Abstract
Polymorphonuclear neutrophils (PMNs) are critical for the formation, maintenance, and resolution of bacterial abscesses. However, the mechanisms that regulate PMN survival and proliferation during the evolution of an abscess are not well defined. Using a mouse model of Staphylococcus aureus abscess formation within a cutaneous wound, combined with real-time imaging of genetically tagged PMNs, we observed that a high bacterial burden elicited a sustained mobilization of PMNs from the bone marrow to the infected wound, where their lifespan was markedly extended. A continuous rise in wound PMN number, which was not accounted for by trafficking from the bone marrow or by prolonged survival, was correlated with the homing of c-kit+-progenitor cells from the blood to the wound, where they proliferated and formed mature PMNs. Furthermore, by blocking their recruitment with an antibody to c-kit, which severely limited the proliferation of mature PMNs in the wound and shortened mouse survival, we confirmed that progenitor cells are not only important contributors to PMN expansion in the wound, but are also functionally important for immune protection. We conclude that the abscess environment provides a niche capable of regulating PMN survival and local proliferation of bone marrow–derived c-kit+-progenitor cells.
Introduction
Neutrophil or polymorphonuclear cell (PMN) abscess formation is a critical event in the innate immune response against invading pathogens and is required for wound resolution and bacterial clearance.1,2 This is especially the case during Staphylococcus aureus infections, in which PMNs are recruited and form an abscess at the site of infection to promote bacterial clearance.3-5 Applying real-time fluorescence imaging of a full-thickness skin wound in mice possessing PMNs engineered to produce enhanced green fluorescence protein (EGFP-PMNs), we previously reported that the numbers of PMNs recruited to the wound bed doubled in response to infection with S aureus or intraperitoneal injection of granulocyte-macrophage colony-stimulating factor (GM-CSF).4 These studies revealed the dynamics of PMNs entering the circulation from the bone marrow pool that enabled them to concentrate in the wound at a density up to 40-fold above that in the blood. Thus, inflammation and infection provide dual signals for the rapid mobilization of PMNs from the bone marrow compartment and recruitment to the site of tissue insult.6,7 However, the mechanisms that regulate this feedback and maintain numbers sufficient to resolve infection while avoiding tissue damage are not entirely known.
PMNs are attracted from the circulation to a site of infection or inflammation by CXC chemokines, including the interleukin-8 (IL-8) homologs keratinocyte chemoattractant (KC) and macrophage inflammatory protein-2 (MIP-2), which are produced by murine endothelium, macrophages, epithelial cells, and fibroblasts.8-10 In addition, we found that other signals, including IL-17, are critical for the production of KC and MIP-2 and for the subsequent recruitment of PMNs to a site of cutaneous S aureus infection.11 PMNs also generate signals that regulate their numbers in the wound; these include tumor necrosis factor-α, and IL-1β, which promote the mobilization of PMNs from the large storage pool in the bone marrow.12-14 Although mature PMNs are reported to have a half-life in the circulation of only ∼12 hours,15,16 inflammatory cytokines can prolong PMN survival in vitro.17 Whether this prolonged survival occurs in vivo during abscess formation is unknown. Cytokines produced within infected wounds may also influence the survival of PMNs within the abscess by down-regulating apoptosis.18,19
It is well established that PMN numbers can be rapidly expanded in the bone marrow from lineage-negative and c-kit receptor–positive (Lin−c-kit+)–progenitor cells. Inflammatory stimuli can induce direct mobilization and infiltration of Lin−c-kit+ cells to peripheral sites.20,21 Furthermore, Lin−c-kit+ cells can be activated by Toll-like receptor (TLR) agonists to trigger proliferation and rapid myeloid differentiation in vitro and in vivo.22,23 Based on these data, we hypothesized that PMN abscess formation and maintenance involves prolonged PMN survival within the abscess and the local generation of PMNs from myeloid-progenitor cells present both in the bone marrow and at the site of the infection.
Methods
Mice
EGFP-lysozyme M (lys) knock-in mice (EGFP-lys-mice24 ; kind gift from Dr Thomas Graf, Center for Genomic Regulation, Spain) were backcrossed for 10 generations onto a C57BL/6 background (The Jackson Laboratory) in an animal facility at the University of California at Davis. Male mice between 8 and 14 weeks of age were used in all experiments. All animal experiments were approved by the institutional animal care and use committee of the University of California at Davis, and were performed following the guidelines of the Animal Welfare Act and the Health Research Extension Act.
Mouse model of S aureus wound infection
Mouse skin wounding and S aureus infection were performed as described previously4 and in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Noninvasive quantification of wound EGFP-PMN trafficking
The trafficking of EGFP-PMNs at wound sites over time was determined noninvasively using the IVIS 100 imaging system (Xenogen), as described previously4 and in supplemental Methods.
Preparation and in vivo bioluminescent imaging of S aureus
Collection of bone marrow cells and blood samples
For murine bone marrow–cell collection, mice were humanely euthanized with CO2 at selected time points and the femurs from both hind limbs were removed. Bone marrow cells were flushed from each bone with Hanks buffered salt solution, pelleted, and resuspended with buffer for further experiments. Mouse whole blood was drawn via cardiac puncture into EDTA (ethylenediaminetetraacetic acid), and red blood cells were depleted with red cell–lysis buffer (BioLegend). The total numbers of bone marrow and blood leukocytes were counted using an automatic cell counter (Beckman Coulter).
Immunodepletion of systemic PMNs and c-kit progenitors
Systemic PMNs were depleted by multiple intraperitoneal injections of anti–Gr-1 monoclonal antibody (mAb; RB6-8C5 clone, 0.1 mg each injection; eBioscience). For early and sustained depletion of PMNs (pre-Gr-1), mice were treated with Gr-1 antibody or isotype control rat immunoglobulin G (eBioscience) every other day starting 24 hours before wounding and continuing up to day 5. For delayed depletion of PMNs (post–Gr-1), mice were injected with Gr-1 antibody every other day starting 24 hours after wounding and continuing up to day 5. For c-kit+-progenitor–cell depletion, 1 mg of the anti–c-kit antibody ACK2 (University of California-San Francisco Hybridoma and Monoclonal Antibody Core) or isotype control rat anti–mouse immunoglobulin G was given intraperitoneally 5 days before wounding and again 1 day after wounding.
Flow cytometric immunophenotyping assay
A flow cytometric immunophenotyping assay to characterize mature PMNs and Lin−c-kit+ cells was performed using bone marrow and blood cells collected from EGFP-lys-mice, as described in supplemental Methods.
FACS cell sorting
Bone marrow cells isolated from EGFP-lys-mice were sorted by fluorescence-activated cell sorting (FACS) into c-kit+ progenitors and mature EGFP-PMNs using the MoFlo cell sorter (Beckman Coulter), as described in supplemental Methods.
Tissue half-life of PMNs
Neutrophil tissue half-life was determined by quantifying the rate of PMN (c-kit−EGFPhighGr-1highCD11bhigh phenotype) clearance from sites of wound infection, as described in supplemental Methods.
In vivo assessment of c-kit+-progenitor–cell trafficking in wounded skin
FACS-sorted EGFP−c-kit+ cells from EGFP-lys-mouse bone marrow (5 × 105 cells in 0.1 mL of sterile saline) were fluorescently labeled with Qtracker 705 (Invitrogen) and then injected via tail vein into C57BL/6 mice 3 hours after wounding and S aureus (1 × 107 CFUs) or sterile saline treatment on wounded skin. At 24 hours after the transfer, Qtracker 705–expressing EGFP−c-kit+ cells within the wound area were visualized using the IVIS 100 imaging system (excitation at 445-490 nm and emission at 695-770 nm) at an exposure time of 1 second.
In vivo assessment of c-kit+-progenitor–cell differentiation to EGFP-PMNs in wounded skin
The FACS-sorted EGFP−c-kit+ cells from EGFP-lys-mouse bone marrow (5 × 105 cells in 0.1 mL of sterile saline) were intravenously injected via the tail vein to C57BL/6 mice. Using the IVIS 100 imaging system, the proliferative capacity of EGFP−c-kit+ cells to differentiate into EGFP-PMNs was assessed by detecting time-dependent increases in the EGFP fluorescence signal. After adoptive transfer of EGFP−c-kit+ cells, circulating EGFP-PMNs were depleted by repetitive treatment with the Gr-1 antibody at days 1, 3, and 5 to block the potential contribution of EGFP-PMNs emigrating into the tissue from outside wounded skin.
CFU assay
To quantify the proliferative capacity of cells isolated from the bone marrow and wounded skin of EGFP-lys-mice, colony-forming unit (CFU) assays were performed in methylcellulose medium supplemented with cytokines to support the growth of myeloid progenitors (MethoCult GF M3434; StemCell Technologies) at a cell density of 3 × 105/mL. For details, see supplemental Methods.
Mathematical modeling to estimate tissue PMN number during S aureus infection
A mathematical model was developed to predict the kinetics of PMN influx, survival, and production during S aureus infection, and is described in detail in supplemental Methods.
Statistical analysis
Data analysis was performed using Prism Version 5.0 software (GraphPad). Statistical significance between 2 groups was determined by 2-tailed unpaired t tests. Mice survival analysis was performed based on the log-rank test. P values < .05 were considered statistically significant.
Results
S aureus infection of skin wounds increase PMN trafficking and mobilization from bone marrow
To investigate the dynamics of PMN recruitment to S aureus–infected cutaneous wounds, the degree of PMN infiltration was measured in wounds infected with a low (2 × 106 CFUs) or a high (1 × 107 CFUs) inoculum and compared with mice treated with a saline vehicle control. Whole-animal fluorescence imaging of EGFP-lys-mice was used to quantify EGFP-PMN infiltration,4 whereas luminescence provided enumeration of a bioluminescent strain of S aureus11 within the wound bed. During the initial 24 hours of skin wounding, the extent of EGFP-PMN infiltration was not significantly different between the 3 experimental groups (Figure 1A). However, between days 1 and 7, mice that received the high inoculum of S aureus exhibited twice the number of PMNs in wounds, nearly a log higher bacterial burden, and wounds took more than 4 days longer to heal than those in mice inoculated with the low inoculum or control wounded mice treated with saline alone (Figure 1A-F). These data show that PMNs infiltrated the wound bed most rapidly over the first 24 hours after a skin wound, and that the extent and persistence of phagocyte recruitment and wound resolution corresponds to the intensity of S aureus infection.
Effects of S aureus inoculum on PMN trafficking and tissue healing. EGFP-lys-mice were inoculated with saline or low-dose S aureus (2 × 106 CFUs, SA-2e6 CFUs), or sublethal high dose S aureus (1 × 107 CFUs, SA-1e7 CFUs) on back skin wounds, and kinetics of wound EGFP-PMN fluorescence (A-B), S aureus (SA) bioluminescence (C-D), and wound closure (E-F) were determined. Depicted are representative images of wound EGFP-PMNs (B), bioluminescent S aureus (D), and wound size (F). Data are derived from 6-8 mice in each group and are expressed as means ± SEM. *P < .05 vs saline group; #P < .05 vs SA-2e6 CFU group.
Effects of S aureus inoculum on PMN trafficking and tissue healing. EGFP-lys-mice were inoculated with saline or low-dose S aureus (2 × 106 CFUs, SA-2e6 CFUs), or sublethal high dose S aureus (1 × 107 CFUs, SA-1e7 CFUs) on back skin wounds, and kinetics of wound EGFP-PMN fluorescence (A-B), S aureus (SA) bioluminescence (C-D), and wound closure (E-F) were determined. Depicted are representative images of wound EGFP-PMNs (B), bioluminescent S aureus (D), and wound size (F). Data are derived from 6-8 mice in each group and are expressed as means ± SEM. *P < .05 vs saline group; #P < .05 vs SA-2e6 CFU group.
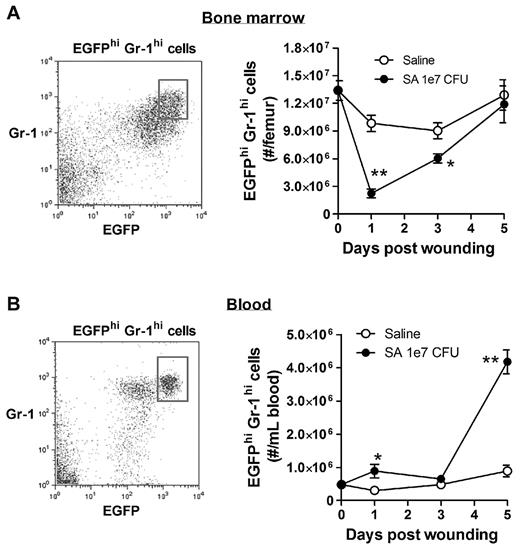
Because the major source of PMNs released into the peripheral circulation is the bone marrow compartment, we quantified the kinetics of EGFP-PMNs leaving the bone marrow and entering the venous blood compartment from mice inoculated with 1 × 107 CFUs of S aureus compared with uninfected wounds. Neutrophils were identified by gating on EGFPhigh, Gr-1high, and CD11bhigh fluorescence as detected by flow cytometry. The majority of EGFPhigh cells (∼ 95%) were Gr-1+ and CD11b+, confirming that they were mature PMNs (supplemental Figure 1). By day 1, mice with a high S aureus inoculum exhibited an 80% reduction in PMNs in the bone marrow that was accompanied by a 2-fold increase in the blood that peaked at day 5 (Figure 2 and supplemental Table 1). The PMN counts returned to baseline levels in the bone marrow between days 3 and 5. In contrast, there were no significant changes in PMN cell numbers in the bone marrow and blood from saline-treated mice. These findings indicate that S aureus infection elicits additional mobilization of PMNs from the bone marrow compartment to the circulation within 24 hours of infection, and is correlated with increased numbers in the wound at later time points.
Bone marrow mobilization of mature PMNs into the peripheral blood circulation in response to S aureus wound infection. Mature EGFP-PMNs were gated on EGFPhighGr-1high cells. Shown are the kinetics of EGFPhighGr-1high cells in the bone marrow (A) and peripheral blood (B) from mice treated with either sterile saline or S aureus (1 × 107 CFUs). Data are derived from 3-4 mice in each group and are expressed as means ± SEM. *P < .05 vs the saline group; **P < .01 vs the saline group.
Bone marrow mobilization of mature PMNs into the peripheral blood circulation in response to S aureus wound infection. Mature EGFP-PMNs were gated on EGFPhighGr-1high cells. Shown are the kinetics of EGFPhighGr-1high cells in the bone marrow (A) and peripheral blood (B) from mice treated with either sterile saline or S aureus (1 × 107 CFUs). Data are derived from 3-4 mice in each group and are expressed as means ± SEM. *P < .05 vs the saline group; **P < .01 vs the saline group.
Early recruitment and prolonged survival of PMNs are essential for S aureus clearance and wound closure
To determine the influence of PMN recruitment and survival within the wound bed on bacterial clearance and wound resolution, mice were systemically depleted of PMNs with an anti-PMN mAb (Gr-1) that was administered beginning 24 hours before or after wounding and S aureus inoculation (SA-pre-Gr-1 and SA-post–Gr-1, respectively; Figure 3A). SA-pre-Gr-1 mice showed a marked decrease in the initial recruitment of EGFP-PMNs to the infected skin wounds, a sustained increase in bacterial burden, and impaired wound closure compared with mice that exhibited normal PMN trafficking (SA-isotype control; Figure 3B-D). These results are consistent with the defective S aureus clearance and larger skin lesions previously reported for mice depleted of PMNs,1 or with impaired PMN recruitment.13 In contrast, SA-post–Gr-1 mice had equal numbers of EGFP-PMNs recruited on day 1 as mice treated with SA-isotype control (Figure 3B). Remarkably, the number of PMNs recruited by day 1 in the SA-post–Gr-1 mice appeared to remain constant through day 8, and this was sufficient to control the S aureus infection to a similar extent as the SA-isotype control, which exhibited a nearly 100% boost in PMN infiltration. Furthermore, wound closure in the SA-post–Gr-1 mice was actually accelerated compared with mice that had no depletion (SA-isotype) or delayed recruitment (SA-pre-Gr-1) of PMNs (Figure 3D). These data indicate that the exuberant PMN recruitment induced by the high S aureus inoculants actually delayed wound healing, and in fact the number of PMNs recruited during the initial 24 hours of the inflammatory phase and maintained in the wound thereafter was sufficient to resolve infection.
Effects of time-dependent immunodepletion of systemic PMNs on S aureus wound infection. (A) Experimental design for early and delayed depletion of systemic EGFP-PMNs. Systemic PMNs were depleted with anti-neutrophil antibody treatment beginning either 24 hours before (pre-Gr-1) or 24 hours after (post–Gr-1) the onset of the infection (1 × 107 CFUs of S aureus) and then every 2 days up to day 5. Shown are the kinetics of wound EGFP-PMNs (B), S aureus burden (C), and wound closure (D). Data are derived from 5-6 mice in each group and are expressed as means ± SEM. *P < .05 vs SA-isotype control group.
Effects of time-dependent immunodepletion of systemic PMNs on S aureus wound infection. (A) Experimental design for early and delayed depletion of systemic EGFP-PMNs. Systemic PMNs were depleted with anti-neutrophil antibody treatment beginning either 24 hours before (pre-Gr-1) or 24 hours after (post–Gr-1) the onset of the infection (1 × 107 CFUs of S aureus) and then every 2 days up to day 5. Shown are the kinetics of wound EGFP-PMNs (B), S aureus burden (C), and wound closure (D). Data are derived from 5-6 mice in each group and are expressed as means ± SEM. *P < .05 vs SA-isotype control group.
Mature neutrophils persist within S aureus–infected wounds
Given the crucial role of PMN number and persistence for controlling bacterial infection and wound resolution, the mechanism for PMN survival in the abscess was investigated. To determine the capacity of PMNs infiltrating the wound to evade apoptosis and survive in response to injury and infection, mature PMNs defined as EGFPhigh, c-kit−, Gr-1high, and CD11bhigh cells were isolated from bone marrow cells by FACS sorting (Figure 4A). These isolated cells were morphologically confirmed to be mature EGFP-PMNs and functionally confirmed to be nonproliferative (supplemental Figure 2A), which is consistent with previous reports.25 A bolus of sorted PMNs was adoptively transferred by intravenous injection into C57BL/6 mice with S aureus–infected or saline-treated wounds. In both groups, the infused EGFP-PMNs were not detected in the circulation after day 3 following the transfer (supplemental Figure 2B), and PMN fluorescence increased equivalently up to day 3, at which point S aureus wounds exhibited sustained survival of PMNs compared with the saline-treated wounds, which rapidly declined to baseline by day 8 (Figure 4B). Estimation of the half-life of EGFP-PMNs based on the rate of fluorescence signal decay after reaching their peak values (supplemental Figure 2C) revealed that PMNs within S aureus–infected wounds exhibited nearly 3-fold longer survival compared with saline treatment (Figure 4C; 4.96 ± 0.38 days for S aureus vs 1.58 ± 0.31 days for uninfected wounds; P < .01). However, this prolonged survival was insufficient to account for the relatively constant EGFP-PMN numbers maintained up to day 8 in the wounds of mice treated with Gr-1 after infection (Figure 3B). Because additional PMN influx from the systemic compartment was blocked in presence of Gr-1, we hypothesized that a dynamic balance between PMN survival, apoptosis, and local production maintained this equilibrium in the EGFP signal.
Adoptive transfer of PMNs and recruitment to S aureus–infected wounds. (A) FACS sorting of mature EGFP-PMNs (c-kit−EGFPhighGr-1highCD11bhigh cells) and cytospin image. To avoid any inclusion of cells with proliferative capacity, c-kit+-progenitor cells were gated out. The FACS-sorted cells were intravenously transferred (5 × 106 cells in 0.1 mL of sterile saline) to C57BL/6 mice whose wounds were treated with either sterile saline or S aureus (1 × 107 CFUs), and the kinetics of EGFP-PMNs within the wound were determined (B). (C) The half-life of wound EGFP-PMNs was quantified from EGFP-PMN kinetic data using the equations described in the supplemental Methods. Data are derived from 4 mice in each group and are expressed as means ± SEM. *P < .01 vs saline control group.
Adoptive transfer of PMNs and recruitment to S aureus–infected wounds. (A) FACS sorting of mature EGFP-PMNs (c-kit−EGFPhighGr-1highCD11bhigh cells) and cytospin image. To avoid any inclusion of cells with proliferative capacity, c-kit+-progenitor cells were gated out. The FACS-sorted cells were intravenously transferred (5 × 106 cells in 0.1 mL of sterile saline) to C57BL/6 mice whose wounds were treated with either sterile saline or S aureus (1 × 107 CFUs), and the kinetics of EGFP-PMNs within the wound were determined (B). (C) The half-life of wound EGFP-PMNs was quantified from EGFP-PMN kinetic data using the equations described in the supplemental Methods. Data are derived from 4 mice in each group and are expressed as means ± SEM. *P < .01 vs saline control group.
Bone marrow–derived c-kit+-progenitor cells proliferate and mobilize to the site of infection and locally differentiate into mature EGFP-PMNs
We next sought to determine whether Lin−c-kit+ cells are recruited from the circulation to the wound and contribute to the increased PMN numbers over the duration of an infection. A 4-fold expansion of Lin−c-kit+ cells was detected in the bone marrow of S aureus–infected mice between days 1 and 3, which significantly exceeded the increase in mice with saline-treated wounds (supplemental Figure 3). One thousand-fold fewer Lin−c-kit+ cells were detected in the blood compared with in the bone marrow, and the number was not different between saline-treated and S aureus–infected mice, suggesting that Lin−c-kit+ cells efficiently home to the wound and in greater numbers in response to infection.
To directly image progenitor-cell recruitment to the site of infection, EGFP−c-kit+ cells were FACS sorted from the bone marrow of EGFP-lys-mice, fluorescently labeled with Qtracker 705, and then injected via the tail vein into C57BL/6 mice (Figure 5A). There was equivalent efficiency of c-kit+-cell recruitment to S aureus–infected or uninfected wounds at 24 hours (Figure 5B). EGFP-PMN numbers in S aureus–infected wounds steadily increased after infusion, reaching a level 30-fold greater than saline control wounds, in which the signal decayed to baseline by day 8 (Figure 5C). It has previously been reported that Lin−c-kit+ cells of EGFP-lys-mice emit green fluorescence after myeloid lineage commitment in the bone marrow.24 Thus, by sorting Lin−c-kit+ progenitors from EGFP-lys-mice bone marrow and infusing them into C57BL/6 wild-type mice, c-kit+ progenitors were shown to home from the circulation to the wound bed and effectively differentiate along the myeloid lineage into mature PMNs in the presence of S aureus infection. To ensure that the EGFP-PMN signal was not due to myeloid progenitors that expanded in other peripheral tissue and subsequently immigrated to the wound, PMNs were depleted from the circulation by injection of Gr-1 mAb at days 1, 3, and 5 after wounding. This treatment effectively eliminated EGFP-PMNs detected in the blood (supplemental Figure 4A), but did not alter the functional capacity of Lin−c-kit+ cells to proliferate into EGFP-PMNs (supplemental Figure 4B). Thus, we conclude that the further rise in EGFP-PMN signal detected in S aureus–infected wounds was derived from the differentiation of c-kit+ progenitors in the wound.
Bone marrow–derived c-kit+ progenitors recruit to wounds and give rise to EGFP-PMNs in the presence of S aureus. (A-C) Adoptive transfer of EGFP−c-kit+ cells from EGFP-lys-mice into C57BL/6 wild-type mice. (A) FACS-sorted EGFP−c-kit+ cells were labeled with Qtracker 705 and intravenously transferred (5 × 105 cells in 0.1 mL of sterile saline) to C57BL/6 mice whose wounds were treated with either sterile saline or S aureus (1 × 107 CFUs). (B) Emigrated Qtracker 705–labeled EGFP−c-kit+ cells were detected at 24 hours after transfer in wounds (n = 2 mice in each group). (C) Kinetics of EGFP-PMNs within the S aureus–infected wound after adoptive transfer of EGFP−c-kit+ cells. *P < .05 vs saline-Gr-1 group. Data are derived from 3-4 mice in each group. (D-E) Ex vivo G-CFU assay of wound cells harvested from EGFP-lys-mice treated with saline or S aureus (1 × 107 CFUs). (D) Three days after wounding, S aureus (1 × 107 CFUs)– or saline-treated skin wounds were collected from EGFP-lys-mice, digested, and plated in methylcellulose medium. Data are from 3 separate experiments and represent mean colony counts from S aureus (n = 5 mice)– and saline (n = 3 mice)–treated wounds analyzed 7 days after plating. *P < .05 vs saline group. GM/GEMM indicates granulocyte-macrophage/granulocyte, erythroid, megakaryocyte, macrophage; G, granulocyte; M, monocyte; and E-BFU, erythroid blast-forming unit. (E) Clockwise from top left, EGFP+ colony from S aureus–infected wound, cytospin of cells extracted from an EGFP+ G-CFU colony, and representative negative control non-EGFP colony. CFU images are overlays of phase-contrast and fluorescent images taken with a 20× objective (bar represents 50 μm). The cytospin image was taken with a 50× objective (bar represents 10 μm). Data are expressed as means ± SEM.
Bone marrow–derived c-kit+ progenitors recruit to wounds and give rise to EGFP-PMNs in the presence of S aureus. (A-C) Adoptive transfer of EGFP−c-kit+ cells from EGFP-lys-mice into C57BL/6 wild-type mice. (A) FACS-sorted EGFP−c-kit+ cells were labeled with Qtracker 705 and intravenously transferred (5 × 105 cells in 0.1 mL of sterile saline) to C57BL/6 mice whose wounds were treated with either sterile saline or S aureus (1 × 107 CFUs). (B) Emigrated Qtracker 705–labeled EGFP−c-kit+ cells were detected at 24 hours after transfer in wounds (n = 2 mice in each group). (C) Kinetics of EGFP-PMNs within the S aureus–infected wound after adoptive transfer of EGFP−c-kit+ cells. *P < .05 vs saline-Gr-1 group. Data are derived from 3-4 mice in each group. (D-E) Ex vivo G-CFU assay of wound cells harvested from EGFP-lys-mice treated with saline or S aureus (1 × 107 CFUs). (D) Three days after wounding, S aureus (1 × 107 CFUs)– or saline-treated skin wounds were collected from EGFP-lys-mice, digested, and plated in methylcellulose medium. Data are from 3 separate experiments and represent mean colony counts from S aureus (n = 5 mice)– and saline (n = 3 mice)–treated wounds analyzed 7 days after plating. *P < .05 vs saline group. GM/GEMM indicates granulocyte-macrophage/granulocyte, erythroid, megakaryocyte, macrophage; G, granulocyte; M, monocyte; and E-BFU, erythroid blast-forming unit. (E) Clockwise from top left, EGFP+ colony from S aureus–infected wound, cytospin of cells extracted from an EGFP+ G-CFU colony, and representative negative control non-EGFP colony. CFU images are overlays of phase-contrast and fluorescent images taken with a 20× objective (bar represents 50 μm). The cytospin image was taken with a 50× objective (bar represents 10 μm). Data are expressed as means ± SEM.
Myeloid progenitors isolated from infected wounds differentiate into mature PMNs
Experiments were performed to further confirm that Lin−c-kit+ progenitors that home to S aureus–infected wounds can differentiate into mature EGFP-PMNs. Cells were harvested on day 3 from infected and uninfected wounds of EGFP-lys-mice and cultured in an ex vivo CFU assay. Infected wounds exhibited a 3-fold increase in both the total number of colonies and in EGFP-expressing granulocytic colonies compared with uninfected wounds (Figure 5D). A cytospin of cells extracted from the CFU assay confirmed that the majority of cells were mature PMNs (Figure 5E). As a control, EGFP-PMNs isolated from the blood and cultured in the CFU assay showed no expansion potential, and fluorescence decayed to background within 3 days (data not shown). These data confirm that Lin−c-kit+ progenitors receive an early signal at the site of S aureus abscess to proliferate and differentiate into mature PMNs as early as day 3 after infection.
Depletion of c-kit+ progenitors diminishes PMN expansion and mice survival compared with PMN depletion alone
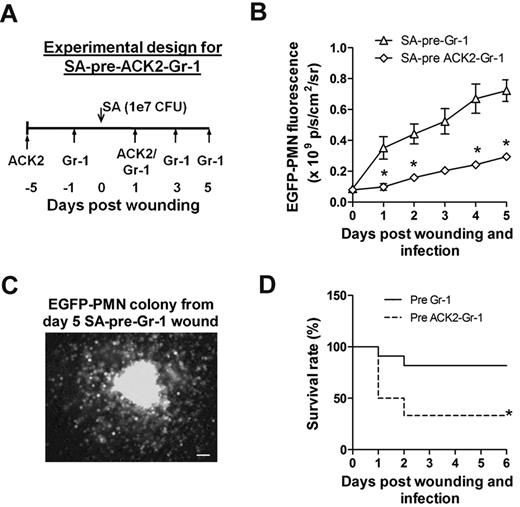
A final set of studies were carried out to reveal the functional significance of c-kit+ myeloid-progenitor trafficking from the bone marrow to the wound for sustaining PMN numbers and resolution of S aureus infection. Antibody to c-kit+ cells (denoted ACK2) was injected 5 days before and 1 day after wounding and infection (Figure 6A). This duration of pretreatment effectively depleted c-kit+-progenitor cells in the bone marrow by more than 60%, whereas PMN numbers decreased by only ∼ 30% compared with injection of an isotype control antibody (supplemental Figure 5). Mice were simultaneously pretreated with ACK2 and Gr-1 to block the trafficking of both c-kit+ progenitors and mature PMNs. This resulted in a 3-fold decrease in the EGFP-PMN signal at day 1 and a significantly diminished rate of PMN proliferation over time compared with depletion of PMNs alone (Figure 6B). We confirmed that cells recovered from wounds of SA-pre-Gr-1 mice exhibited the capacity to proliferate into EGFP-PMN colonies ex vivo in the MethoCult culture assay (Figure 6C). Blocking c-kit+ trafficking in the ACK2-treated mice resulted in a diminished rate of survival of infected mice, which dropped from 80% to 40% by day 2 for SA-pre-ACK2-Gr-1 mice compared with SA-pre-Gr-1 mice (Figure 6D). These data provide a direct demonstration of the importance of myeloid-progenitor trafficking and local expansion within the site of infection to provide adequate numbers of PMNs to prevent dissemination of the bacteria and host survival.
C-kit+-progenitor proliferation within wounds contributes to immune protection against S aureus infection. (A) Experimental design for immunodepletion of c-kit+ cells and PMNs before S aureus infection (1 × 107 CFUs) and skin wounding (SA-pre-ACK2-Gr-1). (B) Comparison of kinetics of wound EGFP-PMNs in S aureus–infected EGFP-lys-mice with SA-pre-ACK2-Gr-1 and SA-pre-Gr-1 treatment (refer to Figure 3A for the experimental design of SA-pre-Gr-1 treatment). (C) EGFP+ colony derived from cells harvested from EGFP-lys-mice with SA-pre-Gr-1 treatment. At day 5 after infection, wounded and S aureus–infected skin biopsies were collected from EGFP-lys-mice with SA-pre-Gr-1 treatment, digested, and plated in methylcellulose medium. Shown is a representative image of an EGFP+ colony 7 days after plating. (D) Comparison of survival rate between EGFP-lys-mice with SA-pre-ACK2-Gr-1 and SA-pre-Gr-1 treatment. *P < .05 vs SA-pre-Gr-1.
C-kit+-progenitor proliferation within wounds contributes to immune protection against S aureus infection. (A) Experimental design for immunodepletion of c-kit+ cells and PMNs before S aureus infection (1 × 107 CFUs) and skin wounding (SA-pre-ACK2-Gr-1). (B) Comparison of kinetics of wound EGFP-PMNs in S aureus–infected EGFP-lys-mice with SA-pre-ACK2-Gr-1 and SA-pre-Gr-1 treatment (refer to Figure 3A for the experimental design of SA-pre-Gr-1 treatment). (C) EGFP+ colony derived from cells harvested from EGFP-lys-mice with SA-pre-Gr-1 treatment. At day 5 after infection, wounded and S aureus–infected skin biopsies were collected from EGFP-lys-mice with SA-pre-Gr-1 treatment, digested, and plated in methylcellulose medium. Shown is a representative image of an EGFP+ colony 7 days after plating. (D) Comparison of survival rate between EGFP-lys-mice with SA-pre-ACK2-Gr-1 and SA-pre-Gr-1 treatment. *P < .05 vs SA-pre-Gr-1.
Discussion
Neutrophils are rapidly mobilized from the bone marrow in sufficient numbers to provide an effective innate immune response against an invading pathogen such as S aureus.1,13,26 However, the mechanisms by which PMNs are recruited and maintain an abscess to control the infection and promote bacterial clearance while promoting wound resolution are not completely defined. In the present study, we applied noninvasive in vivo fluorescence imaging of genetically EGFP-tagged PMNs to provide a quantitative measure of the number present in the wound over time and as a function of S aureus burden. Using this model, we identified 3 distinct mechanisms that act in concert to maintain sufficient numbers of PMNs for host defense in S aureus–infected wounds, including: (1) a robust and sustained mobilization of PMNs from the bone marrow that was correlated with infection burden, (2) a prolonged in vivo survival of PMNs within the abscess, and (3) trafficking of c-kit+-progenitor cells that proliferated and differentiated at the site of the abscess in the skin to produce mature PMNs (Figure 7A). These data demonstrate that S aureus–infected wounds provide important signals that prolong PMN lifespan and, remarkably, the proliferation and differentiation of mature PMNs from bone marrow–derived c-kit+-progenitor cells that have immigrated to the site of infection.
Dynamic contributions of 3 distinct populations of PMNs constitute the innate immune response to S aureus infection. (A) A model depicting the dynamic feedback between the bone marrow compartment and the wound compartment during bacterial infection. Local S aureus infection triggers signals (eg, hematopoietic cytokines) to maintain an enhanced number of PMNs in infected tissue. This can be largely achieved by increased production and mobilization of PMNs in the bone marrow and their recruitment into local infected tissue, where their lifespan is markedly extended to provide sustained immune protection. In addition, bone marrow c-kit+-progenitor expansion and mobilization are activated by S aureus. Progenitor cells infiltrate the site of infection, where they proliferate and differentiate into functional PMNs in part via a MyD88-dependent mechanism to provide additional immune protection. Pinflux represents the number of newly infiltrated PMNs from the blood to the infected tissue during a S aureus infection; Pckit, the number of newly produced PMNs in the tissue by proliferation of c-kit progenitors; and Psurvival, the number of early recruited PMNs that persist after day 1 of infection within the wound. (B) Relative contribution of enhanced PMN survival and their local production by tissue-infiltrated c-kit+ cells that contribute in equal proportion by day 3 to maintain tissue PMN number during S aureus infection. Mathematical modeling predicts the relative contributions of each compartment between days 1 and 5 of S aureus infection (1 × 107 CFUs; for details, see supplemental Methods).
Dynamic contributions of 3 distinct populations of PMNs constitute the innate immune response to S aureus infection. (A) A model depicting the dynamic feedback between the bone marrow compartment and the wound compartment during bacterial infection. Local S aureus infection triggers signals (eg, hematopoietic cytokines) to maintain an enhanced number of PMNs in infected tissue. This can be largely achieved by increased production and mobilization of PMNs in the bone marrow and their recruitment into local infected tissue, where their lifespan is markedly extended to provide sustained immune protection. In addition, bone marrow c-kit+-progenitor expansion and mobilization are activated by S aureus. Progenitor cells infiltrate the site of infection, where they proliferate and differentiate into functional PMNs in part via a MyD88-dependent mechanism to provide additional immune protection. Pinflux represents the number of newly infiltrated PMNs from the blood to the infected tissue during a S aureus infection; Pckit, the number of newly produced PMNs in the tissue by proliferation of c-kit progenitors; and Psurvival, the number of early recruited PMNs that persist after day 1 of infection within the wound. (B) Relative contribution of enhanced PMN survival and their local production by tissue-infiltrated c-kit+ cells that contribute in equal proportion by day 3 to maintain tissue PMN number during S aureus infection. Mathematical modeling predicts the relative contributions of each compartment between days 1 and 5 of S aureus infection (1 × 107 CFUs; for details, see supplemental Methods).
Host-pathogen interactions are initiated through molecular sensing of conserved pathogen-associated molecular patterns of microbes by pattern-recognition receptors such as TLRs, which are expressed on many different types of cells, including innate immune cells (eg, macrophages and dendritic cells) and epithelial cells.27 During the inflammatory phase of the innate immune response, this pathway triggers the release of proinflammatory mediators such as IL-17, IL-1β, tumor necrosis factor-α, and interferon-γ, which leads to the production of chemokines (KC and MIP-2) that trigger the recruitment of circulating PMNs to the site of infection.11,13,26,28 In addition, hematopoietic factors (eg, granulocyte colony-stimulating factor [G-CSF] and GM-CSF) are also produced, which promote granulopoiesis in the bone marrow.29 Our data demonstrate that during the initial 24 hours after skin wounding, signals were released from the wound to elicit a marked increase in PMN recruitment, which was significantly augmented in S aureus–infected wounds by 48 hours. This was accompanied by a concomitant 80% reduction in the bone marrow PMN count and a comparable increase in circulating PMNs that was sustained up to day 5. This is in contrast to the negligible change in PMNs in aseptic wounds, which did not increase in number after 24 hours. These data demonstrate that the presence of S aureus infection promotes an increase in PMN expansion and trafficking from the bone marrow to a cutaneous wound to control the infection.
To understand the contribution of PMNs entering the site of infection from the circulation as opposed to local PMNs that are maintained in the abscess, we depleted PMNs in the circulation with an anti–Gr-1 mAb administered either 24 hours before or after wounding and infection. Immunodepletion before wounding and infection revealed an essential role for the rapid influx of PMNs from the circulation for both controlling S aureus bacterial burden and wound resolution. In contrast, immunodepletion at 24 hours after wounding and infection provided clear evidence that PMN numbers are maintained beyond 8 days in an abscess, and are necessary for resolving the infection. Adoptive transfer of FACS-sorted EGFP-PMNs in wild-type mice demonstrated an equivalent efficiency of recruitment between S aureus–infected and uninfected wounds. The kinetics of the appearance of infused EGFP-PMNs in the wounds of C57BL/6 wild-type mice were similar to those in the EGFP-lys-mice, revealing that the early inflammatory phase provides potent innate signaling that is independent of pathogen-associated molecular patterns. In contrast, PMNs recruited to infected wounds exhibited a tripling in lifespan, and this population was sufficient to control infection without further PMN recruitment from the bone marrow. In fact, limiting the numbers of PMNs recruited to the S aureus–infected wound was not only sufficient in clearing the bacteria, but also allowed the wound to resolve more rapidly than normal control mice exhibiting exuberant PMN recruitment. These data provide evidence that increased PMN numbers do not necessarily have an immune benefit and may in fact be detrimental to wound healing. Furthermore, a PMN-rich environment has previously been shown to potentiate S aureus pathogenesis by facilitating bacterial survival within the PMNs themselves.26 Therefore, a therapeutic advantage against infected wounds may include immunomodulatory strategies aimed at optimizing the numbers of PMNs to clear the infection while minimizing the PMN-rich environment and unwanted PMN-induced inflammation that inhibits wound healing.
In the present study, we demonstrated that S aureus infection effectively extends the half-life of PMNs within the wounds by 3-fold. Applying a simple mathematical model to predict the dynamics of 3 separate populations, we estimate that the prolonged survival of PMNs recruited from the inflammatory phase (Psurvival) make up 60% of total numbers at day 2 and diminish to ∼ 20% by day 5 (Figure 7B and supplemental Figure 6). The remaining 40% are equally divided between PMNs recruited from the systemic compartment (Pinflux) and those differentiated from the c-kit+ myeloid progenitors within the wound (Pckit). Whereas the rate of c-kit+-progenitor proliferation up to day 5 provides a relatively constant source of PMNs, the robust rate of PMN influx from the peripheral blood appears to balance the loss due to clearance and apoptosis. The extended half-life of PMNs is presumably mediated by antiapoptotic signals that are generated during an infection.30 Some of these antiapoptotic signals have been described in vitro, including proinflammatory cytokines (eg, IL-6, interferon-γ, G-CSF, and GM-CSF)31 and bacterial products (eg, lipopolysaccharide and lipoteichoic acid).17 A recent report demonstrated that IL-2–activated natural killer cells produce chemokines that prolong PMN survival and boost their phagocytic and oxidative microbicidal capacity.32 Other in vitro studies have demonstrated that the concentration of the inoculum can directly influence PMN apoptosis. For example, human PMNs undergo rapid apoptosis under culture conditions with a high ratio of bacteria (S aureus or E coli) to PMNs (100:1), but undergo delayed apoptosis at a low ratio (1:1).19 Furthermore, in vivo studies have demonstrated that the concentration of the inoculum can influence PMN survival in a manner similar to our results with S aureus–infected skin wounds. Mice challenged in vivo with a lethal inoculum of Listeria monocytogenes had rapid PMN apoptosis via a TLR2-dependent mechanism.33 In contrast, infection with lower sublethal inocula of L monocytogenes resulted in robust PMN trafficking to infected tissue, which was associated with successful bacterial clearance. Our findings are consistent with these previous studies demonstrating that the bacterial burden plays a key role in PMN survival and extent of influx, which is likely triggered by antiapoptotic signals generated during the infection and local production of chemokines.
We have demonstrated that adoptive transfer of Lin−c-kit+-progenitor cells home to the site of S aureus infection, where they expand the population of mature PMNs. Evidence supporting this was that the depletion of c-kit+ cells with ACK2 antibody nearly abolished the increase in wound PMNs, and that cells recovered from S aureus–infected wounds contained myeloid-progenitor cells that had the potential to give rise to mature PMNs. We conclude that bone marrow–derived c-kit+-progenitor cells traffic to sites of robust inflammation and infection and directly participate in PMN abscess formation. Based on antibody-blocking studies and a mathematical model, we predict that c-kit+-progenitor cells home from the bone marrow early during the inflammatory phase and sustain PMN numbers during abscess formation.
Although the precise mechanism by which S aureus induced local c-kit+-progenitor–cell proliferation and differentiation into functional PMNs in infected wounds was not directly assessed, previous reports suggest that their proliferation was induced by either direct interactions between bacteria and c-kit+ progenitors or indirectly by hematopoietic cytokines. For example, Lin−c-kit+-progenitor cells express TLRs on their surface, and the direct binding of bacterial proteins to Lin−c-kit+ cells can trigger rapid myeloid differentiation in vitro.23 Another study demonstrated that TLRs can induce Lin−c-kit+ cells to expand into a dendritic-cell phenotype, yet the proliferative potential for PMN expansion and their functional role during the progression of bacterial infection was not evaluated.22 To provide insight into the molecular mechanisms underlying the differentiation and expansion of the progenitor cells in the infected wounds, we evaluated the potential role of TLRs in inducing Lin−c-kit+-cell expansion from mice deficient in myeloid differentiation factor 88 (MyD88), an adaptor protein central to signaling via the TLR2 and IL-1 receptor pathways.34 We found that c-kit+ progenitors isolated from the bone marrow of MyD88-knockout mice formed 2-fold fewer granulocytic colonies than wild-type in the presence of heat-killed S aureus in the ex vivo culture assay (supplemental Figure 7). These results point to several pathways by which S aureus may promote c-kit+-progenitor activation. Within the wound, S aureus sheds membrane lipoproteins that, once bound by TLR2, activate MyD88, which signals transcription factor nuclear factor-κB activity in target cells. Because Lin−c-kit+-progenitor cells express TLRs on their surface, one mechanism may involve the direct binding of bacterial lipoproteins to Lin−c-kit+ cells to trigger rapid myeloid differentiation23 (Figure 7A); another mechanism may involve TLR-induced Lin−c-kit+-cell differentiation into a dendritic-cell phenotype, as was recently reported.22 We are currently engaged in studies to determine the differential contribution of TLR and IL-1 receptor ligands and how they may directly or indirectly elicit the differentiation and proliferation of c-kit+ progenitors in the wound.
An alternate mechanism explaining PMN expansion is the production of G-CSF, which is significantly increased during bacterial infection30,35 and can specifically direct the proliferation and differentiation of bone marrow granulocyte-macrophage progenitors into granulocytes in vitro.36 Future study will be directed toward understanding the mechanisms by which S aureus infection promotes c-kit+-progenitor–cell emigration from the bone marrow into the circulation and subsequently into a wound, and the generation of the local signals within the abscess that elicit myeloid-progenitor expansion.
In summary, these studies demonstrate that in an S aureus–infected wound, the formation and maintenance of the PMN abscess involves not only continuous recruitment of PMNs from the circulation and bone marrow, but also prolonged PMN survival in combination with local c-kit+-progenitor–cell proliferation and differentiation into mature PMNs at the site of infection. These findings represent an important demonstration of infection-induced PMN survival in vivo and of the pivotal role of myeloid-progenitor cells in the resolution of a PMN abscess. From a clinical point of view, the identification of the mechanisms that govern PMN abscess formation raises the possibility that PMN and c-kit+-progenitor–cell recruitment, circulating numbers, or activation state could be modulated so that they are sufficient to clear a microbial infection while preventing aberrant inflammation that could exacerbate the infection and delay wound healing.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Thomas Graf for generously providing the EGFP-lys-mice.
This work was supported by National Institutes of Health (NIH) grant AI42794 (to S.I.S.) and by NIH grants AI078910 and AR054534 (to L.S.M.). J.L.G. was supported by a Floyd and Mary Schwall Fellowship in Biomedical Research from University of California-Davis.
National Institutes of Health
Authorship
Contribution: M.-H.K. and J.L.G. designed experiments and performed the research, collected and analyzed data, and wrote the manuscript; C.K. and N.J.W. assisted with the experiments; D.L.B. and F.-R.E.C. designed experiments and reviewed the manuscript; L.S.M. designed the research and wrote the manuscript; and S.I.S. designed the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott I. Simon, Department of Biomedical Engineering, University of California at Davis, 451 E Health Sciences Dr, Davis, CA 95616; e-mail: sisimon@ucdavis.edu.
References
Author notes
M.-H.K. and J.L.G. contributed equally to this study.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal