In this issue of Blood, Al-Tamimi and colleagues demonstrate that activated coagulation factor X (FXa) efficiently triggers the down-regulation of the platelet-activating collagen receptor glycoprotein VI (GPVI), thereby providing a novel negative feedback mechanism in the thrombotic cascade.1
Vascular injury triggers adhesion and aggregation of platelets (primary hemostasis) and coagulation-dependent fibrin formation (secondary hemostasis). These processes are essential for hemostasis but may also lead to occlusion of diseased vessels by thrombus formation and potentially fatal embolization. Therefore, coagulation as well as platelet inhibitors are routinely used in the clinic to treat or prevent thrombotic disease states such as myocardial infarction or stroke.
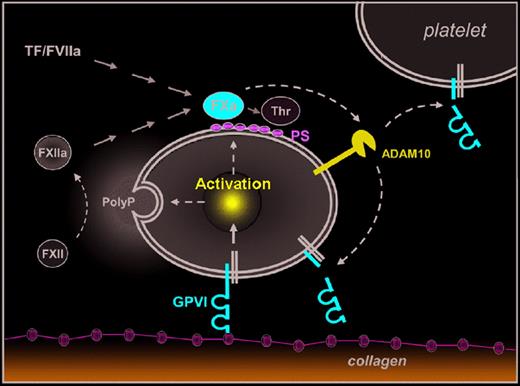
A new molecular link between coagulation and platelet activation. Emphasis is on the novel pathway. Platelet activation on subendothelial collagens is mediated by GPVI and leads to release of inorganic polyphosphates (PolyP) and surface exposure of procoagulant phosphatidylserine (PS). PolyP-triggerd FXII activation (intrinsic coagulation pathway) and tissue factor (TF)/FVIIa (extrinsic coagulation pathway) lead to activation of FX and finally thrombin (Thr) generation. Al-Tamimi and coworkers1 now demonstrate that FXa also directly or indirectly activates ADAM10 (and possibly other metalloproteinases) on the platelet surface leading to ectodomain shedding of GPVI, which may limit further activation of adherent platelets and/or reduce reactivity of circulating platelets.
A new molecular link between coagulation and platelet activation. Emphasis is on the novel pathway. Platelet activation on subendothelial collagens is mediated by GPVI and leads to release of inorganic polyphosphates (PolyP) and surface exposure of procoagulant phosphatidylserine (PS). PolyP-triggerd FXII activation (intrinsic coagulation pathway) and tissue factor (TF)/FVIIa (extrinsic coagulation pathway) lead to activation of FX and finally thrombin (Thr) generation. Al-Tamimi and coworkers1 now demonstrate that FXa also directly or indirectly activates ADAM10 (and possibly other metalloproteinases) on the platelet surface leading to ectodomain shedding of GPVI, which may limit further activation of adherent platelets and/or reduce reactivity of circulating platelets.
Collagens are among the most thrombogenic constituents of the vessel wall and they potently activate platelets. This activation is mediated by GPVI, a type I transmembrane protein of ∼ 68 kDa that signals through the noncovalently associated Fc receptor (FcR) γ-chain. Ligation of GPVI results in full cellular activation characterized by a rise in cytosolic [Ca2+]i, shape change, activation of integrins and granule secretion.2 Furthermore, platelets stimulated via GPVI activate the coagulation system by 2 principal mechanisms: first, they release inorganic polyphosphates, which trigger the intrinsic coagulation pathway through activation of coagulation factor XII (FXII),3 and second, they expose procoagulant phosphatidylserine (PS), which stimulates the assembly of tenase and prothrombinase complexes.4 Both pathways converge in the generation of FXa and finally thrombin, which converts plasma fibrinogen to fibrin, but is also a potent activator of platelets (see figure). Thus, GPVI is in a central position in the regulation of primary and secondary hemostasis, making it an interesting target for antithrombotic therapy.
GPVI can be down-regulated from the platelet surface by shedding, which releases a soluble ectodomain fragment (∼ 55 kDa) that is detected in plasma of humans and mice. Similar to other platelet surface glycoproteins, such as GPIb and GPV, GPVI shedding is mediated by metalloproteinases, including a disintegrin and metalloproteinase 10 (ADAM10)5 and ADAM17.6 The only natural trigger of GPVI shedding identified so far is occupation of the receptor by ligands or antibodies. While the latter efficiently deplete GPVI in circulating platelets in vivo leading to sustained antithrombotic protection,2 it is unclear whether platelet contact with subendothelial collagens does indeed induce significant GPVI shedding. However, increased levels of soluble GPVI ectodomain (sGPVI) have been detected in plasma from patients with acute stroke7 or acute coronary syndrome,8 indicating that other mechanisms may exist to induce GPVI shedding.
Al-Tamimi and coworkers1 now provide compelling evidence that FXa slowly but efficiently induces GPVI ectodomain shedding in platelets, thereby revealing a novel molecular link between the coagulation system and platelet activation. In a series of elegant experiments they show that initiation of the extrinsic coagulation pathway in human platelet-rich plasma progressively induces metalloproteinase- dependent shedding of GPVI and that this can occur independently of active thrombin. Remarkably, sGPVI release was abolished by FXa inhibitors (eg, Rivaroxaban) and strongly reduced in FX-depleted plasma. Conversely, direct activation of FX in plasma or addition of FXa to washed platelets produced sGPVI. Interestingly, in a washed system FXa still provoked GPVI cleavage in the apparent absence of platelet activation. Furthermore, the authors exclude a direct effect of FXa on GPVI or an involvement of GPVI signaling in this process, but rather provide evidence that FXa directly or indirectly activates ADAM10 (and possibly other metalloproteinases) on the platelet surface. In line with this, limited shedding of GPV, another ADAM10 substrate, was detectable in FXa-treated platelets, whereas no shedding of GPIb, a substrate of ADAM17, was observed. In the second part of the paper, the authors provide initial data suggesting that the proposed coagulation-induced GPVI shedding indeed occurs in vivo. They show that plasma sGPVI levels are significantly increased in patients with disseminated intravascular coagulation (DIC) compared with healthy controls or patients with thrombocytopenia. As collagen-induced GPVI shedding may not be relevant in DIC, it appears likely that the increased coagulant activity in these patients triggered the release of sGPVI.
These intriguing observations demonstrate for the first time the existence of a negative feedback of coagulation on a platelet activation pathway, which may have major clinical implications but also raises important questions. First, what is the physiologic function of GPVI shedding? One may speculate that it constitutes a mechanism to regulate platelet reactivity, but no evidence in support of this hypothesis has been provided to date. It will be interesting to study whether shedding of GPVI occurs in collagen-bound platelets, and if so, whether those receptors that are in direct contact with the matrix protein are affected. Alternatively, this cleavage mechanism might have a function to shutdown GPVI to prevent activation by (unknown) noncollagen agonists, but again there is no evidence to support this idea.
Another emerging question is whether proteolytic activity of FXa is required for GPVI shedding. In thrombi formed in vivo and in vitro, FXa is concentrated at the surface of activated platelets where it is also formed.9 Its activity here on prothrombin cleavage is greatly enhanced by the cofactor FVa. Apparently, the shedding of GPVI occurs independently of FVa (although factor V is present in platelets), which points to a mechanism different from that of prothrombin activation. On the other hand, the authors' data with Russell viper venom and inhibitors indicate that exposure of the catalytic site of FXa is essential. This resembles the mechanism of another established activity of FXa, namely activation of protease-activated receptors on endothelial cells that also relies on exposure of the FXa catalytic site.10 Being proteolytically active or not, an unexpected feature of FXa is that it appears to trigger its own protease cascade, turning on metalloproteinase-dependent receptor cleavage.
The conventional view of the coagulation system is as a linear cascade of proteolytic reactions with a tight connection of FXa and thrombin generation by the tenase and prothrombinase complexes, respectively. Hence, the clinical use of FXa inhibitors is often seen as just an alternative, or perhaps more effective, way to suppress thrombin generation. However, the present findings confront us with the fact that FXa also has downstream (proteolytic) effects that are independent of thrombin. It is conceivable that in the near future other thrombin-independent effects of FXa will be discovered as well, which will place FXa at a more central place in the coagulation cascade. This clearly may have implications for the use of novel FXa inhibitors.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal