Abstract
In contrast to the established role of blood vessel remodeling in inflammation, the biologic function of the lymphatic vasculature in acute inflammation has remained less explored. We studied 2 established models of acute cutaneous inflammation, namely, oxazolone-induced delayed-type hypersensitivity reactions and ultraviolet B irradiation, in keratin 14-vascular endothelial growth factor (VEGF)-C and keratin 14-VEGF-D transgenic mice. These mice have an expanded network of cutaneous lymphatic vessels. Transgenic delivery of the lymphangiogenic factors VEGF-C and the VEGFR-3 specific ligand mouse VEGF-D significantly limited acute skin inflammation in both experimental models, with a strong reduction of dermal edema. Expression of VEGFR-3 by lymphatic endothelium was strongly down-regulated at the mRNA and protein level in acutely inflamed skin, and no VEGFR-3 expression was detectable on inflamed blood vessels and dermal macrophages. There was no major change of the inflammatory cell infiltrate or the composition of the inflammatory cytokine milieu in the inflamed skin of VEGF-C or VEGF-D transgenic mice. However, the increased network of lymphatic vessels in these mice significantly enhanced lymphatic drainage from the ear skin. These results provide evidence that specific lymphatic vessel activation limits acute skin inflammation via promotion of lymph flow from the skin and reduction of edema formation.
Introduction
Acute and chronic inflammatory processes are associated with the growth and/or enlargement of blood and lymphatic vessels.1 Indeed, vascular remodeling is a hallmark of a plethora of inflammatory diseases, such as chronic airway inflammation, rheumatoid arthritis, inflammatory bowel disease, and the chronic inflammatory skin disease psoriasis.2-5 We previously identified an important role of the blood vasculature and in particular of vascular endothelial growth factor (VEGF)-A in the promotion of acute and chronic inflammatory reactions in different experimental skin inflammation models.6-11 Recently, we found that activation of VEGFR-3 had a potent anti-inflammatory effect in a mouse model of psoriasis.12 Conversely, inhibition of VEGFR-3 significantly prolonged edema and inflammation during chronic bacterial airway inflammation, in chronic inflammatory arthritis, and in chronic skin inflammation.3,12,13 However, it has also been reported that the lymphatic vasculature plays an active role in promoting corneal and kidney transplant rejections, in part by facilitating dendritic cell transport to draining lymph nodes.14,15 Furthermore, the inflamed lymphatic endothelium might actively modulate immune responses.16,17 Together, these results indicate an important role of blood vessel angiogenesis in sustaining inflammation, whereas the functional role of the lymphatic vasculature in acute inflammation has remained less explored.
The cutaneous lymphatic vasculature is involved in the afferent immune response and also maintains tissue fluid homeostasis.18-20 Among the VEGF family members, VEGF-C and VEGF-D are the best described lymphangiogenic factors to date. Their receptor, VEGFR-3, is mainly expressed on the lymphatic endothelium in the adult.21 Transgenic overexpression of VEGF-C or of VEGF-D in keratinocytes under control of the keratin 14 (K14) promoter leads to the increased formation of lymphatic vessels in the skin, whereas mice that overexpress a soluble VEGFR-3 under control of the K14 promoter show edema and lack lymphatic vessels in the skin.22-24
To investigate the biologic role of lymphatic vessels in acute inflammation, we first induced acute delayed-type hypersensitivity (DTH) reactions in K14-VEGF-C and K14-VEGF-D transgenic (Tg) mice and compared the course of skin inflammation with that observed in wild-type mice. During DTH reactions, antigen-presenting cells take up the antigen in the skin and migrate to the regional lymph nodes where they interact with T helper cells to initiate the inflammatory reaction.25 We then investigated the acute inflammatory reactions induced by a single irradiation of the skin of transgenic and wild-type mice with a physiologically relevant dose of ultraviolet B (UVB; 200 mJ/cm2 ∼ 3 minimal erythema doses7 ). Antigen-presenting cells are dispensable for UVB-induced inflammation, and exposure to UVB light might even suppress DTH reactions.26 We further investigated VEGFR-3 expression, lymphatic and blood vessel morphology, inflammatory cell infiltration, and lymphatic drainage function during acute inflammation and compared those parameters with noninflamed skin. Overall, our studies reveal that an expanded lymphatic network limits acute skin inflammation and reduces dermal edema formation, without major effects on the inflammatory cell infiltration and the production of inflammatory cytokines.
Methods
Mouse models of acute skin inflammation
The generation of K14-VEGF-C Tg mice that express human VEGF-C under control of the K14 promoter and of K14-VEGF-D Tg mice that express mouse VEGF-D has been described previously.22,23 K14-VEGF-C and K14-VEGF-D Tg mice (both FVB genetic background) were bred and housed in the animal facility of ETH Zurich. The mice were genotyped as previously described.22,23 FVB wild-type littermates were used as controls. All experiments were initiated when the mice were between 6 and 8 weeks of age. Experiments were performed in accordance with animal protocols approved by the local veterinary authorities (Kantonales Veterinäramt Zürich).
Acute DTH reactions and UVB irradiation of the skin of mice were performed as described in supplemental Data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Mice were killed 2 days after oxazolone challenge or UVB irradiation, and ear and back skin samples were embedded in optimal cutting temperature compound (Sakura Finetek). Other ear and back skin samples were stored in RNAlater solution (Applied Biosystems) for subsequent RNA and protein extractions. All experiments were performed at least twice with comparable results.
Immunofluorescence
Tissues were embedded in optimal cutting temperature compound, frozen on liquid nitrogen, and 7-μm cryostat sections were cut. Specimens were placed on glass slides, air dried, and fixed with acetone for 2 minutes at −20°C. After rehydration with 80% methanol at 4°C, phosphate-buffered saline (PBS), and PBS with 12% bovine serum albumin, the specimens were incubated with the respective antibodies. Standard hematoxylin and eosin stainings were performed, and immunofluorescence was performed as described,9,12 using the following antibodies: anti–mouse LYVE-1 (Angiobio), anti–mouse CD31 (BD Biosciences), anti–mouse MECA-32 (BD Pharmingen), anti–mouse VEGFR-3 (AF743; R&D Systems), and biotin anti–mouse CD11b (BD Biosciences PharMingen). Alexa488- or Alexa594-coupled secondary antibodies and Hoechst 33342 were purchased from Invitrogen.
Image acquisition and preparation
All digital images were examined on an Axioshop 2 mot plus microscope (Carl Zeiss), equipped with an AxioCam MRc camera and a Plan-APOCHROMAT 10×/0.45 (for blood and lymphatic vessel analysis) and a Plan-NEOFLUAR 20×/0.50 objective (for all figure preparations; both Carl Zeiss). Images were acquired using Axio-Vision software Version 4.7.1 (Carl Zeiss). All figures were prepared using Adobe Photoshop CS4 extended Version 11.0.2.
Computer-assisted morphometric analyses
Immunofluorescence stains of ear and back skin sections for CD31+/LYVE-1+ lymphatic vessels and for MECA-32+ blood vessels27 were examined on an Axioskop 2 mot plus microscope (Carl Zeiss), equipped with an AxioCam MRc camera and a Plan-APOCHROMAT 10×/0.45 and a Plan-NEOFLUAR 20×/0.50 objective (Carl Zeiss). Images of 5 individual fields of view were acquired per section using AxioVision software, Version 4.7.1 (Carl Zeiss). Computer-assisted analyses of digital images were performed using the IP-LAB software (Scanalytics) as described.10,12 The average number of CD31+/LYVE-1+ lymphatic vessels and MECA-32+ blood vessels per millimeter epidermal basement membrane and the average size of vessels were determined in the area between cartilage and stratum corneum. In back skin, the vessels were quantified in a defined area, protruding 200 μm into the dermis from the basement membrane. The results are expressed as vessel number per millimeter epidermal basement membrane and not as vessel number per area, because the formation of inflammatory edema (increase in area) would confound the vessel number if it were calculated per area. To quantify CD11b+ cell numbers per millimeter epidermal basement membrane, images of 3 or 4 individual fields of view were acquired per sample (covering the entire field of view, between the cartilage backbone and the epidermis in the ear, and in an area 250 μm distant from the basement membrane in back skin samples).
Isolation of dermal lymphatic endothelial cells by FACS
Isolation, RNA extraction, and amplification of sorted lymphatic endothelial cells were performed as described in supplemental Data.
Quantitative real-time RT-PCR
Total cellular RNA was isolated from mouse ears or back skin using a TissueLyser, stainless steel beads, and the RNeasy Mini Kit (all from QIAGEN), and was treated with RQ1 RNase-free DNase (Promega). A total of 1 μg RNA was used to synthesize cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The expression of mouse CCL2, CXCL2, interferon-γ, interleukin-1β (IL-1β), S100A8, S100A9, tumor necrosis factor-α, and VEGFR-3 was investigated by TaqMan or SYBR Green real-time reverse-transcribed polymerase chain reaction (RT-PCR) using the AB 7900 HT Fast Real-Time PCR System (Applied Biosystems) and quantified using the 2−ΔΔCt method.28 The probes and primers for CCL2 (Mm99999056_m1), CXCL2 (Mm00436450_m1), interferon-γ (Mm00801778_m1), IL-1β (Mm99999061_mH), S100A8 (Mm00496696_g1), S100A9 (QuantiTect; QT00105252), tumor necrosis factor-α (QuantiTect; QT00104006), and VEGFR-3 (Mm00433337_m1) were predesigned. Each reaction was multiplexed (TaqMan) or run (SYBR Green) with β-actin (reference sequence NM_007393.1 or QT01136772; all Applied Biosystems or QIAGEN) as a reference gene, and all data were normalized based on the expression levels of β-actin; N = 3 to 10 per group.
ELISA
Skin lysates were obtained from ear skin of wild-type, K14-VEGF-C, and K14-VEGF-D Tg mice at 2 days after oxazolone challenge or UVB irradiation (n = 5-7 per group), and of untreated wild-type, K14-VEGF-C, and K14-VEGF-D Tg mice (n = 3 per group). Tissues were homogenized in lysis buffer (150mM NaCl, 50mM Tris, pH 7.5) with a protease inhibitor cocktail (Roche Diagnostics). Homogenates were centrifuged for 10 minutes at 14 000g. Supernatants were stored at −80°C until assayed. The fluorescence-activated cell sorting (ELISA) kits for CCL2 and IL-1β were purchased from RayBiotech, the VEGF-A Quantikine ELISA kit from R&D Systems. The absorbance was measured with an Infinite M200 microplate reader (Tecan). Protein levels were normalized per milligram of tissue.
Lymph flow assessment using Evans blue dye
A total of 3 μL of 1% Evans blue dye solution in PBS (Sigma-Aldrich) was injected into the ear skin of anesthetized wild-type (n = 10), K14-VEGF-C (n = 5), and K14-VEGF-D Tg (n = 5) mice without inflammation, or into noninflamed back skin of wild-type (n = 6) and K14-VEGF-C Tg mice (n = 7) using a Hamilton syringe. After 16 hours, the mice were killed. Evans blue dye was extracted from the ears or from 6-mm punch biopsies of the back skin by incubating them at 55°C for 5.5 hours in formamide (Fluka). The background-subtracted absorbance was measured with an Infinite M200 microplate reader by measuring at 620 nm and 740 nm. The concentration of dye in the extracts was calculated from a standard curve of Evans blue in formamide and is presented as absolute amount of dye that remained in the ear skin or back skin punch biopsy.
In addition, wild-type (n = 20), K14-VEGF-C (n = 10), and K14-VEGF-D (n = 9) Tg mice were sensitized and challenged with oxazolone solution or irradiated with 200 mJ/cm2 UVB light. Then, 32 hours after oxazolone challenge or UVB irradiation, the mice were anesthetized and injected into the ear skin with Evans blue dissolved in PBS as described in the previous paragraph. Evans blue was extracted 16 hours after injection of the dye using formamide. The absorbance was measured as described in the previous paragraph.
Measurement of vascular leakage
Oxazolone sensitized and challenged wild-type (n = 4) and K14-VEGF-C Tg (n = 5) mice were anesthetized as described in supplemental Data and were injected into the tail vein with 1% Evans blue dye solution in PBS at 2 days after oxazolone challenge. After 30 minutes, the mice were killed and Evans blue was extracted by incubating the ears at 55°C for 24 hours in formamide. The background-subtracted absorbance was measured as described in “Lymph flow assessment using Evans blue dye,” and the concentration of Evans blue in formamide was indicated.
Statistical analyses
Statistical analyses were performed using SPSS, Version 16.0 software or the statistical functions of Excel 2002 (Microsoft Corporation). Data are shown as mean ± SD or ± SEM as indicated and were analyzed with a 2-tailed, unpaired Student t test. When more than 2 groups were compared, analysis of variance was applied and the individual groups were compared using the Tukey-HSD post-hoc test. Homogeneity of variances was assessed using the Levene test, and normalized distribution was assessed using Q-Q plots. Differences were considered statistically significant when P was ≤ .05.
Results
Activation of lymphatic vessels reduces edema formation during acute skin inflammation
We first investigated the biologic role of lymphatic vessels in 2 different models of acute skin inflammation, using K14-VEGF-C and K14-VEGF-D Tg mice that are characterized by a dense lymphatic vessel network in their skin.22,23 K14-VEGF-C and K14-VEGF-D Tg mice and their wild-type littermates were either subjected to DTH reactions, induced by sensitization with oxazolone solution and, 5 days later, challenged on both sides of the ears by topical application of oxazolone,9 or were irradiated with a physiologic dose of UVB light, an established model of non–immune-mediated acute inflammation.29
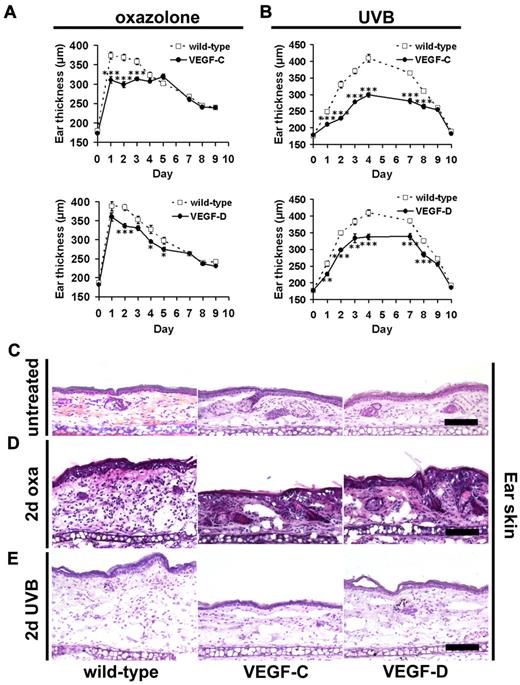
We found that K14-VEGF-C mice showed significantly less pronounced inflammatory ear swelling during the first 3 days after oxazolone challenge, compared with their wild-type littermates (Figure 1A). Inflammatory ear swelling was also significantly lower in K14-VEGF-D Tg mice at days 2, 4, and 5 after challenge (Figure 1A). Because the increased preexisting lymphatic vessel network might have modulated the sensitization phase in the DTH model, we next irradiated mice with 200 mJ/cm2 UVB light, as a second model of acute skin inflammation. We found that the increase in ear thickness that occurs within 24 to 48 hours after UVB irradiation as a result of inflammation was significantly lower in both K14-VEGF-C and K14-VEGF-D Tg mice compared with wild-type littermates, and remained lower until day 8 (Figure 1B). Because we observed the strongest reduction of ear thickness in the DTH model after 2 days (K14-VEGF-C, 19%, P ≤ .001; K14-VEGF-D, 13%, P ≤ .001); and because there were major differences in ear thickness also at 2 days after UVB irradiation (K14-VEGF-C, 31%, P ≤ .001; K14-VEGF-D, 14%, P ≤ .01), we performed all further morphologic characterizations at this time point. Hematoxylin and eosin stains of inflamed ear tissue sections obtained from wild-type mice showed the typical epidermal hyperplasia at 2 days after oxazolone challenge compared with noninflamed wild-type mice (Figure 1C-D). Furthermore, there was a marked inflammatory cell infiltration and dermal edema. In contrast, dermal edema and epidermal thickening were markedly less pronounced in K14-VEGF-C and in K14-VEGF-D Tg mice (Figure 1D). In general, the reduction of swelling appeared to be more pronounced in K14-VEGF-C Tg mice than in K14-VEGF-D Tg mice. Similarly, hematoxylin and eosin stains of sections from UVB-irradiated mouse ears of wild-type mice showed mild epidermal hyperplasia, inflammatory cell infiltration, and dermal edema at 2 days after irradiation (Figure 1E). In contrast, dermal edema was markedly less pronounced in K14-VEGF-C and K14-VEGF-D Tg mice (Figure 1E).
Lymphatic vessel activation reduces edema during acute skin inflammation. (A) K14-VEGF-C (●, n = 7), K14-VEGF-D (●, n = 8) Tg mice, and their wild-type littermates (□, n = 12) were painted with 2% oxazolone and challenged, 5 days later, with 1% oxazolone on the ears. The ear thickness of K14-VEGF-C and K14-VEGF-D Tg mice was significantly reduced compared with wild-type controls at the indicated time points. (B) K14-VEGF-C (●, n = 7), K14-VEGF-D (●, n = 5) Tg mice, and their wild-type littermates (□, n = 11) were irradiated with 200 mJ/cm2 UVB light, and the ear thickness was measured using calipers. The ear thickness of K14-VEGF-C and K14-VEGF-D Tg mice was significantly reduced compared with wild-type controls until day 8 after UVB irradiation. (A-B) Data are mean ± SEM. *P ≤ .05. **P ≤ .01. ***P ≤ .001. (C-E) Hematoxylin and eosin stains of untreated mouse ears (C), at day 2 after oxazolone challenge (2-day oxa, D) or UVB irradiation (2-day UVB, E) revealed reduced edema in inflamed K14-VEGF-C and K14-VEGF-D Tg mice, compared with inflamed skin of wild-type mice. One ear-half is shown. Bars represent 100 μm.
Lymphatic vessel activation reduces edema during acute skin inflammation. (A) K14-VEGF-C (●, n = 7), K14-VEGF-D (●, n = 8) Tg mice, and their wild-type littermates (□, n = 12) were painted with 2% oxazolone and challenged, 5 days later, with 1% oxazolone on the ears. The ear thickness of K14-VEGF-C and K14-VEGF-D Tg mice was significantly reduced compared with wild-type controls at the indicated time points. (B) K14-VEGF-C (●, n = 7), K14-VEGF-D (●, n = 5) Tg mice, and their wild-type littermates (□, n = 11) were irradiated with 200 mJ/cm2 UVB light, and the ear thickness was measured using calipers. The ear thickness of K14-VEGF-C and K14-VEGF-D Tg mice was significantly reduced compared with wild-type controls until day 8 after UVB irradiation. (A-B) Data are mean ± SEM. *P ≤ .05. **P ≤ .01. ***P ≤ .001. (C-E) Hematoxylin and eosin stains of untreated mouse ears (C), at day 2 after oxazolone challenge (2-day oxa, D) or UVB irradiation (2-day UVB, E) revealed reduced edema in inflamed K14-VEGF-C and K14-VEGF-D Tg mice, compared with inflamed skin of wild-type mice. One ear-half is shown. Bars represent 100 μm.
Acute skin inflammation induces lymphatic and blood vessel remodeling
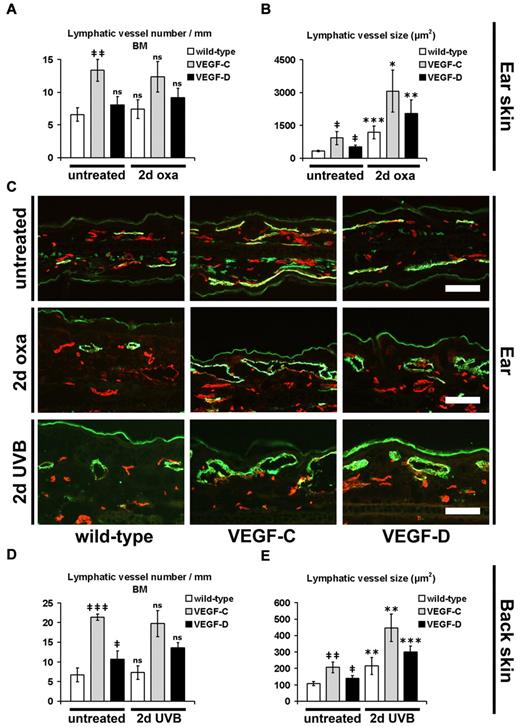
Because we observed a strongly reduced edema formation in the inflamed skin of K14-VEGF-C and K14-VEGF-D Tg mice and because fluid drainage represents a major function of lymphatic vessels, we next investigated whether there might be changes in the lymphatic vasculature of these mice. Computer-based morphometric analyses of immunofluorescence stains for the lymphatic marker LYVE-1 revealed that untreated K14-VEGF-C mice had a significantly increased number (2.0-fold, P ≤ .01) and average size (2.8-fold, P ≤ .05) of cutaneous CD31+/LYVE-1+ lymphatic vessels compared with their wild-type littermates (Figure 2A-C). K14-VEGF-D Tg mice also showed an increase in the average size (1.5-fold, P ≤ .05) of lymphatics, although these changes were less pronounced than in VEGF-C Tg mice (Figure 2B-C). During acute skin inflammation, 2 days after oxazolone challenge, the average lymphatic vessel size was significantly increased in wild-type, K14-VEGF-C, and K14-VEGF-D Tg mice, compared with their noninflamed corresponding genotypes (Figure 2B-C): 334 μm2 versus 1179 μm2, P ≤ .001 (noninflamed vs inflamed wild-type); 919 μm2 versus 3065 μm2, P ≤ .05 (noninflamed vs inflamed K14-VEGF-C); and 514 μm2 versus 2044 μm2, P ≤ .01 (noninflamed vs inflamed K14-VEGF-D), whereas their numbers were not further increased over those observed in noninflamed skin (Figure 2A). Interestingly, although there was less edema in acutely inflamed K14-VEGF-C and K14-VEGF-D Tg mice and although the size of lymphatic vessels was already larger in the normal skin of these mice than in wild-type mice, the relative fold increase of lymphatic vessel size was comparable in all genotypes (Figure 2B): 3.5-fold in wild-type mice, 3.3-fold in K14-VEGF-C Tg mice, and 4.0-fold in K14-VEGF-D Tg mice. Similarly, untreated K14-VEGF-C and K14-VEGF-D Tg mice had a significantly increased number (K14-VEGF-C 3.2-fold, P ≤ .001; K14-VEGF-D 1.6-fold, P ≤ .05) and average size (K14-VEGF-C 1.9-fold, P ≤ .01; K14-VEGF-D 1.3-fold, P ≤ .05) of lymphatic vessels in their back skin, compared with their wild-type littermates (Figure 2D-E). At 2 days after UVB irradiation, the average lymphatic vessel size was significantly increased in wild-type, K14-VEGF-C, and K14-VEGF-D Tg mice, compared with their noninflamed corresponding genotypes (Figure 2E): 107 μm2 versus 214 μm2, P ≤ .01 (noninflamed vs inflamed wild-type); 207 μm2 versus 446 μm2, P ≤ .01 (noninflamed vs inflamed K14-VEGF-C); and 140 μm2 versus 298 μm2, P ≤ .001 (noninflamed vs inflamed K14-VEGF-D), whereas their numbers were not further increased over those observed in noninflamed skin (Figure 2D).
Enlargement of cutaneous lymphatic vessels during acute inflammation. (A-B) Quantitative image analyses of CD31+/LYVE-1+ lymphatic vessels in the ear skin of mice revealed a significantly increased number per millimeter basement membrane (BM, A) and size (B) of lymphatic vessels in untreated K14-VEGF-C Tg mice, compared with untreated wild-type mice (n = 3 mice per group). The lymphatic vessel size was also increased in untreated K14-VEGF-D Tg mice, compared with untreated wild-type mice (n = 3 mice per group). The average number of lymphatic vessels in wild-type mice was not significantly different at 2 days after oxazolone challenge (2-day oxa) compared with untreated wild-type mice. The lymphatic vessel number was also not significantly different between untreated and oxazolone-challenged K14-VEGF-C and K14-VEGF-D Tg mice, respectively (A; wild-type, n = 10; K14-VEGF-C, n = 5; K14-VEGF-D, n = 5). At 2 days after oxazolone challenge, the average size of lymphatic vessels was significantly increased in K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice, compared with untreated mice of the same genotype (B). (C) Representative images of CD31+/LYVE-1+ lymphatic vessels (green) in the ear skin. CD31+/LYVE-1− structures represent blood vessels (red). The positive staining of LYVE-1 in the stratum corneum in panel C is unspecific. Bars represent 100 μm. (D-E) The average lymphatic vessel number (D) and size (E) were significantly increased in the back skin of untreated K14-VEGF-C and K14-VEGF-D Tg mice, compared with untreated wild-type mice. At 2 days after UVB irradiation (2-day UVB), lymphatic vessel size (E), but not lymphatic vessel number (D), was significantly increased in K14-VEGF-C, K14-VEGF-D, and wild-type mice compared with untreated mice of the same genotype. (A-B,D-E) Data are mean ± SD. ‡P ≤ .05. ‡‡P ≤ .01. ‡‡‡P ≤ .001. ns indicates not significant versus untreated wild-type. *P ≤ .05. **P ≤ .01. ***P ≤ .001. ns indicates not significant versus untreated mice (untreated wild-type vs 2-day oxa/2-day UVB wild-type; untreated VEGF-C vs 2-day oxa/2-day UVB VEGF-C; untreated VEGF-D vs 2-day oxa/2-day UVB VEGF-D).
Enlargement of cutaneous lymphatic vessels during acute inflammation. (A-B) Quantitative image analyses of CD31+/LYVE-1+ lymphatic vessels in the ear skin of mice revealed a significantly increased number per millimeter basement membrane (BM, A) and size (B) of lymphatic vessels in untreated K14-VEGF-C Tg mice, compared with untreated wild-type mice (n = 3 mice per group). The lymphatic vessel size was also increased in untreated K14-VEGF-D Tg mice, compared with untreated wild-type mice (n = 3 mice per group). The average number of lymphatic vessels in wild-type mice was not significantly different at 2 days after oxazolone challenge (2-day oxa) compared with untreated wild-type mice. The lymphatic vessel number was also not significantly different between untreated and oxazolone-challenged K14-VEGF-C and K14-VEGF-D Tg mice, respectively (A; wild-type, n = 10; K14-VEGF-C, n = 5; K14-VEGF-D, n = 5). At 2 days after oxazolone challenge, the average size of lymphatic vessels was significantly increased in K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice, compared with untreated mice of the same genotype (B). (C) Representative images of CD31+/LYVE-1+ lymphatic vessels (green) in the ear skin. CD31+/LYVE-1− structures represent blood vessels (red). The positive staining of LYVE-1 in the stratum corneum in panel C is unspecific. Bars represent 100 μm. (D-E) The average lymphatic vessel number (D) and size (E) were significantly increased in the back skin of untreated K14-VEGF-C and K14-VEGF-D Tg mice, compared with untreated wild-type mice. At 2 days after UVB irradiation (2-day UVB), lymphatic vessel size (E), but not lymphatic vessel number (D), was significantly increased in K14-VEGF-C, K14-VEGF-D, and wild-type mice compared with untreated mice of the same genotype. (A-B,D-E) Data are mean ± SD. ‡P ≤ .05. ‡‡P ≤ .01. ‡‡‡P ≤ .001. ns indicates not significant versus untreated wild-type. *P ≤ .05. **P ≤ .01. ***P ≤ .001. ns indicates not significant versus untreated mice (untreated wild-type vs 2-day oxa/2-day UVB wild-type; untreated VEGF-C vs 2-day oxa/2-day UVB VEGF-C; untreated VEGF-D vs 2-day oxa/2-day UVB VEGF-D).
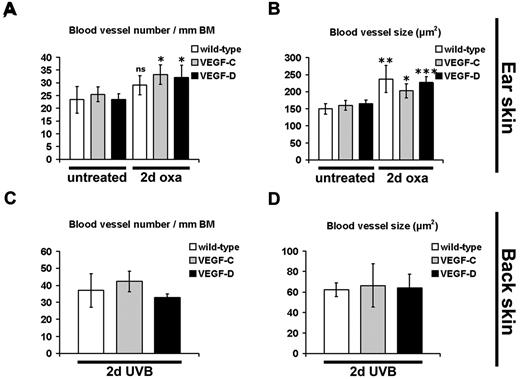
We next investigated morphologic changes of cutaneous blood vessels, using immunostains for the blood vessel marker MECA-32.27 We found that, in contrast to the lymphatic vasculature, the number and size of blood vessels were not significantly different in the untreated, noninflamed skin of K14-VEGF-C and of K14-VEGF-D Tg mice, compared with their wild-type littermates (Figure 3A-B). There was a slight increase in the number of blood vessels in mice of all genotypes at 2 days after oxazolone challenge (wild-type, 1.2-fold, P = .08; K14-VEGF-C, 1.3-fold, P ≤ .05; K14-VEGF-D, 1.4-fold, P ≤ .05), whereas the average size of cutaneous blood vessels was significantly increased in mice of all genotypes (Figure 3B; wild-type, 1.6-fold, P ≤ .01; K14-VEGF-C, 1.3-fold, P ≤ .05; K14-VEGF-D, 1.4-fold, P ≤ .001). However, there was no significant difference in blood vessel number and size between wild-type, K14-VEGF-C, and K14-VEGF-D Tg mice at 2 days after oxazolone challenge (Figure 3A-B). Furthermore, there was also no difference in blood vessel number and blood vessel size between all genotypes at 2 days after UVB irradiation (Figure 3C-D).
Enlargement of blood vessels during acute skin inflammation. (A-B) Immunofluorescence analyses for the blood vessel-specific marker MECA-32 and subsequent morphometric quantification showed a significant increase in blood vessel number per millimeter basement membrane (BM) in K14-VEGF-C and K14-VEGF-D Tg mice at 2 days after oxazolone challenge (2-day oxa), compared with untreated mice (A). The blood vessel size was also increased in all 3 groups of mice at 2 days of oxazolone challenge, compared with untreated mice of the same genotype (B). There was no significant difference in blood vessel number (A,C) or blood vessel size (B,D) at 2 days after oxazolone challenge or UVB irradiation (2-day UVB) between K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice. (A-B) Quantification of ear skin. (C-D) Quantification of back skin. (A-D) Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001. ns indicates not significant versus untreated mice of the same genotype.
Enlargement of blood vessels during acute skin inflammation. (A-B) Immunofluorescence analyses for the blood vessel-specific marker MECA-32 and subsequent morphometric quantification showed a significant increase in blood vessel number per millimeter basement membrane (BM) in K14-VEGF-C and K14-VEGF-D Tg mice at 2 days after oxazolone challenge (2-day oxa), compared with untreated mice (A). The blood vessel size was also increased in all 3 groups of mice at 2 days of oxazolone challenge, compared with untreated mice of the same genotype (B). There was no significant difference in blood vessel number (A,C) or blood vessel size (B,D) at 2 days after oxazolone challenge or UVB irradiation (2-day UVB) between K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice. (A-B) Quantification of ear skin. (C-D) Quantification of back skin. (A-D) Data are mean ± SD. *P ≤ .05. **P ≤ .01. ***P ≤ .001. ns indicates not significant versus untreated mice of the same genotype.
Down-regulation of VEGFR-3 during acute skin inflammation
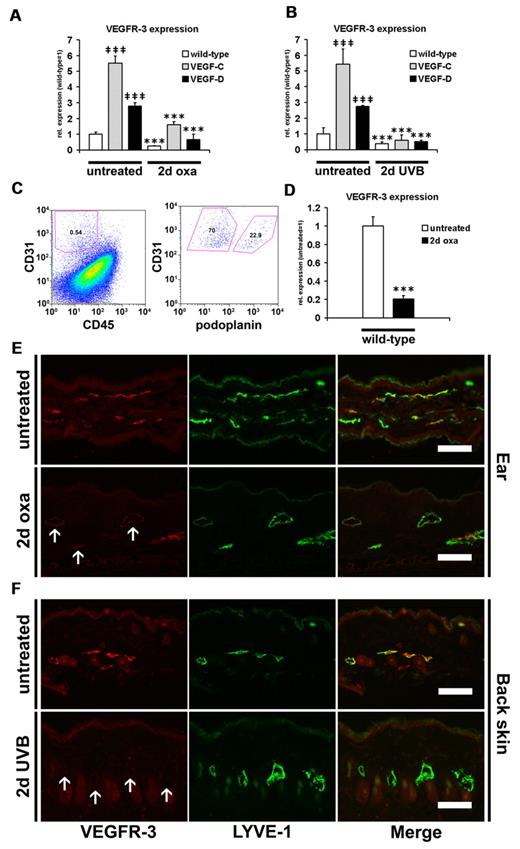
To investigate whether the chronic release of the lymphangiogenic factors VEGF-C or VEGF-D in the Tg mice affects the expression of their common receptor VEGFR-3 on lymphatic endothelium, we next investigated VEGFR-3 expression by real-time RT-PCR, fluorescence-activated cell sorting (FACS), and immunofluorescence stains. We found that VEGFR-3 mRNA levels were significantly increased in untreated ear and back skin of K14-VEGF-C and K14-VEGF-D Tg mice compared with their wild-type littermates (Figure 4A-B): ear skin (K14-VEGF-C, 5.52-fold, P ≤ .001; and K14-VEGF-D, 2.78-fold, P ≤ .001) and back skin (K14-VEGF-C, 5.44-fold, P ≤ .001; and K14-VEGF-D, 2.75-fold, P ≤ .001). The VEGFR-3 transcript levels were significantly reduced 2 days after oxazolone challenge in all types of mice (Figure 4A; wild-type, 4.06-fold, P ≤ .001; K14-VEGF-C, 3.47-fold, P ≤ .001; K14-VEGF-D, 4.24-fold, P ≤ .001). A similar down-regulation was seen 2 days after UVB irradiation in mice of all genotypes (Figure 4B; wild-type, 2.52-fold, P ≤ .001; K14-VEGF-C, 9.30-fold, P ≤ .001; K14-VEGF-D, 5.33-fold, P ≤ .001).
Down-regulation of VEGFR-3 during acute skin inflammation. (A-B) TaqMan-based real-time RT-PCR analyses were performed on whole ear and back skin extracts after 2 days of oxazolone challenge (2-day oxa; n = 6-10 per group) or UVB irradiation (2-day UVB; n = 5-8 per group) and in untreated K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice (n = 3-5 per group). VEGFR-3 was significantly up-regulated in untreated K14-VEGF-C and K14-VEGF-D Tg mouse ear (A) and back skin (B) compared with untreated wild-type mice. VEGFR-3 was significantly down-regulated at 2 days of oxazolone challenge (A) or UVB irradiation (B) in all 3 groups of mice compared with untreated mice of the same genotype. (C-D) Single-cell suspensions from the ear of normal and oxazolone challenged mice were analyzed by FACS. (C) CD31+/CD45− cells represent endothelial cells, whereas CD31+/podoplanin+/CD45− cells are lymphatic endothelial cells. (D) Cutaneous lymphatic endothelial cells from inflamed ears of wild-type mice (2 days after oxazolone challenge) showed a 5-fold decrease of VEGFR-3 mRNA transcript levels compared with lymphatic endothelial cells from untreated mice. (A-B,D) Data are mean ± SD. ‡‡‡P ≤ .001 versus untreated wild-type. ***P ≤ .001 versus untreated mice of the same genotype. (E-F) Double immunofluorescence analyses of VEGFR-3 (red) and LYVE-1 (green) stains demonstrated that VEGFR-3 was strongly down-regulated on LYVE-1+ lymphatic vessels at 2 days after oxazolone challenge (E) or UVB irradiation (F) in the ear and back skin of wild-type mice (arrows). The positive staining of LYVE-1 in the stratum corneum (E) is unspecific. Bars represent 100 μm.
Down-regulation of VEGFR-3 during acute skin inflammation. (A-B) TaqMan-based real-time RT-PCR analyses were performed on whole ear and back skin extracts after 2 days of oxazolone challenge (2-day oxa; n = 6-10 per group) or UVB irradiation (2-day UVB; n = 5-8 per group) and in untreated K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice (n = 3-5 per group). VEGFR-3 was significantly up-regulated in untreated K14-VEGF-C and K14-VEGF-D Tg mouse ear (A) and back skin (B) compared with untreated wild-type mice. VEGFR-3 was significantly down-regulated at 2 days of oxazolone challenge (A) or UVB irradiation (B) in all 3 groups of mice compared with untreated mice of the same genotype. (C-D) Single-cell suspensions from the ear of normal and oxazolone challenged mice were analyzed by FACS. (C) CD31+/CD45− cells represent endothelial cells, whereas CD31+/podoplanin+/CD45− cells are lymphatic endothelial cells. (D) Cutaneous lymphatic endothelial cells from inflamed ears of wild-type mice (2 days after oxazolone challenge) showed a 5-fold decrease of VEGFR-3 mRNA transcript levels compared with lymphatic endothelial cells from untreated mice. (A-B,D) Data are mean ± SD. ‡‡‡P ≤ .001 versus untreated wild-type. ***P ≤ .001 versus untreated mice of the same genotype. (E-F) Double immunofluorescence analyses of VEGFR-3 (red) and LYVE-1 (green) stains demonstrated that VEGFR-3 was strongly down-regulated on LYVE-1+ lymphatic vessels at 2 days after oxazolone challenge (E) or UVB irradiation (F) in the ear and back skin of wild-type mice (arrows). The positive staining of LYVE-1 in the stratum corneum (E) is unspecific. Bars represent 100 μm.
The differences observed at the mRNA levels in samples obtained from total mouse skin might have at least in part been the result of the different amount of lymphatic vessels in these samples, and possibly to the different extent of immune cell infiltration in the inflamed situation. Thus, we next performed FACS analyses in mice to specifically assess VEGFR-3 expression on lymphatic endothelial cells. CD31+/podoplanin−/CD45− cells represent blood vascular endothelial cells, whereas CD31+/podoplanin+/CD45− cells represent lymphatic endothelial cells (Figure 4C). Importantly, we found, by real-time RT-PCR, that the VEGFR-3 transcript levels in lymphatic endothelial cells of wild-type mice were 4.86-fold (P ≤ .001) lower at 2 days after oxazolone challenge than in noninflamed skin (Figure 4D).
In agreement with these findings, double immunofluorescence analyses for the expression of VEGFR-3 and LYVE-1 revealed a pronounced down-regulation of VEGFR-3 protein on LYVE-1+ lymphatic vessels in wild-type mice at 2 days after oxazolone challenge, compared with noninflamed skin (Figure 4E). In addition, VEGFR-3 protein was almost completely absent on LYVE-1+ lymphatic vessels in wild-type mice 2 days after UVB irradiation, whereas lymphatic vessels express VEGFR-3 in noninflamed skin of wild-type mice (Figure 4F). VEGFR-3 expression was also absent on cutaneous MECA-32+ blood vessels and CD11b+ cells in normal and inflamed wild-type mice (data not shown). Furthermore, K14-VEGF-C and K14-VEGF-D Tg mice showed a pronounced down-regulation of VEGFR-3 on LYVE-1+ lymphatic vessels 2 days after oxazolone challenge or UVB irradiation, compared with noninflamed transgenic littermates (supplemental Figures 1-2).
Differential effects of VEGF-C and VEGF-D on inflammatory cytokine expression
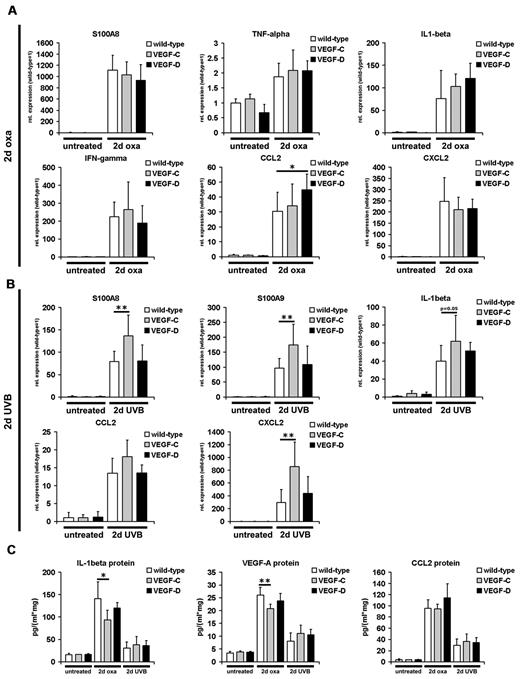
We next investigated whether the reduced edema formation in the inflamed skin of K14-VEGF-C and K14-VEGF-D Tg mice was associated with reduced expression of acute inflammation markers. TaqMan- or SYBR Green-based real-time RT-PCR of total mouse ear and back skin revealed that the inflammation markers S100A8, S100A9, tumor necrosis factor-α, IL-1β, interferon-γ, CCL2, and CXCL2 were all significantly up-regulated at 2 days after oxazolone challenge or UVB irradiation, respectively (Figure 5A-B). Interestingly, K14-VEGF-C and K14-VEGF-D Tg mice did not show reduced transcript levels of inflammation markers in the skin at 2 days after oxazolone challenge or UVB irradiation, compared with wild-type mice, despite strongly reduced edema (Figure 5A-B). The transcript levels of S100A8, S100A9, and CXCL2 were increased in K14-VEGF-C Tg mice at 2 days after UVB irradiation (Figure 5B), compared with wild-type mice. By contrast, protein levels of IL-1β and VEGF-A (assessed by ELISA), which were both increased at 2 days after oxazolone challenge, were slightly reduced in K14-VEGF-C Tg mice compared with challenged wild-type mice (Figure 5C; IL-1β, P ≤ .05; VEGF-A, P ≤ .01), whereas no such changes were seen in K14-VEGF-D Tg mice (Figure 5C). The reduction of IL-1β and VEGF-A proteins was not associated with reduced vascular permeability in K14-VEGF-C Tg mice compared with wild-type mice at 2 days after oxazolone challenge (15.1 ± 1.2 μg Evans blue/mL formamide vs 16.4 ± 1.9 μg Evans blue/mL formamide; wild-type vs VEGF-C). The levels of all 3 proteins analyzed were not different between UVB irradiated Tg and wild-type mice, although they were all significantly up-regulated compared with noninflamed mice (Figure 5C).
Inflammation marker expression in the skin during acute inflammation. (A-B) Real-time RT-PCR analyses were performed using RNAs from whole ear and back skin harvested 2 days after oxazolone challenge (2-day oxa; n = 6-10 per group) or UVB irradiation (2-day UVB; n = 5-8 per group) and from skin of untreated K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice (n = 3-5 per group). All inflammation markers shown were significantly up-regulated in K14-VEGF-C, K14-VEGF-D Tg, and wild-type mouse ear (A) and back skin (B) 2 days after oxazolone challenge (A) or UVB irradiation (B), compared with untreated wild-type or transgenic mice. S100A8, S100A9, and CXCL2 mRNA levels were slightly increased in K14-VEGF-C Tg mice 2 days after UVB irradiation compared with irradiated wild-type mice (B). (C) ELISA analyses of ear lysates showed significantly increased levels of IL-1β, VEGF-A, and CCL2 at 2 days after oxazolone challenge or UVB irradiation in the ear skin of K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice compared with untreated mice. The protein levels of IL-1β and VEGF-A were slightly but significantly reduced in the ear skin of oxazolone challenged K14-VEGF-C Tg mice compared with oxazolone challenged wild-type mice. (A-C) Data are mean ± SD. *P ≤ .05 versus oxazolone challenged or UVB irradiated wild-type mice. **P ≤ .01 versus oxazolone challenged or UVB irradiated wild-type mice.
Inflammation marker expression in the skin during acute inflammation. (A-B) Real-time RT-PCR analyses were performed using RNAs from whole ear and back skin harvested 2 days after oxazolone challenge (2-day oxa; n = 6-10 per group) or UVB irradiation (2-day UVB; n = 5-8 per group) and from skin of untreated K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice (n = 3-5 per group). All inflammation markers shown were significantly up-regulated in K14-VEGF-C, K14-VEGF-D Tg, and wild-type mouse ear (A) and back skin (B) 2 days after oxazolone challenge (A) or UVB irradiation (B), compared with untreated wild-type or transgenic mice. S100A8, S100A9, and CXCL2 mRNA levels were slightly increased in K14-VEGF-C Tg mice 2 days after UVB irradiation compared with irradiated wild-type mice (B). (C) ELISA analyses of ear lysates showed significantly increased levels of IL-1β, VEGF-A, and CCL2 at 2 days after oxazolone challenge or UVB irradiation in the ear skin of K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice compared with untreated mice. The protein levels of IL-1β and VEGF-A were slightly but significantly reduced in the ear skin of oxazolone challenged K14-VEGF-C Tg mice compared with oxazolone challenged wild-type mice. (A-C) Data are mean ± SD. *P ≤ .05 versus oxazolone challenged or UVB irradiated wild-type mice. **P ≤ .01 versus oxazolone challenged or UVB irradiated wild-type mice.
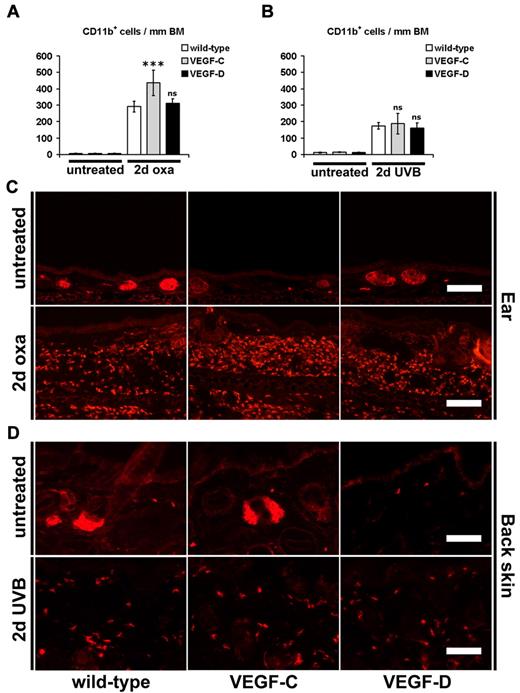
Immunofluorescence and computer-assisted image analyses of the monocyte/granulocyte marker CD11b in the skin revealed that there was a strong infiltration of dermal CD11b+ cells 2 days after oxazolone challenge compared with untreated mice (Figure 6A,C). This increase was not reduced in the skin of K14-VEGF-C and K14-VEGF-D Tg mice at 2 days after oxazolone challenge compared with wild-type littermates (Figure 6A,C). Indeed, there were even more dermal CD11b+ cells in the ear skin of K14-VEGF-C Tg mice (Figure 6A,C; 291 ± 13 cells in wild-type mice vs 436 ± 30 cells in K14-VEGF-C Tg mice, P ≤ .001). The number of CD11b+ cells was also significantly increased 2 days after UVB irradiation (Figure 6B,D). However, there was no difference in the number of infiltrated CD11b+ cells between mice of all genotypes 2 days after UVB irradiation (Figure 6B,D).
Inflammatory cell infiltration during acute skin inflammation. (A-D) Immunofluorescence analyses for the monocyte/granulocyte marker CD11b and subsequent computer-based quantification showed a significantly increased number of dermal CD11b+ cells per millimeter basement membrane (BM) 2 days after oxazolone challenge (2 days oxa, A,C; ear skin) or UVB irradiation (2 days UVB, B,D; back skin) in K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice, compared with untreated mice of the same genotype. K14-VEGF-C Tg mice had even more infiltrated dermal CD11b+ cells in their ear skin 2 days after oxazolone challenge compared with oxazolone challenged wild-type mice (A,C). The hair follicle sebaceous glands are stained red (C-D) because of endogenous biotin. (A-D) Data are mean ± SD. ***P ≤ .001. ns indicates not significant versus oxazolone challenged or UVB-irradiated wild-type mice. Bars represent 100 μm (C), 50 μm (D).
Inflammatory cell infiltration during acute skin inflammation. (A-D) Immunofluorescence analyses for the monocyte/granulocyte marker CD11b and subsequent computer-based quantification showed a significantly increased number of dermal CD11b+ cells per millimeter basement membrane (BM) 2 days after oxazolone challenge (2 days oxa, A,C; ear skin) or UVB irradiation (2 days UVB, B,D; back skin) in K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice, compared with untreated mice of the same genotype. K14-VEGF-C Tg mice had even more infiltrated dermal CD11b+ cells in their ear skin 2 days after oxazolone challenge compared with oxazolone challenged wild-type mice (A,C). The hair follicle sebaceous glands are stained red (C-D) because of endogenous biotin. (A-D) Data are mean ± SD. ***P ≤ .001. ns indicates not significant versus oxazolone challenged or UVB-irradiated wild-type mice. Bars represent 100 μm (C), 50 μm (D).
Increased lymph flow in VEGF-C and VEGF-D Tg mice
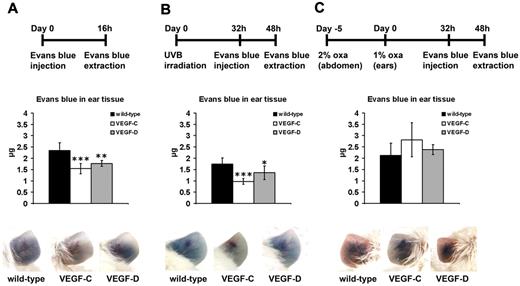
We next investigated whether enhanced lymphatic drainage might have contributed to the reduced ear thickness and edema formation in K14-VEGF-C and K14-VEGF-D Tg mice. To this end, we used Evans blue dye that was intradermally injected into the noninflamed ear skin of wild-type, K14-VEGF-C, or K14-VEGF-D Tg mice. Evans blue is specifically taken up by the lymphatic vasculature after intradermal injection.30 The extraction of Evans blue from the mouse ear, 16 hours after the injection, revealed that K14-VEGF-C and K14-VEGF-D Tg mice had significantly less Evans blue remaining in their noninflamed ear skin than wild-type mice (Figure 7A; VEGF-C, 34%, P ≤ .001; VEGF-D, 25%, P ≤ .01), indicating an enhanced lymphatic clearance function. Because there are studies indicating that K14-VEGF-C Tg mice have retrograde filling of their initial lymphatics,31 we additionally analyzed the lymph drainage from the back skin of untreated K14-VEGF-C Tg versus wild-type mice. There was significantly less Evans blue dye remaining in the back skin of K14-VEGF-C Tg mice than in wild-type mice 16 hours after injection of the dye (0.71 ± 0.36 μg vs 2.01 ± 0.52 μg; VEGF-C vs wild-type; P ≤ .001). We also assessed whether there are still differences of lymph flow between Tg and wild-type mice during acute skin inflammation. Interestingly, we found that K14-VEGF-C and K14-VEGF-D Tg mice still drained significantly faster after UVB irradiation than irradiated wild-type mice (Figure 7B; VEGF-C, 44%, P ≤ .001; VEGF-D, 22%, P ≤ .05). There was no difference in lymph draining capacity between all types of mice during acute oxazolone challenge (Figure 7C).
Increased lymph flow from the ear in K14-VEGF-C and K14-VEGF-D Tg mice. (A-C) Evans blue dye was intradermally injected into the ear of untreated or inflamed K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice and was extracted 16 hours after the dye injection. The total dye remaining in the ear skin is indicated. Representative pictures before Evans blue extraction are shown at the bottom. (A) Untreated K14-VEGF-C (n = 5) and K14-VEGF-D (n = 5) Tg mice had significantly less Evans blue remaining in their ear skin 16 hours after injection compared with wild-type mice (n = 10). (B) Evans blue was also injected 32 hours after UVB irradiation and extracted 16 hours later. UVB irradiated K14-VEGF-C (n = 5) and K14-VEGF-D (n = 4) Tg mice showed a faster lymph flow than irradiated wild-type mice (n = 10). (C) There was no difference in Evans blue clearance from the inflamed ear during acute inflammation after oxazolone challenge between all mice (wild-type, n = 10; K14-VEGF-C, n = 5; K14-VEGF-D, n = 5). Data are mean ± SD. *P ≤ .05 versus wild-type mice. **P ≤ .01 versus wild-type mice. ***P ≤ .001 versus wild-type mice.
Increased lymph flow from the ear in K14-VEGF-C and K14-VEGF-D Tg mice. (A-C) Evans blue dye was intradermally injected into the ear of untreated or inflamed K14-VEGF-C, K14-VEGF-D Tg, and wild-type mice and was extracted 16 hours after the dye injection. The total dye remaining in the ear skin is indicated. Representative pictures before Evans blue extraction are shown at the bottom. (A) Untreated K14-VEGF-C (n = 5) and K14-VEGF-D (n = 5) Tg mice had significantly less Evans blue remaining in their ear skin 16 hours after injection compared with wild-type mice (n = 10). (B) Evans blue was also injected 32 hours after UVB irradiation and extracted 16 hours later. UVB irradiated K14-VEGF-C (n = 5) and K14-VEGF-D (n = 4) Tg mice showed a faster lymph flow than irradiated wild-type mice (n = 10). (C) There was no difference in Evans blue clearance from the inflamed ear during acute inflammation after oxazolone challenge between all mice (wild-type, n = 10; K14-VEGF-C, n = 5; K14-VEGF-D, n = 5). Data are mean ± SD. *P ≤ .05 versus wild-type mice. **P ≤ .01 versus wild-type mice. ***P ≤ .001 versus wild-type mice.
Discussion
In this study, we found that specific promotion of lymphatic vessel function limits acute skin inflammation in mice, without major effects on the composition of the inflammatory infiltrate and the tissue cytokine milieu. We used 2 independent experimental models of acute inflammation, namely, induction of cutaneous DTH reactions and UVB irradiation of the skin. The DTH model is an immune-based model where antigen-presenting cells are thought to be essential to initiate the inflammatory reaction.32 Because modulation of lymphatic vessel function in this model might potentially also have affected the induction phase of acute inflammation via modulation of the transport of antigen-presenting cells to the draining lymph nodes, we also performed all studies in parallel in a second acute inflammation model (ie, UVB irradiation of the skin). In this model, a single exposure to UVB irradiation (290-320 nm wavelength) induces inflammatory skin alterations that include erythema, vascular hyperpermeability, dilation of dermal blood vessels, and epidermal hyperplasia without the requirement for prior antigen sensitization.7,25
In both models, VEGF-A probably represents the major driver of vascular remodeling and hyperpermeability, leading to edema formation. The amount of VEGF-A is increased in the skin during acute oxazolone-induced DTH reactions.9 Acute UVB irradiation also increases cutaneous VEGF-A levels, and mice that overexpress VEGF-A are more sensitive to DTH reactions and UVB irradiation than wild-type mice.7,9,33 Conversely, we previously found that systemic blockade of VEGF-A reduces UVB-induced inflammation and vascular enlargement and that combined blockade of VEGFR-1 and VEGFR-2 partially reduced acute oxazolone-mediated inflammation and vascular remodeling.7,9
In addition to VEGF-A, VEGF-C has also been implicated in the inflammation-induced vascular remodeling, in particular regarding the lymphatic vasculature.18,34 Epidermal keratinocytes and macrophages secrete both VEGF-A and VEGF-C, and they participate in lymphatic vessel remodeling during inflammation.5,35,36 Whereas VEGF-A binds to VEGFR-1 and VEGFR-2, VEGF-C specifically binds to VEGFR-3 and, after proteolytic cleavage of the propeptides, also binds and activates VEGFR-2.18 In contrast, murine VEGF-D only activates VEGFR-3.37 Importantly, VEGFR-2 is expressed by both the lymphatic and the blood vascular endothelium, whereas VEGFR-3 is mainly restricted to the lymphatic vasculature in the adult.21,38 In our studies, we used a genetic approach (chronic transgenic overexpression of either VEGF-C or of murine VEGF-D in the skin) to investigate the biologic effects of an expanded lymphatic vascular network on acute inflammation. Thus, VEGF-C derived from K14-VEGF-C Tg mice might theoretically also affect the blood vasculature, whereas mouse VEGF-D specifically activates lymphatic vessels.22 However, in agreement with a previous report,23 we did not detect any changes of the blood vasculature in K14-VEGF-C Tg mice. Therefore, our findings that Tg expression of VEGF-C more potently reduced edema formation than Tg expression of murine VEGF-D were probably not caused by effects on blood vessels but by the denser network of dermal lymphatic vessels in K14-VEGF-C mice. The denser network of lymphatics in K14-VEGF-C Tg mice might be caused by the different binding affinities of VEGF-C (dissociation constant = 1.35 × 10−10M) and VEGF-D (dissociation constant = 8.9 × 10−8M) to VEGFR-3 or by potential copy number differences.37,39
The expression of VEGFR-3 has been generally thought to be restricted to the lymphatic vascular system with the exception of corneal dendritic cells and some angiogenic blood vessels in tumors, healing wounds, and during early embryonic development.38,40,41 Our current findings reveal that, in the setting of acute skin inflammation in mice, VEGFR-3 is strongly down-regulated (at the mRNA and the protein level) in inflamed, enlarged lymphatic vessels and is completely absent from the blood vascular endothelium and from cutaneous CD11b+ monocytes/granulocytes. These findings are in contrast to those recently reported in a model of inflammatory peritonitis, where VEGFR-3 up-regulation on lymphatic endothelium was detected several days before the onset of lymphangiogenesis.42 These differences might be explained by the different models and organs studied. Moreover, the repeated application of thioglycollate every 48 hours in the inflammatory peritonitis model42 might rather reflect a chronic inflammation setting, in contrast to the acute inflammation model (48 hours after induction) of our study, which is characterized by lymphatic enlargement but not by sprouting lymphangiogenesis. A potential mechanism might be that down-regulation of Prox1 that also occurs during acute skin inflammation, at least on the mRNA level (data not shown), directly down-regulates VEGFR-3 expression on the lymphatic endothelium by binding to the VEGFR-3 promoter.42-45 Our finding that down-regulation of the lymphatic-specific VEGFR-3 in acute inflammation was comparable in total ear skin (4-fold) and in FACS-isolated lymphatic endothelial cells (5-fold) further confirms its lymphatic-specific expression in the skin. The higher expression of VEGFR-3 in the skin of noninflamed K14-VEGF-C and K14-VEGF-D Tg mice was probably the result of the increased lymphatic vascular network in these mice and not to a specific enhancement of VEGFR-3 expression on lymphatic endothelium. There is additional evidence that lymphatic function is reduced in acute skin inflammation via a down-regulation of the VEGF-C/VEGFR-3 axis because epidermal VEGF-C expression is also reduced after acute UVB irradiation.46 Moreover, specific inhibition of VEGFR-3 in K14-VEGF-A Tg mice significantly increased edema formation after UVB irradiation, which was reduced by injection of the VEGFR-3-specific mutant VEGF-C156S.46,47
Several aspects of cutaneous inflammation could have been modulated by the increased lymphatic network in K14-VEGF-C or VEGF-D Tg mice, thereby limiting the inflammatory response. Interestingly, we found that inflammatory cell infiltration and the expression of molecular markers and mediators of inflammation were largely unchanged in K14-VEGF-C and K14-VEGF-D Tg mice, despite their reduced ear swelling compared with wild-type littermates. These findings are in agreement with a recent report that Tg VEGF-C reduced LPS-induced edema but did not reduce inflammatory cell migration to the draining lymph node.48 Further studies are needed to investigate whether the increased lymphatic network in the Tg mice might have trapped inflammatory chemokines by the lymphatic-specific decoy receptor D6.49
Importantly, our findings that the clearance of intradermally injected Evans blue from the ear skin was significantly faster in both K14-VEGF-C and K14-VEGF-D Tg mice clearly indicate that the increased preexisting lymphatic network and the increased lymphatic drainage were largely responsible for the reduced edema formation. The drainage-promoting function of VEGF-C/VEGF-D stimulation in our model is in agreement with recent findings that application of a soluble VEGFR-3 (which blocks both VEGF-C and VEGF-D from reaching their receptor on lymphatic endothelium) decreased lymph flow in a model of bacterial inflammation,48 whereas genetic overexpression of soluble VEGFR-3 in the skin of mice resulted in a lymphedema-like phenotype.24 Although not assessed in this study, it would be of interest to specifically knock out VEGFR-3 on adult lymphatic vessels and to compare the course of induced inflammation with normal wild-type mice, to further clarify the specific role of VEGFR-3 and its down-regulation in the acute inflammatory process.
In conclusion, our study provides the first evidence that specific lymphatic vessel activation limits acute skin inflammation via promotion of fluid drainage from the skin and reduction of edema formation, without major effects on inflammatory cell recruitment and the inflammatory cytokine milieu. Therefore, the role of lymphatic vessels in acute and chronic skin inflammation might differ in that lymphatic vessels also help resolving proinflammatory cells from the site of inflammation in the latter.12 Our findings that increased lymphatic function reduces inflammation-induced edema are indeed in line with the well-established clinical observation that patients with impaired lymphatic function (eg, in congenital or acquired lymphedemas of the extremities) are more prone to develop inflammatory skin reactions.50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jeannette Scholl and Cornelius Fischer for excellent technical assistance and Carlos Ochoa for help with the animal studies.
This work was supported by the National Institutes of Health (grant CA69184), Swiss National Science Foundation (grants 3100A0-108207 and 31003A-130627), Commission of the European Communities (grant LSHC-CT-2005-518178), Oncosuisse, and Krebsliga Zurich (M.D.).
National Institutes of Health
Authorship
Contribution: R.H. designed the research, performed experiments, analyzed results, and wrote the manuscript; S.S.S., D.B., S.U., and K.Z. performed experiments and analyzed results; M.A. performed experiments; S.W. designed the research and analyzed results; K.A. contributed material; and M.D. designed the research, analyzed results, and wrote the manuscript.
Conflict-of-interest disclosure: K.A. is the Chairman of the Scientific Advisory Council of Circadian Technologies. The remaining authors declare no competing financial interests.
Correspondence: Michael Detmar, Institute of Pharmaceutical Sciences, Swiss Federal Institute of Technology, ETH Zurich, Wolfgang Pauli-Str 10, HCI H303, CH-8093 Zurich, Switzerland; e-mail: michael.detmar@pharma.ethz.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal