Abstract
In patients with chronic lymphocytic leukemia (CLL), lenalidomide can promote humoral immune responses but also induces a distinct disease-specific toxicity of tumor flare and cytokine release. These CLL-specific events result from increased expression of costimulatory molecules on B cells. Here we demonstrate that lenalidomide activation of CLL cells depends on the phosphatidylinositol 3-kinase p110δ (PI3K-δ) pathway. Inhibition of PI3K-δ signaling by the PI3K-δ-inhibiting drug, CAL-101, or by siRNA knockdown of p110δ, abrogates CLL cell activation, costimulatory molecule expression, and vascular endothelial growth factor and basic fibroblast growth factor gene expression that is induced by lenalidomide. In addition, CAL-101 attenuates lenalidomide-mediated increases in immunoglobulin M production by normal B cells. Collectively, these data demonstrate the importance of PI3K-δ signaling for lenalidomide immune modulation. These findings may guide development of strategies for the treatment of CLL that combine lenalidomide with CAL-101, with other inhibitors of the PI3K-δ pathway, or with other agents that target downstream kinases of this signaling pathway.
Introduction
Lenalidomide is an immune modulatory agent currently approved for marketing in multiple myeloma and myelodysplasia. Lenalidomide is also clinically active in lymphoma, acute myeloid leukemia, and chronic lymphocytic leukemia (CLL).1,2 The potential mechanisms of action of lenalidomide in these different diseases are multiple.3 Application of lenalidomide in CLL has been associated with development of antitumor antibodies and reversal of hypogammaglobulinemia4 but can also induce a disease-specific side effect of tumor flare and cytokine release.3,5 The downstream manifestations of cytokine release, including increased serum basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF), have previously been associated with promoting CLL survival6-11 and also with decreased response to lenalidomide in CLL.2 Understanding the mechanisms of these responses to lenalidomide is relevant to the development of this agent in CLL and to the logical design of future combination studies.
We have recently demonstrated that lenalidomide can increase surface expression of CD154 on CLL cells while promoting normal B cells to produce immunoglobulin G (IgG) and IgM.4 After clinical treatment with lenalidomide, we were also able to demonstrate a similar phenotype in CLL cells in patients receiving CD154 gene therapy,12 including up-regulation of DR5 and BID. In one patient with pretreatment evidence of residual normal B cells, lenalidomide induced the development of antibodies, including generation of antitumor-directed ROR-1 antibodies.4 The mechanism by which this occurred was shown in vitro to involve upstream activation of the phosphatidylinositol 3-kinase (PI3K) pathway.4 Immune activation of CLL cells with up-regulation of costimulatory molecules, such as CD40, CD80, and CD86,4,5,13 may also be responsible for lenalidomide-induced tumor flare. Given that CLL cell activation could have both favorable immune-modulating effects and detrimental clinical effects from tumor flare and also production of antiapoptotic cytokines, such as bFGF and VEGF, we sought to characterize whether a specific PI3K isoform was responsible for CLL activation by lenalidomide. This work is highly relevant given the observed preclinical14 and clinical activity15 of the PI3K p110δ (PI3K-δ) inhibitor, CAL-101, in the treatment of CLL, the development of other compounds targeting B-cell receptor signaling,16,17 and the potential interest in administering these newer drugs with lenalidomide as therapy for CLL.
Methods
Cell culture and treatment reagents
Written, informed consent was obtained in accordance with the Declaration of Helsinki to procure cells from patients with CLL following a blood collection protocol approved by The Ohio State University Institutional Review Board,18 and selection was performed as previously described.19 CAL-101 was supplied by Calistoga Pharmaceuticals. Lenalidomide (Revlimid; Celgene) was obtained as previously described.5 The 0.5μM dose of lenalidomide and 1μM dose of CAL-101 used in the in vitro experiments are reached clinically. In addition, as we reported,14 CAL-101 has a modest effect on cell viability; thus, the conditions used in these experiments show little effects on viability.
Flow cytometry
Surface staining with antibodies to CD20, CD40, CD80, CD86, or IgG1 (BD Biosciences) was done as previously described.4
Immunoblot analysis
Immunoblots were performed as previously described.20 Antibodies included: anti-AKT, antiphospho-AKT (Ser473), anti-GSK3β, antiphospho-GSK3β (Ser 9; Cell Signaling), anti-p110δ (Santa Cruz Biotechnology), and anti-GAPDH (Millipore).
Quantitative RT-PCR
RNA was extracted using TRIzol reagent (Invitrogen), and cDNA was prepared using a SuperScript First-Strand Synthesis System (Invitrogen) as previously described.21 Real-Time PCR was performed using predesigned TaqMan Gene Expression Assays and an ABI Prism 7700 sequence detection system (Applied Biosystems).
PI3K assay
The PI3K assay was performed on whole-cell lysates from CLL cells. The enzyme-linked immunosorbent assay was performed according to the manufacturer's instructions as previously described14 (Echelon Biosciences).
siRNA transfection
CLL cells were transfected as previously described.22 PI3K-δ-specific siRNA (Ambion) was used at a final concentration of 50nM.
Ig detection
Quantitation of IgM was determined as previously described.4 Briefly, lenalidomide-treated or vehicle-treated CLL cells were irradiated and placed in culture with target, normal B cells in the absence or presence of pokeweed mitogen (5 μg/mL).
Statistical analysis
All reported statistical evaluations were performed with methods previously described.14 Because the same patient samples were treated under different conditions, linear mixed effects models were used for all analyses to account for the correlations of observations from the same patient. Reverse-transcribed polymerase chain reaction (RT-PCR) data were first normalized to internal controls before analysis. Log transformation was also used for some experiments to reduce variance and skewness. The Holm procedure was used to adjust for multiple comparisons. P values ≤ .05 (including those adjusted for multiple comparisons) were considered significant.
Results and discussion
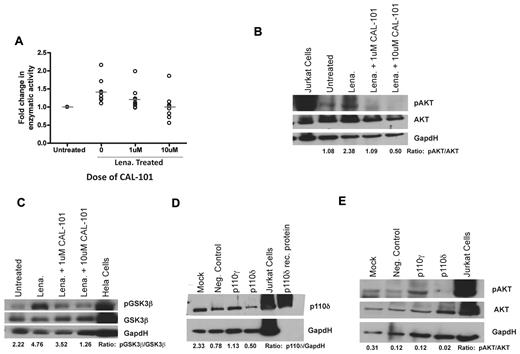
Previous studies have demonstrated that lenalidomide up-regulates CD154 protein expression on CLL cells by activation of AKT, I-κ kinase, and by nuclear factor-κB nuclear translocation with subsequent increased mRNA transcription and stabilization without inducing cell death.4 Treatment of CLL cells with the pan-PI3K inhibitor LY294002 antagonized this CD154 up-regulation.4 PI3K signaling by lenalidomide could occur through any of the 4 catalytic isoforms of PI3K: p110α, p110β, p110γ, and p110δ. We therefore sought to determine whether a specific isoform of PI3K was responsible for this. We first confirmed that lenalidomide directly increased the enzymatic activity of PI3K (P = .0012; Figure 1A). We found that inhibition of PI3K-δ via the p110δ-specific small-molecule inhibitor CAL-101 prevented the increase in PI3K enzymatic activity (P = .0152; Figure 1A). Next, we sought to determine whether inhibition of PI3K-δ with CAL-101 could prevent the increase in downstream phosphorylation of AKT induced by lenalidomide; we found that inhibition of PI3K-δ by CAL-101 prevented phospho-AKT formation (Figure 1B). To confirm these results, we evaluated the phosphorylation of GSK3β, another downstream protein in the PI3K pathway. We found that lenalidomide provoked an increase in phospho-GSK3β that was preventable by cotreatment with CAL-101, again suggesting a link between PI3K-δ and lenalidomide-dependent PI3K activity (Figure 1C). To confirm that these results were indeed the result of PI3K-δ inhibition, we knocked down PI3K-δ in CLL cells (Figure 1D). We were able to show that lenalidomide was unable to induce phosphorylation of AKT when PI3K-δ-specific siRNA was expressed (Figure 1E). Given our desire to focus on selectively expressed hematopoietic isoforms, we did not target p110α and p110β. Such investigation with pan-PI3K inhibitors warrants future studies. These findings demonstrate that lenalidomide-mediated PI3K activation and subsequent effects on nuclear factor-κB and CD1544 in CLL cells use a PI3K-δ-dependent pathway.
Lenalidomide leads to activation of the PI3K pathway via a PI3K-δ-dependent mechanism. (A) CD19+ cells from CLL patients (N = 9) treated with or without 0.5μM lenalidomide were examined for PI3K activity with and without the addition of 1 or 10μM CAL-101 to the lysate. Results were calculated relative to micrograms of protein. (B) CD19+ cells from CLL patients (N = 6) were incubated with or without 0.5μM lenalidomide and/or CAL-101 for 48 hours. AKT phosphorylation at Ser473 was assessed by immunoblot. Results are shown from one of 6 experiments. (C) CD19+ cells from CLL patients (N = 4) were incubated with or without 0.5μM lenalidomide and/or CAL-101 for 48 hours. GSK3β phosphorylation at Ser9 was assessed by immunoblot. Results are shown from one of 4 experiments. (D) CD19+ cells from CLL patients (N = 3) were transfected with siRNA targeted to PI3K-δ, PI3K-γ, or a nonsense target. p110δ protein expression was assessed by immunoblot. Results are shown from one of 3 experiments. (E) CD19+ cells from CLL patients (N = 3) were transfected with siRNA targeted to PI3K-δ, PI3K-γ, or a nonsense target and then incubated with or without 0.5μM lenalidomide for 48 hours. AKT phosphorylation at Ser473 was assessed by immunoblot. Results are shown from one of 3 experiments. (B-E) Quantification was done using the Alpha Innotech FluorChemQ MultiImage III System.
Lenalidomide leads to activation of the PI3K pathway via a PI3K-δ-dependent mechanism. (A) CD19+ cells from CLL patients (N = 9) treated with or without 0.5μM lenalidomide were examined for PI3K activity with and without the addition of 1 or 10μM CAL-101 to the lysate. Results were calculated relative to micrograms of protein. (B) CD19+ cells from CLL patients (N = 6) were incubated with or without 0.5μM lenalidomide and/or CAL-101 for 48 hours. AKT phosphorylation at Ser473 was assessed by immunoblot. Results are shown from one of 6 experiments. (C) CD19+ cells from CLL patients (N = 4) were incubated with or without 0.5μM lenalidomide and/or CAL-101 for 48 hours. GSK3β phosphorylation at Ser9 was assessed by immunoblot. Results are shown from one of 4 experiments. (D) CD19+ cells from CLL patients (N = 3) were transfected with siRNA targeted to PI3K-δ, PI3K-γ, or a nonsense target. p110δ protein expression was assessed by immunoblot. Results are shown from one of 3 experiments. (E) CD19+ cells from CLL patients (N = 3) were transfected with siRNA targeted to PI3K-δ, PI3K-γ, or a nonsense target and then incubated with or without 0.5μM lenalidomide for 48 hours. AKT phosphorylation at Ser473 was assessed by immunoblot. Results are shown from one of 3 experiments. (B-E) Quantification was done using the Alpha Innotech FluorChemQ MultiImage III System.
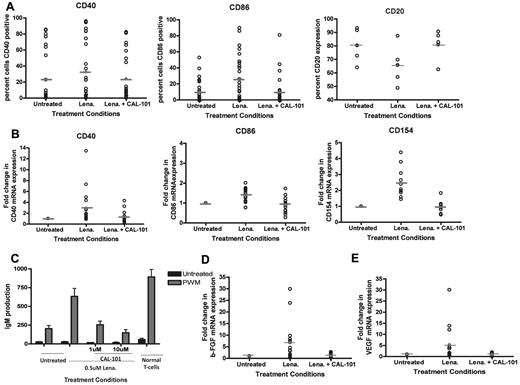
We have previously shown that lenalidomide activates CLL cells. To extend our findings, we sought to determine whether inhibition of PI3K-δ prevented lenalidomide-induced up-regulation of other costimulatory molecules important to B-cell antigen presentation.4 We found that treatment with the PI3K-δ inhibitor CAL-101 was able to prevent the up-regulation of CD40 and CD86 induced by lenalidomide (P = .0102 and < .0001, respectively; Figure 2A). Similarly, we found that inhibition of PI3K-δ could also prevent the increase in mRNA of CD40, CD86, CD154, and CD80 promoted by lenalidomide (P < .009 for all genes; Figure 2B; and data not shown). Activation of CLL cells by lenalidomide has also been shown to induce internalization of the CD20 antigen on CLL cells21 similar to that observed in activated normal B cells23,24 ; concurrent treatment with CAL-101 prevented this effect (P = .0057; Figure 2A). The CD40-CD154 axis is important in allowing lenalidomide-treated CLL cells to promote Ig production by normal B cells; thus, we next assessed whether CAL-101 could prevent the occurrence of this effect. As we have also previously shown,4 lenalidomide treatment of CLL cells increased IgM levels. However, pretreatment of CLL cells with CAL-101 could completely prevent production of IgM by normal B cells (P < .0001; Figure 2C). Cytokines, such as bFGF6-8 and VEGF,6,9-11 support the survival of CLL cells. Moreover, it has been observed that circulating levels of bFGF can correlate with response or nonresponse to therapy for CLL.2 Thus, we sought to determine whether lenalidomide treatment of CLL cells in culture could promote conditions favoring enhanced tumor cell production of these factors. We found that lenalidomide treatment increased bFGF mRNA in all patients (P = .0004) and that coincubation with CAL-101 prevented this up-regulation (P < .001; Figure 2D). Similarly, we found that lenalidomide treatment increased VEGF mRNA in a subset (8 of 13) of patients and that this effect was also reversed by CAL-101 cotreatment (P = .002; Figure 2E).
Inhibition of PI3K-δ prevents CLL cell immune activation induced by lenalidomide. (A) CD19+ cells from CLL patients (N = 25) were treated with or without 0.5μM lenalidomide and/or 10μM CAL-101 for 48 hours. Surface expression of CD20, CD40, or CD86 was evaluated by flow cytometry using CD20-phycoerythrin, CD40-phycoerythrin, or CD86-phycoerythrin antibodies and IgG1-phycoerythrin isotype control. (B) CD19+ cells from CLL patients (N = 15) were treated with or without 0.5μM lenalidomide and/or 10μM CAL-101 for 48 hours. RNA was extracted and converted to cDNA, and RT-PCR analysis was done to determine quantities of CD40, CD86, and CD154 mRNA. (C) CD19+ cells from CLL patients (N = 6) were treated with or without 0.5μM lenalidomide and/or CAL-101 for 48 hours. CLL cells were irradiated (20 Gy) and placed in culture with purified B cells, in the absence or presence of 5 μg/mL pokeweed mitogen. Quantification of IgM was determined by enzyme-linked immunosorbent assay. (D) CD19+ cells from CLL patients (N = 15) were treated with or without 0.5μM lenalidomide and/or 10μM CAL-101 for 48 hours. RNA was extracted and converted to cDNA, and RT-PCR analysis was done to determine quantities of bFGF. (E) CD19+ cells from CLL patients (N = 13) were treated with or without 0.5μM lenalidomide and/or 10μM CAL-101 for 48 hours. RNA was extracted and converted to cDNA, and RT-PCR analysis was done to determine quantities of VEGF.
Inhibition of PI3K-δ prevents CLL cell immune activation induced by lenalidomide. (A) CD19+ cells from CLL patients (N = 25) were treated with or without 0.5μM lenalidomide and/or 10μM CAL-101 for 48 hours. Surface expression of CD20, CD40, or CD86 was evaluated by flow cytometry using CD20-phycoerythrin, CD40-phycoerythrin, or CD86-phycoerythrin antibodies and IgG1-phycoerythrin isotype control. (B) CD19+ cells from CLL patients (N = 15) were treated with or without 0.5μM lenalidomide and/or 10μM CAL-101 for 48 hours. RNA was extracted and converted to cDNA, and RT-PCR analysis was done to determine quantities of CD40, CD86, and CD154 mRNA. (C) CD19+ cells from CLL patients (N = 6) were treated with or without 0.5μM lenalidomide and/or CAL-101 for 48 hours. CLL cells were irradiated (20 Gy) and placed in culture with purified B cells, in the absence or presence of 5 μg/mL pokeweed mitogen. Quantification of IgM was determined by enzyme-linked immunosorbent assay. (D) CD19+ cells from CLL patients (N = 15) were treated with or without 0.5μM lenalidomide and/or 10μM CAL-101 for 48 hours. RNA was extracted and converted to cDNA, and RT-PCR analysis was done to determine quantities of bFGF. (E) CD19+ cells from CLL patients (N = 13) were treated with or without 0.5μM lenalidomide and/or 10μM CAL-101 for 48 hours. RNA was extracted and converted to cDNA, and RT-PCR analysis was done to determine quantities of VEGF.
Our data demonstrate that the PI3K-δ pathway is involved in lenalidomide-mediated activation of CLL cells. These findings have potential relevance to the clinical use of the combination of lenalidomide with CAL-101, pan-PI3K inhibitors, and potentially other B-cell receptor-signaling agents. Our data suggest that such agents may antagonize the immune-modulating properties of lenalidomide. Thus, if lenalidomide-mediated immune modulation is considered desirable, coadministration of such drugs with lenalidomide should be approached with caution. Conversely, concomitant therapy with agents, such as CAL-101, might be attractive for preventing the immune activation, tumor flare, and cytokine-release syndrome associated with lenalidomide treatment. The addition of CAL-101 or similar drugs to lenalidomide might also reduce the induction of cytokines, such as bFGF and VEGF, which has been associated with poor response to treatment, thus offering the prospect of enhanced antitumor activity with combination therapy.2
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Specialized Center of Research from the Leukemia & Lymphoma Society, the National Cancer Institute (K12 CA133250, P50 CA140158, P01 CA95426, and P01 CA101956), and the D. Warren Brown Foundation. A.J.J. is a Paul Calabresi Scholar.
National Institutes of Health
Authorship
Contribution: S.E.M.H. planned the research, performed experiments, analyzed data, drafted the first and subsequent drafts of the paper, and approved the final version of the paper; R.L. planned the research, performed experiments, analyzed data, assisted in drafting the paper, and approved the final version of the paper; A.L.G. and A.R. were involved in planning components of the research, performed experiments, reviewed drafts, and approved the final version of the paper; K.A.B. and J.J. were involved in recruitment of patients and sample collection, reviewed drafts, and approved the final version of the paper; X.Z. was involved in planning components of the research, did all the statistical analysis, reviewed drafts, and approved the final version of the paper; B.J.L. and K.D.P. provided input and suggestions to the presentation of the data and a critical reagent (CAL-101) essential for completion of the work and reviewed and approved the final version of the paper; and N.M., J.C.B., and A.J.J. planned every aspect of the proposal, supervised the research, analyzed data, reviewed drafts, obtained funding for the research work, and approved the final version of the paper.
Conflict-of-interest disclosure: J.C.B. has financial interest in Calistoga Pharmaceuticals (stock options). B.J.L. and K.D.P. are employees of Calistoga Pharmaceuticals and have financial interest in this company (stock options and salary). The remaining authors declare no competing financial interests.
Correspondence: John C. Byrd, The Ohio State University, B302 Starling-Loving Hall, 320 West 10th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu; and Amy J. Johnson, The Ohio State University Comprehensive Cancer Center Bldg, Rm 455C, 410 West 12th Ave, Columbus, OH 43210; e-mail: amy.johnson@osumc.edu.
References
Author notes
S.E.M.H. and R.L. contributed equally to this study.
J.C.B. and A.J.J. contributed equally to this study and are the senior authors.