Abstract
Research applications and cell therapies involving genetically modified cells require reliable, standardized, and cost-effective methods for cell manipulation. We report a novel nanomagnetic method for integrated cell separation and gene delivery. Gene vectors associated with magnetic nanoparticles are used to transfect/transduce target cells while being passaged and separated through a high gradient magnetic field cell separation column. The integrated method yields excellent target cell purity and recovery. Nonviral and lentiviral magselectofection is efficient and highly specific for the target cell population as demonstrated with a K562/Jurkat T-cell mixture. Both mouse and human enriched hematopoietic stem cell pools were effectively transduced by lentiviral magselectofection, which did not affect the hematopoietic progenitor cell number determined by in vitro colony assays. Highly effective reconstitution of T and B lymphocytes was achieved by magselectofected murine wild-type lineage-negative Sca-1+ cells transplanted into Il2rg−/− mice, stably expressing GFP in erythroid, myeloid, T-, and B-cell lineages. Furthermore, nonviral, lentiviral, and adenoviral magselectofection yielded high transfection/transduction efficiency in human umbilical cord mesenchymal stem cells and was fully compatible with their differentiation potential. Upscaling to a clinically approved automated cell separation device was feasible. Hence, once optimized, validated, and approved, the method may greatly facilitate the generation of genetically engineered cells for cell therapies.
Introduction
The feasibility of using genetically engineered cells for therapy in humans has been demonstrated using various cell types, including tumor cells,1-3 lymphocytes, dendritic cells,4,5 fibroblasts,6,7 hematopoietic stem cells (HSCs),8,9 and mesenchymal stem cells (MSCs).10,11 Actual or potential applications are as diverse as immune gene therapy for the treatment of cancer,1-3 cancer therapy with T cells expressing chimeric T-cell receptors,12,13 the treatment of hereditary diseases,8,9,14-16 and a plethora of applications in regenerative medicine.17 With the emerging field of induced pluripotent stem cells, research in genetically engineered cell therapies has reached yet another level of pace and dimension. Clinical applications will require efficient, reliable, standardized methods for cell manipulation. For optimized reproducibility and wide practicality in a decentralized manner, such methods should compose a minimum number of handling steps and be cost-effective and amenable to automation in a closed system.
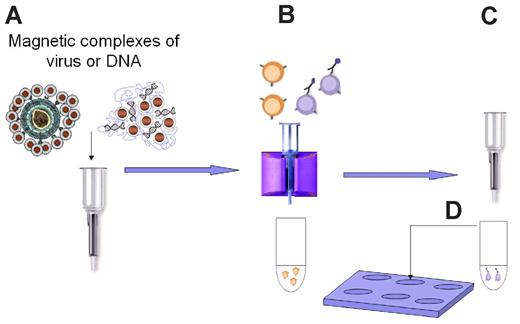
The goal of this work is to provide a novel methodology for performing genetic modification and cell isolation in a single standardized procedure, which we call “magselectofection” (Figure 1). It integrates nanomagnetic cell separation, which is an approved clinical application,18,19 and nanomagnetically guided nucleic acid delivery known as magnetofection.20-22 For magnetic cell separation, magnetic nanoparticles modified with target cell-specific antibodies are used to capture target cells and to separate them from nontarget cells using a high gradient magnetic field. For magnetofection, vectors for nucleic acid delivery are associated with magnetic nanoparticles. A gradient magnetic field is used to rapidly concentrate and internalize the full applied vector dose into the target cells,23,24 drastically improving kinetics and the overall efficiency of the nucleic acid delivery process in vitro and in vivo.20-22,25-28
Schematic representation of the magselectofection procedure. (A) A vector, viral or nonviral, is associated with magnetic nanoparticles. In this manner, one can immobilize the vector on magnetic cell separation devices, such as shown here on a magnetic cell separation column from Miltenyi Biotec. (B) Magnetic particles binding specifically to target cells by virtue of affinity ligands bound to the particle surface are used to magnetically label target cells. The cells are then loaded to the vector-modified cell separation device while it is exposed to a magnetic field and are thus retained. Nontarget cells are not retained and are flushed from the device. During this procedure, the target cells bind the magnetically retained vector. (C) Finally, the cell separation device is removed from the magnetic field, and the selected cells are flushed from the device and (D) cultivated until further use. This general scheme was implemented on MACS cell separation devices (Miltenyi Biotec) and led to rapid, efficient, and target cell-specific gene delivery.
Schematic representation of the magselectofection procedure. (A) A vector, viral or nonviral, is associated with magnetic nanoparticles. In this manner, one can immobilize the vector on magnetic cell separation devices, such as shown here on a magnetic cell separation column from Miltenyi Biotec. (B) Magnetic particles binding specifically to target cells by virtue of affinity ligands bound to the particle surface are used to magnetically label target cells. The cells are then loaded to the vector-modified cell separation device while it is exposed to a magnetic field and are thus retained. Nontarget cells are not retained and are flushed from the device. During this procedure, the target cells bind the magnetically retained vector. (C) Finally, the cell separation device is removed from the magnetic field, and the selected cells are flushed from the device and (D) cultivated until further use. This general scheme was implemented on MACS cell separation devices (Miltenyi Biotec) and led to rapid, efficient, and target cell-specific gene delivery.
Optimized magnetic nonviral and viral vector compositions and commercially available magnetic-activated cell sorting (MACS) cell separation columns were used to establish magselectofection. This demonstrated that the integrated procedure works effectively and can be practiced with nonviral, lentiviral, and adenoviral vectors. Magselectofection is highly efficient in genetically modifying hematopoietic and mesenchymal-like stem cells from umbilical cord while maintaining their differentiation potential. We provide evidence that upscaling in an automated cell separation device is feasible. Once optimized, validated, and approved, the method can facilitate future clinical applications of genetically engineered cells.
Methods
Modification of Miltenyi LS cell separation columns with magnetic transfection/transduction complexes
All handling steps were carried out at room temperature. Magnetic vectors were generally prepared in a final volume of 400 μL, the approximate void volume of LS columns (Miltenyi Biotec). The vector suspensions were allowed to seep into the columns, which were subsequently positioned in the MidiMACS Separator magnet. This procedure ensures complete immobilization and homogeneous distribution of the magnetic vector within the MACS column. Magnetically labeled cells were applied to vector-modified columns within 30 minutes after vector loading.
Magnetic lipoplexes
PEI-Mag2 and SO-Mag2 magnetic nanoparticles were synthesized as previously described29,30 (supplemental Data; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The particle stock concentration in terms of iron content was determined as described previously.20 Magnetic lipoplexes were prepared with eGFP or luciferase plasmid DNA and DreamFect-Gold lipid transfection reagent (DF-Gold, OZ Biosciences). Magnetic nanoparticles (MNPs, 20 μg in 100 μL water) were added to plasmid DNA (20 μg in 100 μL RPMI) and mixed followed by addition of DF-Gold (80 μL diluted to 100 μL with water). After addition of 100 μL RPMI 1640 media without additives, the suspension was kept at room temperature for 20 minutes to allow complex assembly. The resulting ratio of components in the complex (MNPs/DF-Gold/pDNA) is 1:4:1 (iron weight/volume/weight). The characteristics of the complexes are summarized in supplemental Table 2.
Magnetic virus complexes
A third-generation self-inactivating lentiviral vector pRRL.PPT.SF.eGFP.bPRE4*.SIN (LV.eGFP) containing the spleen focus-forming virus promoter or the phosphoglycerate kinase (PGK) promoter (LV-PGK-eGFP) was produced as described previously.16 The vector backbone was kindly provided by Dr Naldini with modifications by Dr Schambach.16
Lentivirus vector stock physical titers ranged between 1.2 × 109 and 9 × 1012 p24 vector particles (VP/mL) determined by QuickTiter Lentivirus Quantitation Kit (Cell Biolabs, Inc) for the detection and quantitation of lentivirus-associated HIV-1 p24 core protein. Biologic titers ranged between 1.7 × 106 and 1.9 × 109 transducing units (TU/mL), determined on CMS531 cells or HeLa cells as described previously,16,32 and are referred to as applied multiplicity of infection (MOI).
For lentiviral magselectofection of human umbilical cord blood mesenchymal-like stem cells (hUC-MSCs) and hCB-CD34+ cells, virus vector was mixed with SO-Mag2, resulting in a composition up to 20 fg Fe/VP and kept at room temperature for 20 minutes to allow complex assembly. The characteristics of the complexes are summarized in supplemental Table 3. For reducing the volume to 400 μL, supernatant was removed after magnetic sedimentation of the complexes using an Nd-Fe-B permanent magnet. The complexes were resuspended by gentle vortexing and were then applied to an LS column. Subsequently, hUC-MSCs or hCB-CD34+ cells were loaded and transduced at an MOI of 1 to 10.
For lentiviral magselectofection of mouse bone marrow (BM) Sca-1+ cells, virus vector was mixed with SO-Mag2, resulting in a composition of 0.02 up to 20 fg Fe/VP. Then, 2 × 106 enriched cells were loaded on vector modified MS columns and transduced at an MOI of 3.
When carrying out magselectofection with different numbers of target cells or at different MOIs or at different MNP/VP ratios, the relative volumes of ingredients were adjusted proportionally. In all cases, the final volume of the complexes was adjusted to 400 μL for loading on LS columns.
Magnetic labeling of the cells before magselectofection
Magnetic labeling of Jurkat T cells, K562, hUC-MSCs, hCB-CD34+ cells, mouse Sca-1+ cells with anti-CD45 and CD2, CD33, CD105, CD34, or Sca-1+ MicroBeads (Miltenyi Biotec), respectively, was performed according to the protocol of the manufacturer (supplemental Data).
Magselectofection general protocol
When adopting the 2-column cell separation protocol for achieving increased purity of target cells, magnetically labeled cells or cell mixtures were first passed through an unmodified LS column according to the instructions of the manufacturer. This was applied for mixtures of Jurkat T and K562 cells or on isolation of the CD34+ HSCs from the cord blood mononuclear cell (CBMC) Ficoll gradient fraction from the UC blood, and similarly for Sca-1+ mouse cells isolated from BM, with the exception that these cells were loaded onto MS columns. After the first positive selection, the cells were applied to vector-modified columns.
MicroBead-labeled target cells or preselected cell mixtures, suspended in 2 mL complete culture medium, were loaded and allowed to infiltrate into the column positioned within a MidiMACS Separator magnet. The columns were washed with 3 × 3 mL of cell culture medium after cell loading to collect nontarget cells. The columns were then left positioned in the MidiMACS magnet for 30 minutes at room temperature. Subsequently, the target cells were flushed from the columns with 2 mL cell culture medium into 15 mL test tubes by pressure-enforced elution, according to the manufacturer's standard protocol. Nontarget and target cells were then cultivated in standard cell culture dishes at 37°C in a humidified atmosphere containing 5% CO2 until evaluation of cell separation and/or gene transfer efficiency. Reporter gene expression analysis was carried out 48 hours after magselectofection with the nonviral and adenoviral vectors and 72 hours after infection with the lentiviral vectors by fluorescence-activated cell sorter (FACS) analysis (eGFP) or luciferase assay.
Magnetic cell separation and gene transfer efficiency on nonviral and lentiviral magselectofection with Jurkat/K562 cell mixtures
Before the experiment, the CD2+/CD3+/CD33− status of the Jurkat T cells and the CD2−/CD3−/CD33+ status of the K562 cells were confirmed by FACS analysis (supplemental Figure 4). The purity of the selected cell population after separation with 2 unmodified LS columns was tested against separation using an unmodified column and a vector-modified column sequentially. Vector-modified columns were prepared as described in “Modification of Miltenyi LS cell separation columns with magnetic transfection/transduction complexes” with complexes comprising 20 μg plasmid DNA in magnetic DF-Gold formulation (DNA/DF-Gold/MNP = 1:4:1; weight/volume/Fe weight) or SO-Mag2/LV.eGFP complexes up to 20 fg Fe/VP at various MOIs with respect to the target cell (Jurkat or K562) numbers. Jurkat T cells were labeled with CD2 MicroBeads (Miltenyi Biotec) according to the instructions of the manufacturer and were mixed 1:1 (cell/cell) with unlabeled K562 cells in RPMI. For the nonviral experiment, 2 portions composed of 2.5 × 106 cells of each species were subjected to a first round of selection on unmodified LS columns. Retained cells were eluted by pressure enforced elution with 2 mL medium. Then, one portion was passed through a second unmodified column, whereas the other portion was applied to a vector modified column as described in “Magselectofection general protocol.” For the lentiviral experiment with Jurkat T target cells, cell mixtures composed of 5 × 105 cells of each species were applied in the same manner. Aliquots of the positively selected cells were treated with an anti-CD3-phycoerythrin (PE) antibody (AbD Serotec) immediately after magselectofection, and the purity of the CD2+/CD3+ cell population (Jurkat T cells) was determined by FACS analysis. For the lentiviral experiment with K562 target cells, the cell mixture composed of 9 × 106 Jurkat cells and 1 × 106 K562 cells was labeled with CD2 MicroBeads and applied to unmodified LS columns to deplete Jurkat cells. The cells in the effluent were labeled with CD33 MicroBeads (Miltenyi Biotec) and applied to a vector modified column as described in “Magselectofection general protocol.” The selected and nontarget cells were cultivated until evaluation of reporter gene expression by FACS analysis or luciferase assay at different time points.
Human cord blood and mice
Human cord blood was obtained on the receipt of informed consent of the mother in accordance with legislation in The Netherlands and in Poland. All mice were bred in the Experimental Animal Center of Erasmus MC, The Netherlands. Il2rg−/− single KO mice with a targeted γc deletion were derived from a 10th generation backcross of BALB/c Rag2−/−/γc−/− mice, kindly provided by Dr H. Spits33 with syngenic BALB/c wild-type mice. All mice were used at 6 to 10 weeks of age and were maintained in specified pathogen-free conditions. All animal experiments were approved by the institutional Animal Ethical Committee of Erasmus MC in accordance with legislation in The Netherlands.
Lentiviral magselectofection versus standard infection of human cord blood CD34+ cells at low cell density (1.5 × 105 cells/mL)
Ficoll gradient freshly isolated CBMCs (as described in the online supplemental Data) were pooled with thawed CBMCs in phosphate-buffered saline solution containing 2mM ethylenediaminetetraacetic acid, plus 400 μL of FcR Blocking Reagent (to avoid unspecific labeling of the cells via Fc receptors) plus 400 μL CD34+ MicroBeads to label the CD34+ hCB cells magnetically. Subsequently, the cells were incubated at 4°C for 30 minutes, washed with phosphate-buffered saline buffer containing 2mM ethylenediaminetetraacetic acid, centrifuged at 300g for 10 minutes, and resuspended in 1 mL of serum-free modified Dulbecco modified Eagle medium as described.34,35 hCB-CD34+ cells in the resulting CBMC mixture were positively selected by 2 consecutive rounds of magnetic cell separation on unmodified LS columns. Retained hCB-CD34+ cells were eluted with 2 mL of serum-free Dulbecco modified Eagle medium without growth factors after the first round and 3 mL of medium supplemented with 50 ng/mL FMS-like tyrosine kinase 3, 100 ng/mL thrombopoietin, and 100 ng/mL stem cell factor in the second round.
For magselectofection, LS columns were loaded with SO-Mag2/LV.eGFP magnetic complexes prepared by mixing LV.eGFP vector (1.5 × 106 TU) with SO-Mag2 stock followed by dilution to 400 μL with RPMI medium (without additives) to result in magnetic particle/VP ratios of 2 and 20 fg Fe/VP, respectively. Preselected hCB-CD34+ cells were loaded at 150 000 cells per column positioned in the MidiMACS magnet, incubated for 30 minutes, followed by pressure-enforced elution with growth factor supplemented medium. The samples were cultivated for 2 days in a 24-well plate until analyzing eGFP expression by FACS.
For comparison, 150 000 positively selected cells were treated in the same manner on an unmodified LS column but were infected with virus vector at the same MOI 10 in a 24-well plate (standard infection with 8 μg/mL polybrene) without virus/magnetic complexes, and cultivated for 2 days until FACS analysis.
Colony-forming cell assay
In vitro colony-forming cell assays were performed as described35,36 and initiated 12 hours after magselectofection of hCB-CD34+ cells or mouse BM cells. Fourteen days after magselectofection, erythroid (burst-forming units-erythroid [BFU-E]) and granulocytic (colony-forming units granulocyte-macrophage [CFU-GM]) colonies were counted.
Transduction and transplantation of lineage-negative Sca-1+ BM cells in mice
BM cells from male congenic wild-type mice were purified by lineage depletion (Lin−; BD Biosciences) and subsequently further enriched for Sca-1+. Lin−Sca-1+ BM cells were transduced with LV-PGK-eGFP overnight at MOI 3 with or without magselectofection at 106 cells/mL in serum-free modified Dulbecco medium with supplements37 in the presence 100 ng/mL of murine stem cell factor, 50 ng/mL human FMS-like tyrosine kinase 3-ligand, and 10 ng/mL murine thrombopoietin. Subsequently, 30 000 Lin−Sca-1+ were injected into the tail vein of 6-Gy irradiated female Il2rg−/− recipients.
Immunophenotyping by flow cytometry
Flow cytometric analyses were performed on cells obtained from blood. Peripheral blood was collected monthly in ethylenediaminetetraacetic acid tubes by retro-orbital puncture under isoflurane anesthesia. Complete blood cell counts were measured using a Vet ABC hematology analyzer (scil animal care company GmbH). Blood was lysed, and leukocytes were washed 3 times with Hanks balanced salt solution (Invitrogen) containing 0.5% (weight/volume) bovine serum albumin and 0.05% (weight/volume) sodium azide. Cells were incubated for 30 minutes at 4°C in Hanks balanced salt solution (Invitrogen) containing 0.5% (weight/volume) bovine serum albumin and 0.05% (weight/volume) sodium azide containing 2% heat-inactivated normal mouse serum and antibodies against CD3, CD4, CD8, B220, IgM, IgD, CD11b, Gr-1 directly conjugated to R-PE, peridinin chlorophyll protein, or allophycocyanin (all antibodies, BD Biosciences). Subsequently, cells were washed and measured on a FACSCalibur (BD Biosciences). In addition, GFP expression was measured in mice treated with the LV-PGK-eGFP vectors.
Upscaling: magselectofection of hUC-MSCs using CliniMACS
A prototype CliniMACS tubing set was manufactured allowing manual application of magnetic SO-Mag2/LV.eGFP complexes to the CliniMACS separation column by 3-way taps at each end of the column. A total of 107 hUC-MSCs were labeled with CD105 MicroBeads, resuspended in 10 mL of CliniMACS buffer containing 2% human AB serum, and placed in a sample application bag. This was connected to the prototype CliniMACS tubing set, and the cells were separated in the CliniMACS device using the CD34 Selection Program. During this standard cell processing procedure, the cells are loaded and eluted from the column 3 times. Before the third application of cells to the separation column, the program was paused and 8-mL lentiviral magnetic SO-Mag2/LV.eGFP complexes, formulated at 20 fg Fe/VP and containing 107 infectious particles, were added to the nonmagnetized column by way of the 3-way taps. Flow-through was collected in a waste bag. After flushing an additional 8 mL buffer through the tubing, the 3-way taps were closed, the program was resumed, and the cells were reloaded onto the column. At this point, the program was again paused and the cells were incubated with the complexes for 30 minutes at room temperature. The program was then resumed, and the cells were eluted and cultivated until FACS analysis for eGFP+ cells.
Additional methods
Acquisition and manipulation of images given in Figures 5A and 6B, C, E and F was performed using an Axiovert 135 Microscope equipped with an AxcioCamMRcRev.2 camera and Axiovision Re. 4.5 software from Carl Zeiss Imaging Solutions GmbH. Data on type, magnification, and numerical aperture of the objective lens used are provided in corresponding figure legends. Figure 5c was captured on an inverted Leica DM IRB microscope, using a 5×/0.12 objective, a Photometrics SenSys camera, and Leica QWin V3 acquisition software. All cell images were acquired in cell culture plates under cell culture medium. In the supplemental Data, we provide a detailed description of the materials, magnetic nanoparticles and magnetic vectors, lentivirus vector association, and magnetic sedimentation in complexes with the magnetic nanoparticles, packaging of the lentivirus vector LV.eGFP, titer determination, construction and purification of the recombinant adenoviral vector AdmCMVeGFPLuc, cell isolation and culture, adenoviral magselectofection as well as adenoviral, lentiviral, and nonviral magnetofection and standard transfection/transduction of hUC-MSC, evaluation of the reporter gene expression, and the osteogenesis assay.
Results
Vectors need to be associated with magnetic nanoparticles in a quantitative manner to be retained on magnetic cell separation columns (supplemental Figure 1). Parameters to be optimized for magselectofection are the type and ratio of magnetic nanoparticles to DNA or virus particle, the vector dose on the magnetic cell separation column, the vector dose per target cell, and the sequence of loading vectors and cells to magnetic separation columns. Optimized viral and nonviral magnetic vector compositions were selected in 2D magnetofection format.20,38 Some physical characteristics of vectors and magnetic nanoparticles29,39 used in this study as well as results from optimization procedures are presented as supplemental Data.
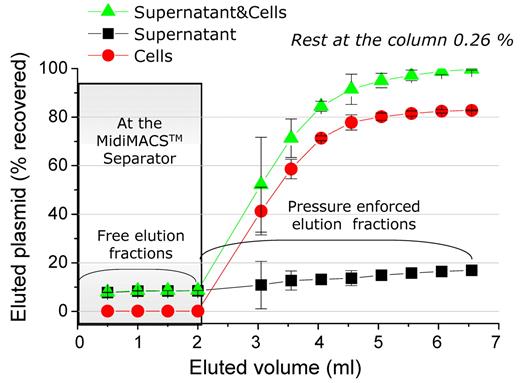
Briefly, we used Jurkat T and K562 as model cell lines to establish magselectofection. The protocol, which was finally adopted, is composed of the following steps: The target cells in a mixed cell population were enriched by a first round of selection on an unmodified separation column followed by “polishing” and gene transfer on a vector-modified column. This yielded the highest target cell purity. In addition, manufacturers propose 2 rounds of selection for maximizing target cell purity. The vector-modified column was prepared by loading magnetic vectors suspended in 400 μL or 60 μL of medium, respectively, which corresponds to the dead volumes of Miltenyi Biotec's LS and MS Cell Separation Columns, respectively. Then, magnetically labeled cells are loaded while the column is positioned within the MidiMACS Separator magnet. This gave consistently better transfection/transduction results than when loading the cells first (supplemental Figure 3D). An incubation of the magnetically labeled cells for 30 to 60 minutes in the modified column was sufficient. A longer incubation time of up to 90 minutes resulted in a more than 2-fold decrease in reporter gene expression (supplemental Figure 3C). With CD45 MicroBead-labeled Jurkat T cells, used when establishing the procedure, there was complete vector retention during cell loading and washing steps. A total of 99% of the applied nonviral vector dose and 100% of the applied cells were recovered on pressure-enforced elution from the column. Eighty percent of the vector dose was associated with the Jurkat T cells (Figure 2).
Magnetic transfection complexes are effectively immobilized within an LS cell separation column and are associated with cells after the magselectofection procedure. The magnetic transfection complex PEI-Mag2/DF-Gold/125I-pBLuc containing 20 μg of plasmid labeled with the 125I isotope was loaded onto the column. The column was placed into the MidiMACSTM Separator. Jurkat T cells that had been magnetically labeled with CD45 MicroBeads were loaded onto the column in 4 portions of 0.5 mL RPMI media each, and free elution fractions were collected. After 30 minutes of incubation, the column was removed from the separator, and the cells that had been retained were flushed out by firmly applying the plunger supplied with the column using 8 portions of 0.5 mL RPMI media each (Pressure enforced elution fractions). The fractions were centrifuged, and the radioactivity was measured in the supernatants and pellets (Cells) to recalculate the DNA fraction associated with the cells, unbound DNA in supernatant and the total DNA recovered from the column (Supernatant&Cells).
Magnetic transfection complexes are effectively immobilized within an LS cell separation column and are associated with cells after the magselectofection procedure. The magnetic transfection complex PEI-Mag2/DF-Gold/125I-pBLuc containing 20 μg of plasmid labeled with the 125I isotope was loaded onto the column. The column was placed into the MidiMACSTM Separator. Jurkat T cells that had been magnetically labeled with CD45 MicroBeads were loaded onto the column in 4 portions of 0.5 mL RPMI media each, and free elution fractions were collected. After 30 minutes of incubation, the column was removed from the separator, and the cells that had been retained were flushed out by firmly applying the plunger supplied with the column using 8 portions of 0.5 mL RPMI media each (Pressure enforced elution fractions). The fractions were centrifuged, and the radioactivity was measured in the supernatants and pellets (Cells) to recalculate the DNA fraction associated with the cells, unbound DNA in supernatant and the total DNA recovered from the column (Supernatant&Cells).
The 2-column cell separation/transfection procedure yields specific gene delivery to the target cell population without compromising the cell separation efficiency
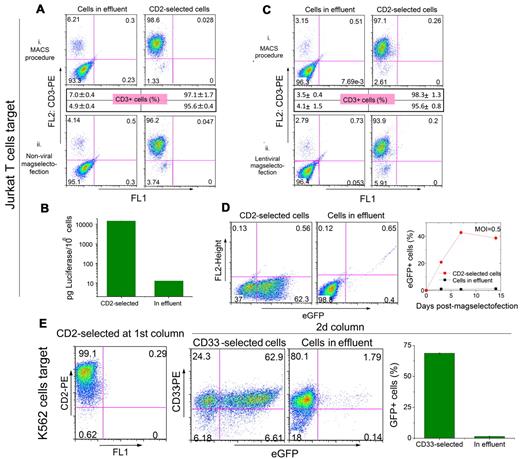
This was demonstrated with nonviral and lentiviral magselectofection in Jurkat/K562 cell mixtures composed of either CD2-MicroBead-labeled Jurkat or CD33-MicroBead-labeled K562 cells as the target cell population. Preliminary experiments confirmed the CD2+/CD3+/CD33− status of the Jurkat and CD2−/CD3−/CD33+ status of the K562 cells, respectively, as well as the similar transducibility of the cell lines by lentiviral magselectofection (supplemental Figure 4). Passing cell mixtures, preselected for the Jurkat target cells on an unmodified LS column, through vector-modified columns (magnetic lipoplexes SO-Mag2/DF-Gold/pDNA or magnetic lentiviral complexes SO-Mag2/LV.eGFP) yielded high target cell purity, cell recovery, and transfection/transduction efficiency specific for the target cell population (Figure 3). Having CD2-MicroBead-labeled Jurkat cells as the target cell population yielded 25.5% ± 3.9% eGFP+ with nonviral magselectofection. When carrying out the experiment with the luciferase reporter gene, 3 orders of magnitude higher transgene expression was observed in the selected cells than in the effluent (Figure 3B). The selected cells were 95.6% ± 0.4% CD3+ (ie predominantly Jurkat T cells). The control experiment with 2 unmodified columns yielded 97.1% ± 1.7% CD3+ cells. In both cases, the recovery of the cells was close to 100%. The lentiviral experiment delivered up to 60.8% ± 3.3% eGFP+ Jurkat cells at MOI of 2 with 95.6% ± 0.8% target cell purity. Having CD33-MicroBead-labeled K562 cells as the target cell population yielded 68.7% ± 0.3% eGFP+ cells with lentiviral magselectofection, of which 90.5% were CD33+ according to FACS analysis (Figure 3E). Also in this case, the target cell recovery was approximately 100%. The transduction efficiency in the effluent nontarget cell population was at most 1.2% ± 0.7% and 1.5% ± 0.3% for having Jurkat and K562 cells as the target, respectively (Figure 3D-E).
Cell separation efficiency and specific transfection/transduction of the target cells using nonviral and viral magselectofection Jurkat T cells as a target. (A) A mixture of 2.5 × 106 Jurkat T cells and 2.5 × 106 K562 cells was treated with CD2 MicroBeads and passed (i) sequentially through 2 LS columns (MACS procedure) or (ii) through one LS column, followed by magselectofection at the second LS column modified with PEI-Mag2/DF-Gold/pBLuc magnetic lipoplexes composed of 20 μg plasmid DNA (DNA/DF-Gold/MNP = 1:4:1; weight/volume/Fe weight). The CD2-cell fraction in the effluent (K562 cells) and the CD2+ cells positively selected in the column (Jurkat T cells) were treated with a CD3-PE antibody and analyzed for the percentage of CD3-PE+ cells using FACS analysis. Dot plots show the data for single measurement and the percentage of CD3+ cells (mean ± SD from triplicates in the inset boxes). (B) Luciferase expression in the effluent (CD3−/CD2− cells) and in the cell fraction that was magnetically selected with CD2 beads (CD3+/CD2+ cells). (C) A mixture of 0.5 × 106 Jurkat T cells and 0.5 × 106 K562 cells was treated with CD2 MicroBeads and passed (i) sequentially through 2 LS columns (MACS procedure) and (ii) through one LS column, followed by magselectofection in the second LS column modified with SO-Mag2/LV.eGFP magnetic lentivirus complexes (0.5 × 106 TU/column, 20 fg Fe/VP). The CD2− cell fraction in the effluent (K562 cells) and the CD2+ cells positively selected within the column (Jurkat T cells) were treated with a CD3-PE antibody and analyzed for the percentage of CD3-PE+ cells using FACS analysis. Dot plots show the data 31 for single measurement, and the percentage of CD3+ cells is given as mean plus or minus SD from triplicates in the inset boxes. (D) The cell fractions in the effluent and the CD2− selected fraction after the second column loaded with SO-Mag2/LV.eGFP magnetic lentivirus complexes formulated at 20 fg Fe/VP were analyzed for eGFP expression using FACS analysis. FACS data are shown for MOI of 2. The right graph shows the percentages of eGFP+ cells in the effluent and in the CD2-selected fraction at different time points after magselectofection using an MOI of 0.5. (E) K562 cells as a target. A mixture of 9 × 106 Jurkat T cells and 1 × 106 K562 was labeled with CD2 MicroBeads for depleting the Jurkat T cells on an unmodified LS column. The cells in the effluent were labeled with CD33 MicroBeads and applied to the second vector-loaded LS column (SO-Mag2/LV.eGFP magnetic lentivirus complexes, 2 × 106 TU/column, formulated at 10 fg Fe/VP). The CD2-selected cells from the first column were analyzed for CD2 expression and the CD33-selected and effluent fractions from the second column were analyzed for eGFP expression using FACS analysis. All experiments were carried out in triplicate.
Cell separation efficiency and specific transfection/transduction of the target cells using nonviral and viral magselectofection Jurkat T cells as a target. (A) A mixture of 2.5 × 106 Jurkat T cells and 2.5 × 106 K562 cells was treated with CD2 MicroBeads and passed (i) sequentially through 2 LS columns (MACS procedure) or (ii) through one LS column, followed by magselectofection at the second LS column modified with PEI-Mag2/DF-Gold/pBLuc magnetic lipoplexes composed of 20 μg plasmid DNA (DNA/DF-Gold/MNP = 1:4:1; weight/volume/Fe weight). The CD2-cell fraction in the effluent (K562 cells) and the CD2+ cells positively selected in the column (Jurkat T cells) were treated with a CD3-PE antibody and analyzed for the percentage of CD3-PE+ cells using FACS analysis. Dot plots show the data for single measurement and the percentage of CD3+ cells (mean ± SD from triplicates in the inset boxes). (B) Luciferase expression in the effluent (CD3−/CD2− cells) and in the cell fraction that was magnetically selected with CD2 beads (CD3+/CD2+ cells). (C) A mixture of 0.5 × 106 Jurkat T cells and 0.5 × 106 K562 cells was treated with CD2 MicroBeads and passed (i) sequentially through 2 LS columns (MACS procedure) and (ii) through one LS column, followed by magselectofection in the second LS column modified with SO-Mag2/LV.eGFP magnetic lentivirus complexes (0.5 × 106 TU/column, 20 fg Fe/VP). The CD2− cell fraction in the effluent (K562 cells) and the CD2+ cells positively selected within the column (Jurkat T cells) were treated with a CD3-PE antibody and analyzed for the percentage of CD3-PE+ cells using FACS analysis. Dot plots show the data 31 for single measurement, and the percentage of CD3+ cells is given as mean plus or minus SD from triplicates in the inset boxes. (D) The cell fractions in the effluent and the CD2− selected fraction after the second column loaded with SO-Mag2/LV.eGFP magnetic lentivirus complexes formulated at 20 fg Fe/VP were analyzed for eGFP expression using FACS analysis. FACS data are shown for MOI of 2. The right graph shows the percentages of eGFP+ cells in the effluent and in the CD2-selected fraction at different time points after magselectofection using an MOI of 0.5. (E) K562 cells as a target. A mixture of 9 × 106 Jurkat T cells and 1 × 106 K562 was labeled with CD2 MicroBeads for depleting the Jurkat T cells on an unmodified LS column. The cells in the effluent were labeled with CD33 MicroBeads and applied to the second vector-loaded LS column (SO-Mag2/LV.eGFP magnetic lentivirus complexes, 2 × 106 TU/column, formulated at 10 fg Fe/VP). The CD2-selected cells from the first column were analyzed for CD2 expression and the CD33-selected and effluent fractions from the second column were analyzed for eGFP expression using FACS analysis. All experiments were carried out in triplicate.
Lentiviral magselectofection yields mouse Lin−Sca1+ cells, which persistently reconstitute T and B cells in Il2rg−/− mice
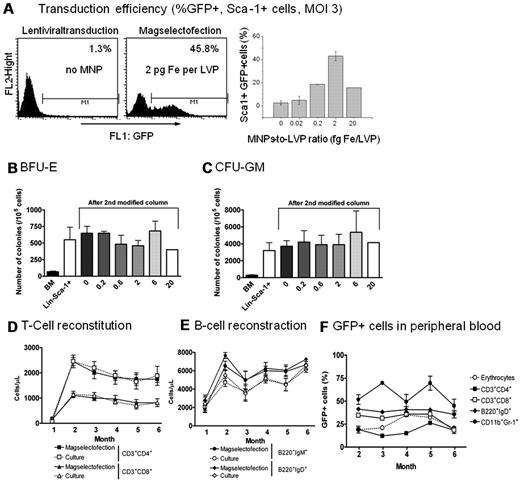
Lentiviral magselectofection with 2 fg Fe/VP combined with a routine magnetic isolation procedure of Sca-1+ mouse cells from BM yielded 50% eGFP+ cells (Figure 4A) and more than 80% cell purity. The latter and cell recovery was equivalent to cell separation only. Using higher iron/VP ratios resulted in a reduction of cell purity (supplemental Figure 5B) and transduction efficiency (Figure 4A). Magselectofection did not affect the progenitors as quantified for BFU-E and CFU-GM (Figure 4B-C). When transplanted in Il2rg−/− mice, magselectofected Lin−Sca-1+ reconstituted T and B cells to similar levels in peripheral blood as control-treated Lin−Sca-1+ cells (Figure 4D-E). GFP expression in multiple lineages in the transplanted mice was stable and ranged from 25% to 50% (Figure 4F), which are significant levels for therapeutic purposes.
Transduction efficiency in Sca-1+ mouse BM cells after lentiviral magselectofection. Sca-1+ cells were enriched over LS columns first. Subsequently, up to 2 × 106 Sca-1+ cells were magselectofected within an MS column modified with SO-Mag2/LV-PGKeGFP complexes at different MNP-to-VP ratios, but a fixed MOI of 3. (A) Positively selected cells were analyzed by FACS. Cell purity on passage of a cell isolate through a first unmodified LS column followed by passage through second lentiviral magnetic vector-modified columns at constant virus dose with increasing Fe/VP ratios. Cell separation efficiency is only impaired at very high magnetic particle-to-virus ratios. (B) Quantification of mouse BFU-E colonies at day 14 after magselectofection. Total BM, Lin−Sca-1+ (first round selection), and second round selection with increasing ratios of Fe per LVP (0-20 fg Fe/LVP) are shown. (C) Quantification of mouse CFU-GM colonies at day 14 after magselectofection. Total BM, Lin−Sca-1+ (first round selection), and second round selection with increasing ratios of Fe per LVP (0–20 fg Fe/LVP) are shown. (D) Reconstitution of T cells in blood of Il2rg−/− mice (N = 4-8) transplanted with 30 000 overnight cultured or magselectofected wild-type Lin−Sca-1+ cells. (E) Reconstitution of B cells in blood of Il2rg−/− mice (N = 4-8) transplanted with 30 000 overnight cultured or magselectofected wild-type Lin-Sca-1+ cells. (F) GFP percentage in peripheral blood of Il2rg−/− mice (N = 4) transplanted with magselectofected wild-type Lin−Sca1+ cells. Long-term GFP expression was detected in erythrocytes, T cells (CD3+CD4+ and CD3+CD8+), B cells (B220+IgD+), and myeloid cells (CD11b+Gr-1+).
Transduction efficiency in Sca-1+ mouse BM cells after lentiviral magselectofection. Sca-1+ cells were enriched over LS columns first. Subsequently, up to 2 × 106 Sca-1+ cells were magselectofected within an MS column modified with SO-Mag2/LV-PGKeGFP complexes at different MNP-to-VP ratios, but a fixed MOI of 3. (A) Positively selected cells were analyzed by FACS. Cell purity on passage of a cell isolate through a first unmodified LS column followed by passage through second lentiviral magnetic vector-modified columns at constant virus dose with increasing Fe/VP ratios. Cell separation efficiency is only impaired at very high magnetic particle-to-virus ratios. (B) Quantification of mouse BFU-E colonies at day 14 after magselectofection. Total BM, Lin−Sca-1+ (first round selection), and second round selection with increasing ratios of Fe per LVP (0-20 fg Fe/LVP) are shown. (C) Quantification of mouse CFU-GM colonies at day 14 after magselectofection. Total BM, Lin−Sca-1+ (first round selection), and second round selection with increasing ratios of Fe per LVP (0–20 fg Fe/LVP) are shown. (D) Reconstitution of T cells in blood of Il2rg−/− mice (N = 4-8) transplanted with 30 000 overnight cultured or magselectofected wild-type Lin−Sca-1+ cells. (E) Reconstitution of B cells in blood of Il2rg−/− mice (N = 4-8) transplanted with 30 000 overnight cultured or magselectofected wild-type Lin-Sca-1+ cells. (F) GFP percentage in peripheral blood of Il2rg−/− mice (N = 4) transplanted with magselectofected wild-type Lin−Sca1+ cells. Long-term GFP expression was detected in erythrocytes, T cells (CD3+CD4+ and CD3+CD8+), B cells (B220+IgD+), and myeloid cells (CD11b+Gr-1+).
The data clearly demonstrate that genetic modification occurs predominantly in the target cell population when using magselectofection, and the procedure is compatible with the biologic function of hematopoietic stem cells.
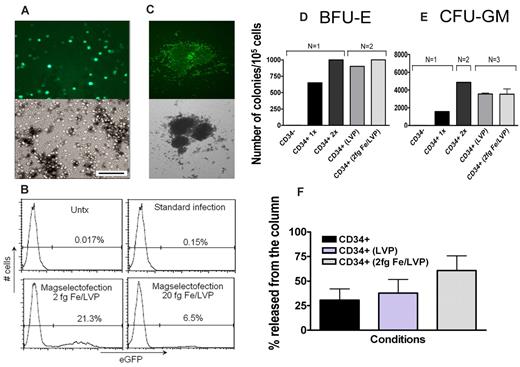
Efficient gene delivery to human cord blood CD34+ cells (hCB-CD34+) was achieved using lentiviral magselectofection, and the progenitor status of the transduced cells was not affected. The target cells enriched using CD34 MicroBeads on unmodified LS columns were passaged through lentiviral vector-modified columns without prior stimulation with growth factors. A standard infection of the cells was performed as a reference procedure. Forty-eight hours after transduction, approximately 21% and 6.5% of hCB-CD34+ cells were eGFP+ when magselectofected with complexes formulated at 2 and 20 fg Fe/VP, respectively (Figure 5A-B). No eGFP+ hCB-CD34+ cells were found after standard infection carried out at the low cell density of 1.5 × 105 cells/mL (Figure 5B). Results from colony-forming assays of day 14 after magselectofection show that the colonies were GFP+ (Figure 5C) and differentiated into multiple lineages (CFU-GM and BFU-E; Figure 5D-E). Magselectofection did not affect the release of cells from the column (Figure 5F) or the colony number (Figure 5D-E). Thus, essential biologic characteristics of progenitor cells are maintained on magselectofection.
hCB-CD34+ cells are transduced with remarkably high efficiency and maintain their progenitor cell phenotype after lentiviral magselectofection. hCB-CD34+ cells were transduced at a low cell density of 1.5 × 105 cells/mL without cell stimulation before magselectofection. (A) Fluorescence (490/509 nm) and bright-field microscopy images of the hCB-CD34+ cells taken on day 3 after magselectofection with the magnetic complexes at 2 fg Fe/VP. Bar represents 100 μm. Objective Achroplan 20×/0.4 NA. (B) Histogram plots of the untreated hCB-CD34+ cells (Untx), cells transduced using the standard infection protocol or viral magselectofection with the complexes formulated at 2 or 20 fg Fe/VP. (C) GFP+ BFU-E colony at 14 days after magselectofection. Objective 5×/0.12. (D-E) Quantification of BFU-E and CFU-GM colonies at 14 days after magselectofection (N = 1-3). (F) Percentage of cells released from the column (N = 3-5) for CD34+ cells alone, with addition of lentiviral vector (LVP) and magselectofection (2 fg Fe/LVP).
hCB-CD34+ cells are transduced with remarkably high efficiency and maintain their progenitor cell phenotype after lentiviral magselectofection. hCB-CD34+ cells were transduced at a low cell density of 1.5 × 105 cells/mL without cell stimulation before magselectofection. (A) Fluorescence (490/509 nm) and bright-field microscopy images of the hCB-CD34+ cells taken on day 3 after magselectofection with the magnetic complexes at 2 fg Fe/VP. Bar represents 100 μm. Objective Achroplan 20×/0.4 NA. (B) Histogram plots of the untreated hCB-CD34+ cells (Untx), cells transduced using the standard infection protocol or viral magselectofection with the complexes formulated at 2 or 20 fg Fe/VP. (C) GFP+ BFU-E colony at 14 days after magselectofection. Objective 5×/0.12. (D-E) Quantification of BFU-E and CFU-GM colonies at 14 days after magselectofection (N = 1-3). (F) Percentage of cells released from the column (N = 3-5) for CD34+ cells alone, with addition of lentiviral vector (LVP) and magselectofection (2 fg Fe/LVP).
Gene delivery to hUC-MSCs using nonviral and lentiviral magselectofection is efficient, and the differentiation potential of the cells is maintained
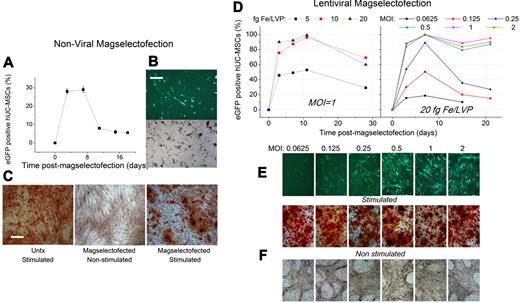
Nonviral magselectofection of hUC-MSC yielded approximately 30% eGFP+ cells 3 to 7 days after transfection (Figure 6A-B) followed by a decrease in the percentage of the eGFP+ cells to 9% and 6% at days 11 and 18, respectively. The cells retained their potential to differentiate into osteoblasts when cultured in osteogenic differentiation medium (Figure 6C). The differentiation capacity was comparable with stimulated untreated cells (Figure 6c). This applies also for hUC-MSCs transduced by lentiviral magselectofection (Figure 6F). In this case, 100% transduction efficiency at MOIs as low as 0.5 TU per cell was achieved (Figure 6D). Maximum transduction efficiency was observed with complexes formulated at 10 and 20 fg Fe/VP, and reporter gene expression was persistent during 21 days after magselectofection (Figure 6D).
hUC-MSCs maintain their differentiation potential and efficiently express the eGFP reporter gene after nonviral and viral magselectofection. For nonviral magselectofection, we labeled 2.5 × 106 hUC-MSCs using CD105 MicroBeads and magselectofected them with the magnetic lipoplexes SO-Mag2/DFGold/eGFP. Two days after magselectofection, the cells were stimulated using an osteogenic medium; and 18 days after stimulation, the cells were analyzed using alizarin red staining. (A) FACS data relating to the percentage of the eGFP+ cells at different time points after magselectofection. (B) Bright-field and fluorescence (490/509 nm) microscopy images of hUC-MSCs 7 days after magselectofection. Bar represents 500 μm. Objective Achroplan 4×/0.10 NA. (C) Microscopy images of the untreated (Untx) stimulated hUC-MSCs and the nonstimulated and stimulated hUC-MSCs 20 days after magselectofection. Bar represents 200 μm. Objective Achroplan 10×/0.25 NA Phl. For viral magselectofection, we labeled 106 hUC-MSCs using CD105 MicroBeads and magselectofected them with the lentiviral magnetic complexes SOMag2/LV.eGFP. (D) FACS data relating to the percentage of eGFP+ hUC-MSCs versus the time after transduction (left graph) at different iron/lentivirus particle ratios in terms of fg Fe/VP with an MOI of 1 and (right graph) at a fixed Fe/VP ratio of 20 fg Fe/VP at different MOIs. (E) Fluorescence microscopy (490/509 nm) images of the hUC-MSCs 7 days after magselectofection with different MOIs at 20 fg Fe/VP. Objective Achroplan 10×/0.25 NA Phl. (F) Two days after magselectofection, the cells were stimulated using an osteogenic medium; and 18 days after stimulation, the cells were analyzed using alizarin red staining. Bright-field microscopy images of the magselectofected stimulated (differentiated) and nonstimulated hUC-MSCs 20 days after magselectofection with different MOIs at 20 fg Fe/VP. Objective Achroplan 4×/0.10 NA.
hUC-MSCs maintain their differentiation potential and efficiently express the eGFP reporter gene after nonviral and viral magselectofection. For nonviral magselectofection, we labeled 2.5 × 106 hUC-MSCs using CD105 MicroBeads and magselectofected them with the magnetic lipoplexes SO-Mag2/DFGold/eGFP. Two days after magselectofection, the cells were stimulated using an osteogenic medium; and 18 days after stimulation, the cells were analyzed using alizarin red staining. (A) FACS data relating to the percentage of the eGFP+ cells at different time points after magselectofection. (B) Bright-field and fluorescence (490/509 nm) microscopy images of hUC-MSCs 7 days after magselectofection. Bar represents 500 μm. Objective Achroplan 4×/0.10 NA. (C) Microscopy images of the untreated (Untx) stimulated hUC-MSCs and the nonstimulated and stimulated hUC-MSCs 20 days after magselectofection. Bar represents 200 μm. Objective Achroplan 10×/0.25 NA Phl. For viral magselectofection, we labeled 106 hUC-MSCs using CD105 MicroBeads and magselectofected them with the lentiviral magnetic complexes SOMag2/LV.eGFP. (D) FACS data relating to the percentage of eGFP+ hUC-MSCs versus the time after transduction (left graph) at different iron/lentivirus particle ratios in terms of fg Fe/VP with an MOI of 1 and (right graph) at a fixed Fe/VP ratio of 20 fg Fe/VP at different MOIs. (E) Fluorescence microscopy (490/509 nm) images of the hUC-MSCs 7 days after magselectofection with different MOIs at 20 fg Fe/VP. Objective Achroplan 10×/0.25 NA Phl. (F) Two days after magselectofection, the cells were stimulated using an osteogenic medium; and 18 days after stimulation, the cells were analyzed using alizarin red staining. Bright-field microscopy images of the magselectofected stimulated (differentiated) and nonstimulated hUC-MSCs 20 days after magselectofection with different MOIs at 20 fg Fe/VP. Objective Achroplan 4×/0.10 NA.
Magselectofection of hUC-MSCs was also highly efficient using an adenoviral vector. At an MOI of 0.5 pfu/cell, the infection efficiency was improved by approximately 3- and 17-fold when using adenoviral vectors compared with magnetofection and standard infection, respectively (supplemental Figure 6).
Upscale of magselectofection to CliniMACS format is feasible
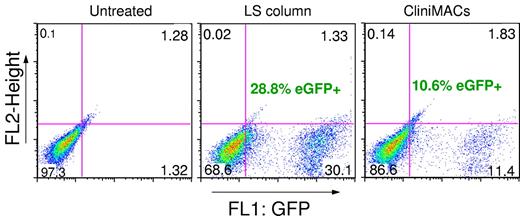
Lentiviral magselectofection of 107 hUC-MSCs was carried out with a semiautomated protocol on the CliniMACS instrument, which is approved for clinical use. This yielded 10.6% of eGFP+ cells 48 hours after transduction at an MOI opf 1 (Figure 7). A parallel experiment with the same cell number and MOI carried out on LS columns yielded 28.8% eGFP+ cells. This shows that upscaling is possible but requires further optimization.
Feasibility of magselectofection using CliniMACS. Percentage of the eGFP+ hUC-MSCs 48 hours after lentiviral magselectofection using LS columns or CliniMACS. hUC-MSCs were labeled with CD105 MicroBeads. LS columns were modified with the magnetic viral complexes formulated at nanoparticle/virus particle ratios of 20 fg Fe/VP (2.5 × 106 infectious particles per column) and 2.5 × 106 cells were applied to the column positioned in the MidiMACS magnet and incubated for 30 minutes at room temperature. For the CliniMACS separation, a prototype CliniMACS tubing set was used, allowing manual application of the same magnetic SOMag2/LV.eGFP complexes to the CliniMACS separation column by 3-way taps at each end of the column. A total of 107 cells were applied to a cell application bag, connected to this tubing set and separated using the standard CD34 selection process. After 2 cycles of selection, the procedure was paused and magnetic viral complexes composed of 107 infectious particles were added to the nonmagnetized column. After restarting the process and reloading the cells onto the modified column, the process was again interrupted for an incubation of 30 minutes at room temperature. The cells were then eluted from the LS columns manually and from the CliniMACS Tubing Sets by resuming the separation program.
Feasibility of magselectofection using CliniMACS. Percentage of the eGFP+ hUC-MSCs 48 hours after lentiviral magselectofection using LS columns or CliniMACS. hUC-MSCs were labeled with CD105 MicroBeads. LS columns were modified with the magnetic viral complexes formulated at nanoparticle/virus particle ratios of 20 fg Fe/VP (2.5 × 106 infectious particles per column) and 2.5 × 106 cells were applied to the column positioned in the MidiMACS magnet and incubated for 30 minutes at room temperature. For the CliniMACS separation, a prototype CliniMACS tubing set was used, allowing manual application of the same magnetic SOMag2/LV.eGFP complexes to the CliniMACS separation column by 3-way taps at each end of the column. A total of 107 cells were applied to a cell application bag, connected to this tubing set and separated using the standard CD34 selection process. After 2 cycles of selection, the procedure was paused and magnetic viral complexes composed of 107 infectious particles were added to the nonmagnetized column. After restarting the process and reloading the cells onto the modified column, the process was again interrupted for an incubation of 30 minutes at room temperature. The cells were then eluted from the LS columns manually and from the CliniMACS Tubing Sets by resuming the separation program.
Discussion
We have established a new method to separate and genetically modify target cell populations in one integrated procedure, and demonstrate that the new approach works efficiently for both viral and nonviral vectors, allowing a high transfection/transduction efficiency of cell lines and primary HSCs and MSCs from the umbilical cord. We show that the performance of cell sorting and cell recovery is not affected by magselectofection and that the function, viability, differentiation, and transplantation potential of the cells are not impaired.
The first essential steps for efficient magselectofection are the generation of nanomagnetic vector formulations and magnetically labeled cells. Lipoplexes with the cationic lipid transfection reagent Dreamfect-Gold and suitable MNPs at an iron/DNA (weight/weight) ratio of 1 yielded high transfection efficiency. For magnetic viral vectors, it is essential to formulate the compositions in terms of iron weight per physical virus particle, and not per infectious virus particle, taking into account that both infectious and noninfectious virus particles associate with appropriate MNPs. Therefore, once a suitable ratio for quantitative virus particle binding has been identified, this ratio can be applied to any other virus preparation, even without knowing its biologic titer. In our study, we have used in-house synthesized magnetic nanoparticles,20,29 but magselectofection can be carried out also with commercially available magnetofection reagents (supplemental Figure 1B).40,41 Similarly, magnetically labeled cells are obtained easily with magnetic nanoparticles that are coated with specific antibodies, which are commercially available for cell separation purposes.18,19 As an alternative, cells can also be nonspecifically labeled with magnetic nanoparticles as described previously42,43 (supplemental Figure 7).
Magselectofection turned out to be an extraordinarily simple procedure with quantitative and reversible magnetic vector retention on LS columns enabling more than 80% association of vectors with magnetically labeled target cells (Figure 2). The method performed excellently with respect to cell recovery, purity of target cells, viability, biologic functionality, transfection/transduction efficiency, and specificity for the target cells.
For obtaining maximum target cell purity, we adopted a 2-column protocol as is recommended for magnetic cell sorting. Cells are sorted on the first column and then brought in contact with transfection/transduction complexes within a second vector-modified column. The one-column procedure (vector-modified only) can be used when working with cell lines or already purified cells. In general, the cell separation efficiency was as high as when using the cell separation-only protocol of the manufacturer (Figure 3), as was observed not only for the Jurkat/K562 model mixture (Figures 3), but also for Sca-1+ mouse hematopoietic stem cells isolated from the BM of C57BL/6 mice (supplemental Figure 5). Importantly, the selectivity of the transfection/transduction process for the target cell population was excellent (Figure 3), and the target cell recovery was high.
Lentiviral magselectofection yielded favorable results with Sca-1+ mouse cells, an antigen that is commonly used for purification of murine pluripotent stem cells.44 Here, magselectofection with low MOIs (≤ 3) yielded up to 50% transduced cells (Figure 4) compared with only 9.5% with an MOI of 5 to 8 using a standard transduction protocol for Lin− BM cells as reported previously.45 Importantly, the magselectofected cells were fully competent in colony-forming assays (Figure 4B-C), and administration in mice also resulted in long-term stable reconstitution and long-term GFP expression (Figure 4D-F).
To demonstrate the utility of magselectofection with human primary cells, we chose CD34+ and mesenchymal cells from umbilical cord because these cells are relevant in ongoing and future clinical applications of genetically engineered cell therapies.9,14,15,46 Lentiviral magselectofection performed with hCB-CD34+ cells at low cell density resulted in 21% eGFP+ cells, whereas standard infection failed under these conditions (Figure 5). This is relevant because low cell number and density of hCB-CD34+ cells reflect the normal situation when working with clinical material. The progenitor ability of the cells was not impaired by the magselectofection procedure as demonstrated in colony-forming assays (Figure 5C-E).
Magselectofection proved highly efficient with hUC-MSCs. The nonviral procedure at a low vector dose of 8 pg plasmid/cell resulted in more than 85% metabolic activity (compared with untreated control) and yielded 29% eGFP-expressing cells 3 to 7 days after magselectofection (Figure 6A-B). This compares favorably with standard transfections as reported by Yang et al47 who obtained 27% transfected cells with 40 pg DNA/cell. Among the nonviral methods, only electroporation (nucleofection) was reported to be superior in terms of transfection efficiency, however, at the expense of cell viability.48 Hence, magselectofection and nucleofection yield approximately the same percentage (25%) of viable genetically modified hUC-MSCs. More importantly, magselectofection does not affect the differentiation potential of hUC-MSCs into the osteogenic lineage, as shown in Figure 6.
Under optimized transduction conditions, lentiviral magselectofection of hUC-MSCs at MOI as low as 0.5 TU/cell resulted in 60% to 100% transduced cells (Figure 6D) depending on the donor. Reporter gene expression was stably maintained during one month for most of the donors, whereas for some of the donors a gradual decrease in the transgene expression was observed (Figure 6D left graph). Significant interdonor variations in transduction efficiency and in the persistence of transgene expression for hUC-MSCs have previously been reported.49,50 A similarly high transduction efficiency as obtained with lentiviral magselectofection of hUC-MSCs was reported earlier only for infection at a high MOI of 20.51 The metabolic activity (compared with untreated control) on adenoviral or lentiviral magselectofection was greater than 85%. The apparently paradoxical result of achieving 100% transduction for hUC-MSCs at 0.5 MOI demonstrates clearly that infectivity is determined by the vector concentration, the internalization efficiency of the particles, and the susceptibility of different cell types to transduction. Magnetofection and magselectofection lead to rapid target cell contact of the major fraction of an applied vector dose23 (Figure 2) and also to improved vector uptake.29,38 With standard protocols, transfection/transduction kinetics and efficiency are dominated by diffusion.52 Hence, protocols that do not enforce vector-target cell contact will necessarily lead to an underestimation of biologic vector titers. We propose that magselectofection or magnetofection can be used as a tool to estimate the biologic virus titers more realistically.
Our findings suggest that magselectofection yields superior results than other transfection/transduction procedures and is broadly applicable with nonviral and viral vectors. Interestingly, adenoviral and lentiviral magselectofection outperformed the same procedure carried out in a 2-dimensional format (magnetofection; supplemental Figure 6). This may be the result of the stronger magnetic forces prevailing in a high gradient field magnetic separation column and also to the high concentration of “reactants” (vectors and cells) under magselectofection conditions. Hence, magselectofection is not only a promising integrated procedure for combined magnetic cell separation and genetic modification, but is a versatile and highly efficient tool for transfecting/transducing cell lines and primary monocell cultures. For example, labeling of hUC-MSCs with SO-Mag2 nanoparticles before magselectofection even resulted in a 2-fold increase in the percentage of transduced cells compared with magselectofection of the same cells labeled with CD105 MicroBeads (supplemental Figure 7). Cells manipulated in this manner can be used for a variety of purposes, such as magnetic positioning and magnetically enforced engraftment in target tissues, which is potentially useful in delivering cell-based therapies.41-43 Another important application is tracking of magnetically labeled cells by magnetic resonance imaging.42,53,54
In conclusion, magselectofection is a versatile integrated procedure for cell sorting and genetic modification. With a minimal number of ex vivo handling steps, and with low vector consumption, it yields high target cell purity and recovery with satisfactory cell viability and biologic functionality. The inherent transfection/transduction is equal or superior to other published methods. As magselectofection can be upscaled to an instrument for automated magnetic cell sorting, which is approved for clinical applications (Figure 7), we envisage that magselectofection can become an affordable and standardized tool for future cell therapies involving genetically engineered cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Ernst Wagner for valuable discussions and Edelburga Hammerschmid for the flow cytometry analysis.
This work was supported by the European Commission (6th Framework Programs, contracts LSHB-CT-2006-19038-Magselectofection and LSHB-CT-2004-005242-CONSERT, 7th FP Grant Agreements 222878-PERSIST and 261387-CELL-PID), The Netherlands Health Research and Development Organization ZonMw (Translational Gene Therapy Program, Projects 43100016 and 43400010), and the German Research Foundation, through DFG Research Unit FOR917 Nanoguide (Projects PL 281/3-1 and AN 333/1-1) and the Excellence Cluster Nanosystems Initiative Munich.
Authorship
Contribution: Y.S.-A., A.C., O.M., and C.P. performed the experiments on establishment and refinement of the method; N.P.v.T., J.H.d.J., M.W.H., and G.W. performed the experiments with Sca1+ cells; I.C.D.J. contributed expertise in magnetic cell separation and examined the sequence of vector/cell loading for magselectofection; O.M. and C.P. designed the magnetic nanoparticles; Z.P. supplied the hUC-MSCs; M.A. designed, produced, and characterized the AdvmCMVeGFPLuc vector, designed and constructed lentiviral vector LVSFeGFP, and advised in viral vector production methods; and all authors contributed to writing the manuscript.
Conflict-of-interest disclosure: I.C.D.J. is an employee of Miltenyi Biotec, Bergisch Gladbach, Germany. Miltenyi Biotec's proprietary magnetic cell separation technology was used in this study. C.P. is a cofounder of OZ Biosciences, SA, Marseille, France. OZ Biosciences commercializes Magnetofection technology. The remaining authors declare no competing financial interests.
The current affiliation of Y.S.-A. is Comprehensive Pneumology Center, Institute of Lung Biology and Disease, Helmholtz Zentrum München, German Research Center for Environmental Health, Neuherberg, Germany.
Correspondence: Christian Plank, Institute of Experimental Oncology and Therapy Research, Technische Universität München, Ismaninger Strasse 22, 81675 Munich, Germany; e-mail: plank@lrz.tum.de; and Gerard Wagemaker, Department of Hematology, Erasmus University Medical Center, Dr Molewaterplein 50, 3015 GE Rotterdam, The Netherlands; e-mail: g.wagemaker@erasmusmc.nl.
References
Author notes
Y.S.-A., O.M., and N.P.v.T. contributed equally to this study.