In this issue of Blood, Chernysh et al examine the pregelation phase of fibrin polymerization, and by demonstrating heterogeneous polymeric structures that progressively coalesce into scaffolds provide novel insights into this process with potential implications on circulating soluble fibrin.1
Initiated by thrombin cleavage of the 2 FpAs (see figure panel A), fibrin polymerization is mainly mediated by the 2 “A” knobs in the central region (E) that bind to complementary “a” pockets constitutively present on each outer (D) region of another molecule. The resulting, staggered 2-molecule-thick structure (see figure panel B) elongates by sequential binding of additional monomers to form the protofibril. Protofibrils self-associate laterally via “B”:“b” and αC:αC contacts.2 Using a rotating disc confocal microscope, Chernysh and colleagues captured images present at progressive time intervals during the lag phase that they defined by turbidity measurements. Each image was characterized by its diffusion coefficient and by transmission electron microscopy, illustrated in Figure 4 of their article.1 What is new is the monomer composition of mobile heterogeneous structures whose progressive growth leads to formation of scaffolds. In addition, the smallest and necessarily most mobile structures continued to attach to the increasingly less mobile and larger structures even at gelation point. Demonstration of growing scaffolds provides a novel view of soluble fibrin, perhaps more complex than previously proposed.3 The study by Chernysh and colleagues substantially advances our understanding of pregelation events, and of the likely composition of circulating fibrin/fibrinogen complexes.
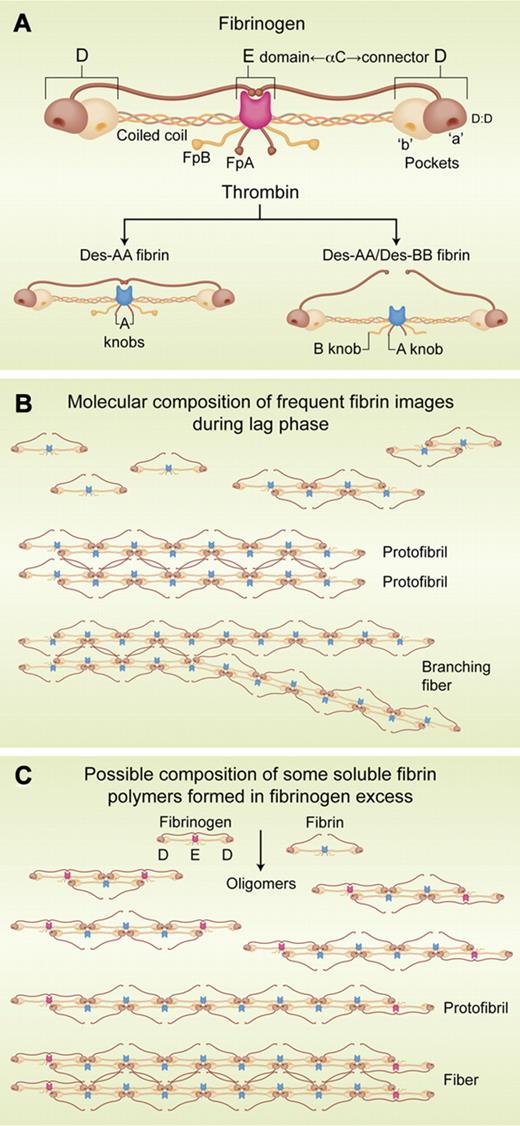
(A) Schemata of fibrinogen and fibrin molecules. D:D: structure on each D region that interfaces with its counterpart on another molecule. αC: carboxyl terminal region of the Aα chain. Coiled coil: interior parts of each chain connecting E and D regions. FpA and FpB: fibrinopeptides A and B, respectively. “a” and “b” pockets: complementary binding sites for “A” and “B” knobs, respectively (bottom schemata). Des-AA and Des-AA/DesBB fibrin: fibrin lacking its FpAs or both its FpAs and FpBs. (B) Molecular composition of some of the fibrin structures observed by Chernysh and colleages. (C) Possible composition of some fibrin oligomers in fibrin/fibrinogen complexes showing that incorporation of fibrinogen interrupts elongation. Fibrinogen E/fibrin D juxtaposition is consistent with fibrinogen self-assembly data.9 Professional illustration by Alice Y. Chen.
(A) Schemata of fibrinogen and fibrin molecules. D:D: structure on each D region that interfaces with its counterpart on another molecule. αC: carboxyl terminal region of the Aα chain. Coiled coil: interior parts of each chain connecting E and D regions. FpA and FpB: fibrinopeptides A and B, respectively. “a” and “b” pockets: complementary binding sites for “A” and “B” knobs, respectively (bottom schemata). Des-AA and Des-AA/DesBB fibrin: fibrin lacking its FpAs or both its FpAs and FpBs. (B) Molecular composition of some of the fibrin structures observed by Chernysh and colleages. (C) Possible composition of some fibrin oligomers in fibrin/fibrinogen complexes showing that incorporation of fibrinogen interrupts elongation. Fibrinogen E/fibrin D juxtaposition is consistent with fibrinogen self-assembly data.9 Professional illustration by Alice Y. Chen.
The lag phase underlies clotting-based coagulation tests and soluble fibrin that reflects limited fibrin levels in high fibrinogen excess (see figure panel C), forming soluble fibrin/fibrinogen complexes.4 These complexes are cryoprecipitable,5 are cleared rapidly when containing des-AA fibrin than when containing des-AA/des-BB fibrin (panel A),3 and are markedly increased ex vivo in donated blood.6 This increase enables harvest of plasma cryoprecipitate that is widely used in clinical medicine.7 In addition, it accounts for the cryoprecipitate in stored blood (4°) that is retained by each blood filter (eg, ∼ 170 μ or smaller average size pore) used invariably during transfusion. Moreover, increased soluble fibrin in some clinical disorders, obtained by cryoprecipitation and termed “cryofibrinogen” or “cryofibrinogenemia,” has long been used as a disease marker.8
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal