells are naive B cells. During the time of maximal B-cell depletion (day 14), switch memory B cells predominate.
B cells are depleted after melphalan conditioning. Plotted are the average absolute B-cell counts (top), absolute numbers of naive B cells (IgM+, CD27−; middle), or percentage of B cells with a switched memory phenotype (IgM−, CD27+; bottom) as a function of time. At most time points, most of the B cells are naive B cells. During the time of maximal B-cell depletion (day 14), switch memory B cells predominate.
Effect of melphalan and T-cell infusion of T-cell subsets. T-cell subsets are differentially affected by conditioning and autologous T-cell infusions. The subsets were identified as described in “Immunologic assays,” and the fraction of each subset within the CD3 population is plotted on the y-axis.
Effect of melphalan and T-cell infusion of T-cell subsets. T-cell subsets are differentially affected by conditioning and autologous T-cell infusions. The subsets were identified as described in “Immunologic assays,” and the fraction of each subset within the CD3 population is plotted on the y-axis.
The provision of an autologous T-cell infusion has been previously shown to quantitatively restore both CD4 and CD8 T cells.30 In this study, we examined functionally distinct T-cell subsets. T-cell recovery differed dramatically between CD4 and CD8 T cells; however, there were no differences between the primed and nonprimed groups. CD4 effector memory T cells were the most highly retained cell type, whereas CD4-naive, CD4 central memory, and CD4 reverted memory cells remained depressed for a sustained period of time. In contrast, CD8-naive, CD8 central memory, and CD8 reverted memory all increased after the infusion of the autologous T-cell product. None of the CD8 T-cell subsets exhibited a group effect when analyzed by GEE. The naive CD8 and reverted memory CD8 T cells exhibited a significant time effect (P < .001 and P = .05, respectively). The apparent difference in CD8 effector memory populations between the primed group and the nonprimed group is not significant and is due to 3 outliers with engraftment syndrome.30 Therefore, there were no differences in T-cell subsets between the 2 arms.
Autologous T-cell infusions have been shown previously to retain a diverse and consistent T-cell repertoire after in vitro expansion42 ; however, the retention of the repertoire after infusion has not been previously investigated. We identified the fraction of abnormal Vβ families (oligoclonal or < 10% of the average signal in controls) after spectratyping.43 Approximately one-half of the Vβ families were abnormal, and there were no differences between the 2 groups or across time points (data not shown). Approximately 60% of the Vβ families exhibited no change from time point to time point. Therefore, the repertoire appears to be largely stable within the host.
Studies of T- and B-cell responsiveness
In principle, the mechanism of enhanced vaccine response after a priming dose could be due to either recruitment of antigen-specific memory T cells carried over from the priming at the time of the post-ASCT vaccine44 or persistence of memory B cells generated at the time of the priming dose followed by expansion and maturation after the post-ASCT vaccine. To examine this question, B-cell responsiveness was assessed by a B-cell ELISPOT and the T-cell responsiveness was assessed by a T-cell ELISPOT (Figure 5). Both assays used the season-specific antigen to match the vaccine administered. The anti-influenza antigen responsive B cells increased in the primed recipients after the priming dose; however, the responses subsequently fell. This may be due to the lower frequency of effector cells once the naive B-cell population has expanded (Figure 3). The B-cell responses to influenza did not change over the time course in the nonprimed patient group. The differences between the 2 groups did not reach statistical significance. The global capacity of the B cells to produce any antibody was measured by a total IgG ELISPOT, and there were no differences between the 2 groups.
Functional analysis of T-cell and B-cell responses. Functional responses to antigen are augmented in the primed group. T-cell and B-cell responses to influenza antigens were measured by antigen-specific ELISPOTS. CD4 T-cell antigen–specific responses (γ-interferon responses to intact protein after CD8 T-cell depletion) were increased in the primed group compared with the nonprimed group at the T-cell harvest time point (P = .02). There were no statistically significant differences between the 2 groups for the CD8-specific responses (responses to influenza peptides), and the global responses (phorbol myristate acetate [PMA] and ionomycin and total IgG) also did not differ between the 2 groups at any time point.
Functional analysis of T-cell and B-cell responses. Functional responses to antigen are augmented in the primed group. T-cell and B-cell responses to influenza antigens were measured by antigen-specific ELISPOTS. CD4 T-cell antigen–specific responses (γ-interferon responses to intact protein after CD8 T-cell depletion) were increased in the primed group compared with the nonprimed group at the T-cell harvest time point (P = .02). There were no statistically significant differences between the 2 groups for the CD8-specific responses (responses to influenza peptides), and the global responses (phorbol myristate acetate [PMA] and ionomycin and total IgG) also did not differ between the 2 groups at any time point.
To assess the T-cell responses, γ-interferon–producing T cells were measured after stimulation with a cocktail of T-cell epitope peptides. The patients in the primed group trended toward a higher CD8 T-cell response than the patients in the nonprimed group; however, the difference did not reach statistical significance. The inactivated influenza vaccine stimulates predominantly CD4 T-cell responses.45 We, therefore, measured T-cell ELISPOT results after depletion of CD8 T cells and found that CD4-specific responses were significantly better in the primed group at the T-cell harvest time point (P = .02) (Figure 5). Finally, to ensure that there were no global differences between the ability of the 2 groups of patients to respond to stimuli, responses to phorbol myristate acetate and ionomycin were measured. There were no differences between the 2 patient groups (Figure 5).
We then analyzed whether early T-cell and B-cell responses were associated with subsequent specific antibody production independently of the study group. Influenza-specific B-cell ELISPOT responses analyzed on the day of T-cell harvest were very strongly associated with subsequent anti-H1N1 and anti-H3N2 responses. The Spearman correlation for influenza-specific B-cell ELISPOT responses on the day of T-cell harvest ranged from 0.03 to 0.006 for H1N1 responses at each subsequent time point. The Spearman correlation for influenza-specific B-cell ELISPOT responses on the day of T-cell harvest was 0.05 for H3N2 responses at the final time point. There were no statistically significant associations between B-cell ELISPOT results and influenza B titers. We similarly investigated the relationship of T-cell ELISPOT results with subsequent titer production and found no consistent relationship. Therefore, although the T-cell infusion quantitatively restores the T-cell compartment, the most significant effect in this study was on the restoration of B-cell functional responses.
Clinical correlates of influenza vaccine responses
No differences were observed between the primed and nonprimed groups in terms of demographics, medications, hospitalizations, or baseline CD3, CD19, absolute lymphocyte count, or absolute neutrophil count. Four patients developed engraftment syndrome, and all were in the nonprimed group. Two of these 4 received short courses of steroids. No subject developed community-acquired influenza and sought medical attention. We considered whether any of the clinical variables might influence HAI responses. Age, sex, race, dexamethasone before enrollment, bortezomib before enrollment, baseline CD3, baseline CD19, baseline absolute neutrophil count, and baseline absolute lymphocyte count were examined with the use of the Wilcoxon test for association with HAI seroconversion status. None of the variables were statistically associated with seroconversion. Study subjects also received the conjugated pneumococcal vaccine as a control, and we evaluated the concordance between pneumococcal responses and HAI seroconversion. We defined a pneumococcal responder by summing the responses to 4 serotypes and defining a 4-fold increase from baseline. There was no association between HAI responders and pneumococcal responders. Overall, 21% of enrolled subjects (all primed with the conjugated pneumococcal vaccine) were pneumococcal responders, much lower than the 73% overall responder frequency seen in the primed group for influenza seroconversion.
Discussion
This study shows that seasonal influenza vaccination priming before autologous T-cell collection, ASCT, infusion of autologous T cells, and repeat influenza vaccination leads to superior influenza vaccine responses in patients with multiple myeloma compared with the nonprimed patients. The importance of this result is 2-fold. Infection is one of the most common causes of death after transplantation for multiple myeloma.26,27 This is thought to be a consequence of the age at the time of transplantation and the natural immune suppression arising after high-dose melphalan. The provision of an autologous costimulated and expanded T-cell product may improve the outcome by augmenting host responses to infection. In this study, the provision of the autologous T-cell product did not restore vaccine responses unless the patient and the T-cell product had been primed by prior antigen exposure. Even though both groups of patients had full T-cell recovery after melphalan, the nonprimed group did not respond to the posttransplantation vaccine. Therefore, this strategy could be broadly applicable in patients who receive a transplant in whom influenza is a common pathogen with high morbidity.27 The second implication of this work is that this strategy could be harnessed for improved tumor vaccine responses, whereby responses to date have been compromised by poor T-cell function after ASCT.18
In a prior study, we explored an approach of combination immunotherapy with the 7-valent pneumococcal conjugate vaccine and adoptive T cells.29 We immunized patients with pneumococcal vaccine before T-cell collection and after ASCT and infusion of the autologous costimulated and expanded T-cell product.46 This corrected the immunodeficiency and lymphopenia and improved the responsiveness to the pneumococcal vaccine compared with similar groups of patients who did not receive this approach.29 The combined results of the current influenza study and the pneumococcal study suggest broad applicability of costimulated and expanded T cells to boost vaccine-induced immunity.
When analyzed without stratifying by study group, B-cell ELISPOT results were significantly associated with subsequent HAI titers. This assay measures only bloodstream B cells responding to antigen and may underestimate the potential in the secondary lymphoid organs where the memory B cells are located. Nevertheless, B cells were clearly affected by the primed autologous T-cell product because antigen-specific antibody production was enhanced. B-cell responses were increased after the priming dose and the B-cell ELISPOT results were strongly associated with subsequent antibody production.
The mechanism underlying this effect remains to be determined. The conditioning regimen and lymphodepletion leads to high levels of interleukin-15 in the serum, which probably contributes to the significant expansion of CD8 T cells.30 Similarly, high levels of interleukin-6 could support B-cell recovery.47 The global expansion of central memory CD8 T cells generically (Figure 3) may explain the previously observed increase in CMV responsiveness seen after autologous T-cell infusion but does not account for the enhanced response to the inactivated influenza vaccine seen exclusively in the primed patients.29,48 The CD4 T cells were less tolerant of melphalan and, in the absence of a primed post-ASCT infusion, may not have been able to supply sufficient help for B-cell differentiation. At the time of the study design, follicular helper T cells were not well-defined, and we did not perform flow cytometric identification of these cells.49 We hypothesize that the autologous T-cell product contained antigen-specific follicular helper T cells, which contributed to the B-cell maturation and subsequent production of HAI antibodies.
This study contributes to the growing body of knowledge about the provision of antigen-specific defenses to immune-compromised patients. Achieving the goal broadly could markedly improve the survival of patients with multiple myeloma who undergo ASCT. In this patient population, infection is the most common cause of morbidity.7,8,18,27 In addition, harnessing antigen-specific immunity for tumor vaccines will become increasingly important as immune-based therapies become a reality.50
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients, physicians, nurses, and research staff at ACC, CHOP, and UMD. Specifically, we thank Adriana Weinberg, Ximena Rivera, Tikettta McIntyre, and Kelly Maurer for their contributions. Certain assays were performed at the Human Immunology Core, and we thank the staff.
This work was supported in part by the National Institute of Allergy and Infectious Diseases (grant NO1-AI-50 024; K.E.S.) and (R21CA130293; A.P.R.) and by the Leukemia & Lymphoma Society (SCOR 7414; R.H.V. and C.H.J.).
National Institutes of Health
Authorship
Contribution: E.A.S., A.P.R., K.E.S., D.T.V., and C.H.J. conceived and designed the study; A.F.J., E.L.P., and J.B. provided statistical and analytical support for the study; B.L.L. and R.H.V. produced the autologous T cells; E.A.V., K.R.M., X.H., H.M., R.B., P.A.M., and A.C. provided clinical trials management, enrollment, and database analyses; and N.A.A. provided T-cell analyses.
Conflict-of-interest disclosure: R.H.V. is an inventor on a patent that relates to human telomerase reverse transcriptase immunotherapy. C.H.J. is an inventor on a patent related to the T-cell manufacturing process that was used for this protocol. This conflict has been disclosed and was managed in accordance with the policies of the University of Pennsylvania. The remaining authors declare no competing financial interests.
Correspondence: Kathleen E Sullivan, The Children's Hospital of Philadelphia, Division of Allergy Immunology, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: sullivak@mail.med.upenn.edu.


![Figure 5. Functional analysis of T-cell and B-cell responses. Functional responses to antigen are augmented in the primed group. T-cell and B-cell responses to influenza antigens were measured by antigen-specific ELISPOTS. CD4 T-cell antigen–specific responses (γ-interferon responses to intact protein after CD8 T-cell depletion) were increased in the primed group compared with the nonprimed group at the T-cell harvest time point (P = .02). There were no statistically significant differences between the 2 groups for the CD8-specific responses (responses to influenza peptides), and the global responses (phorbol myristate acetate [PMA] and ionomycin and total IgG) also did not differ between the 2 groups at any time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/1/10.1182_blood-2010-07-296822/4/m_zh89991062780005.jpeg?Expires=1769097148&Signature=wQcgMCvxlgSH49AyNe1fn6Ex-uZ9yfwenoGQboGSxa84xp9LuV0wRQ-O-TTwN~sk7Mt-G6rADWyjmuYDXoHnVer9yAoS4SdUo9DGTF5jaX5ROCaWeCYiG9qOQqItPUYTBpTxwAxMLdWlA68ft1CuZXis9DQkJkqjJZIv-upzwGQ3tYyxTxy~es43MpdMxbYmk0o457-wcMApvcTr-rqmpaZsZYyJN5Y8pMvSH0veIC2fwaxiSx9iiBDSIoo7guT-HYlXWrH5wRMRGjBIyfAGX14yT3YHtdmpHGYwcXVjkLYf0h3m0BdwG7CbdtcY~FL8QvJm3T8Jvz7qxyecWGKppg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal