Abstract
Viral persistence during chronic viral infections is associated with a progressive loss of T-cell effector function called functional exhaustion. There is therefore a need to develop immunotherapies to remediate the functional deficits of T cells during these infections. We investigated the immunotherapeutic effects of IL-7 during chronic lymphocytic choriomeningitis virus infection in mice. Our results showed that the effects of IL-7 on T cells depend on the viral load, timing, and duration of treatment during the course of the infection. We document that the effectiveness of IL-7 was constrained by high viral load early in the infection, but treatment for at least 3 weeks during declining viral titers mitigated the programmed contraction of CD8 T cells, markedly enhanced the number of high-quality polyfunctional virus-specific CD8 T cells with a nonexhausted phenotype, and accelerated viral control. Mechanistically, the enhancement of CD8 T-cell responses by IL-7 was associated with increased proliferation and induction of Bcl-2, but not with altered levels of PD-1 or Cbl-b. In summary, our results strongly suggest that IL-7 therapy is a potential strategy to bolster the quality and quantity of T-cell responses in patients with chronic viral infections.
Introduction
Acute viral infections in humans and mice often induce potent polyfunctional CD8 T-cell responses, and viral clearance occurs in 1-2 weeks.1 In contrast, in viruses such as HIV, hepatitis B virus, hepatitis C virus, or simian immunodeficiency virus (SIV) that establish chronic infections, strong CD8 T-cell responses are stimulated initially, but virus-specific CD8 T cells effect poor viral control and exhibit various degrees of functional exhaustion during the course of the infection.1 Functional exhaustion of CD8 T cells is characterized by a progressive decline in cytotoxicity and in the ability to produce cytokines such as IL-2, TNFα, and IFNγ.1 Mechanistically, functional exhaustion is multifactorial and can be attributed to inhibitory signaling cascades triggered by engagement of PD-1, LAG-3, TIM-3, IL-10 receptor (IL-10R), or TGF-β receptor (TGF-βR) on CD8 T cells.2-6 There is a need to develop broadly effective immunotherapeutic strategies to counteract multiple inhibitory pathways to remediate functional exhaustion of CD8 T cells in patients with chronic viral infections.
IL-7 is integral to T- and B-lymphocyte development in primary lymphoid organs and to homeostasis of T cells in the periphery.7-15 Therefore, IL-7 is one of the front-runners among cytokines currently being evaluated in human clinical trials for therapeutic potential as immune restorative or enhancing agents in lymphopenic patients with refractory malignancy, in patients after allogenic transplantation for nonlymphoid malignancy, or in those who are HIV-positive.16-19 Clinical trials are also in progress to determine the effectiveness of IL-7 therapy to accelerate viral clearance during chronic viral infections such as hepatitis C in humans.17 However, the effect of IL-7 therapy on T-cell responses or viral clearance in a preclinical tractable animal model of a chronic viral infection has not been studied, nor has the optimal IL-7 treatment regimen determined to treat a chronic viral infection in an experimental model of infection.
Studies using the mouse model of viral infection using lymphocytic choriomeningitis virus (LCMV) have provided seminal insights into the mechanisms that regulate CD8 T-cell responses during acute and chronic viral infections.1 Infection of immunocompetent mice with a rapidly replicating strain of LCMV (LCMV-Clone 13) establishes a chronic infection lasting up to 6 months20 due to ineffective CD8 T-cell responses. Signaling via the PD-1, IL-10R, and TGF-βR is known to promote functional exhaustion of CD8 T cells during a chronic LCMV infection.3,4,21 In a murine tumor model, IL-7 treatment has been demonstrated to counteract the inhibitory effects of TGF-βR signaling and to down-regulate PD-1 expression in activated CD8 T cells.22 Therefore, IL-7 has the potential to antagonize multiple pathways of functional exhaustion and to improve the number and/or function of CD8 T cells during a chronic LCMV infection. However, the effect of IL-7 therapy on viral control or functional exhaustion of CD8 T cells has not been examined. In this study, we determined the effect of IL-7 therapy on viral control and antigen-specific CD8 and CD4 T-cell responses to a chronic LCMV infection in mice. We show that IL-7 administration is an effective therapeutic strategy to expand the number of nonexhausted polyfunctional T cells and to accelerate viral clearance during a chronic viral infection. Furthermore, our results show that the viral load and the timing or duration of treatment dictate the effect of IL-7 therapy on the qualitative and quantitative aspects of virus-specific T cells during a chronic viral infection. These findings are expected to have implications in the therapeutic use of IL-7 to treat patients with chronic viral infections.
Methods
Mice
Six- to 8-week-old C57BL/6 mice were purchased from the National Cancer Institute and housed under conditions free of known rodent pathogens in the animal facility at the University of Wisconsin-Madison. Experiments were conducted in accordance with the approved protocols of the institutional animal care committee.
Virus
IL-7 treatment
Recombinant human IL-7 (kindly provided by Cytheris Inc) was diluted in sterile PBS and administered intraperitoneally daily at a dose of 5μg/mouse, as described previously.14
Flow cytometry
MHC I tetramers specific for Db-restricted LCMV epitopes were prepared and used as described previously.26 Single-cell suspensions of splenocytes were stained with MHC I tetramers in combination with antibodies against CD8, CD127, KLRG-1, PD-1, CD122, CD62L, CD43 (clone 1B11), LAG-3, and CD44. Staining for intracellular Bcl-2 and Cbl-b was as described previously.27 Staining with peptide-blocked anti–Cbl-b antibodies was equivalent to binding of anti–Cbl-b antibodies to CD8 T cells from Cbl-b–deficient mice.27 Antigen-induced CD107a expression on LCMV-specific CD8 T cells was assessed as described previously.28 Staining for Ki-67 was performed as described previously.29 To visualize LCMV-specific CD4 T cells, splenocytes were stained with I-Ab/GP66 MHC II tetramers (kindly provided by the National Institutes of Health Tetramer Facility, Atlanta, GA) at 37°C for 3 hours, followed by staining with anti-CD4 and anti-CD44 antibodies. All antibodies were purchased from BD Biosciences except anti-CD127, which was purchased from eBioscience. The cells were acquired using the FACSCalibur flow cytometer and data were analyzed using FlowJo software Version 8.4 (TreeStar).
Intracellular staining for cytokines
Splenocytes were stimulated with LCMV MHC I- or MHC II–restricted epitope peptides ex vivo at 37°C for 5 hours.26 After culture, cells were stained for cell-surface CD8 or CD4 and for intracellular IFNγ, TNFα, and IL-2 using the Cytofix/Cytoperm kit (BD Biosciences). The percentages of cytokine-producing cells were quantified by flow cytometry.
Statistical analysis
All data were analyzed using statistical analysis software (SYSTAT 10.2; Systat Software). Groups were compared using the 2-tailed Student t test.
Results
Effect of IL-7 treatment during the high-viremic phase on CD8 and CD4 T-cell responses to a chronic LCMV infection
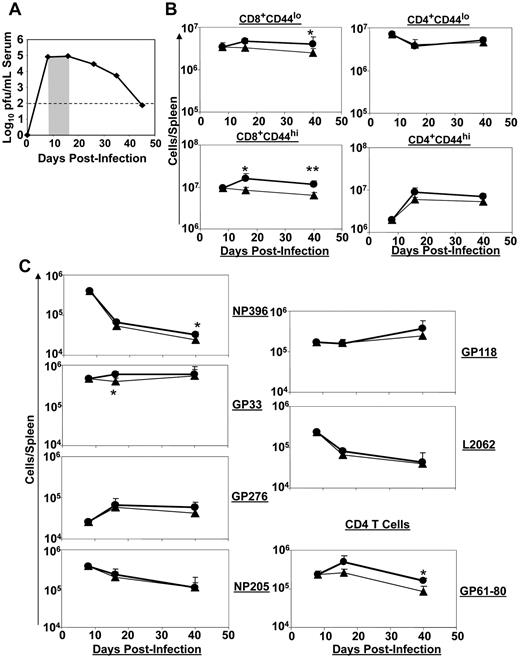
As shown in Figure 1A, infection of immunocompetent mice with LCMV-Clone 13 induces a chronic infection.30 Viral titers peak at day 8 postinfection (PI) and show a gradual decline after day 15 PI. A strong CD8 T-cell response is initially elicited in response to LCMV-Clone 13, which peaks at day 8 PI.31 However, in the ensuing 2-3 weeks, T cells undergo programmed contraction, and the high viral load induces various levels of functional exhaustion in the remaining virus-specific CD8 T cells.30-33 We evaluated the effect of IL-7 treatment on host response to a chronic LCMV infection when IL-7 was administered during the early phase of T-cell clonal contraction. Mice were infected with LCMV-Clone 13 and treated with PBS or IL-7 daily between days 8 and 15 PI, when the viral load continued to be high in the circulation (Figure 1A). T-cell responses were analyzed between days 8 and 40 PI (Figure 1B-C). The total numbers of naive or activated CD8 T cells remained relatively constant between days 8 and 40 PI in PBS-treated mice, but IL-7 administration led to a small but sustained increase in the number of naive and activated CD8 T cells in the same interval (Figure 1B). However, IL-7 treatment did not have discernible effects on naive or activated CD4 T cells (Figure 1B). Figure 1C shows the dynamics of LCMV epitope–specific CD8 and CD4 T-cell responses in PBS- and IL-7–treated mice. Between days 8 and 40 PI, there was a substantial decline in the number of NP396-, NP205-, and L2062-specific CD8 T cells, but the number of CD8 T cells that were specific to the other epitopes (GP33, GP276, and GP118) either increased or remained relatively constant during the same interval in PBS-treated mice. IL-7 administration did not significantly alter the magnitude or the dynamics of CD8 T-cell responses to MHC I–restricted epitopes of LCMV. Interestingly, IL-7 administration significantly (P < .05) enhanced the number of MHC II–restricted CD4 T-cell responses to the LCMV epitope GP61 (Figure 1C). Consistent with the lack of a substantive effect on LCMV-specific CD8 T-cell responses, IL-7 treatment did not affect serum viral titers at days 16 or 40 PI (data not shown). IL-7 treatment during the first 8 days PI only transiently increased the number of NP396-specific CD8 T cells, which are typically deleted during a chronic LCMV infection,34 and had no effect on viral titers (supplemental Figures 1-2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These results suggest that IL-7 therapy was ineffective when administered during the first 15 days after LCMV infection.
Effect of IL-7 treatment during early contraction phase on the CD8 T-cell response. Cohorts of LCMV-Clone 13–infected mice were treated daily with either IL-7 or PBS between days 8 and 15 PI, as depicted by the shaded area (A). At days 8 (before IL-7 therapy), 16 (1 day after completion of therapy), and 40 (25 days after cessation of IL-7 therapy) PI, the numbers of naive (CD44lo) and activated (CD44hi) CD8 or CD4 T cells (B) and LCMV epitope-specific, IFNγ-producing CD8 or CD4 T cells (C) in IL-7- (●) or PBS (▴)-treated mice were quantified by flow cytometry. Data for each time point were obtained from 4-5 mice per group. *P ≤ .05; **P ≤ .005.
Effect of IL-7 treatment during early contraction phase on the CD8 T-cell response. Cohorts of LCMV-Clone 13–infected mice were treated daily with either IL-7 or PBS between days 8 and 15 PI, as depicted by the shaded area (A). At days 8 (before IL-7 therapy), 16 (1 day after completion of therapy), and 40 (25 days after cessation of IL-7 therapy) PI, the numbers of naive (CD44lo) and activated (CD44hi) CD8 or CD4 T cells (B) and LCMV epitope-specific, IFNγ-producing CD8 or CD4 T cells (C) in IL-7- (●) or PBS (▴)-treated mice were quantified by flow cytometry. Data for each time point were obtained from 4-5 mice per group. *P ≤ .05; **P ≤ .005.
IL-7 treatment during declining viremia augments antigen-specific CD8 and CD4 T-cell responses to a chronic LCMV infection
Data presented in Figure 1 and supplemental Figures 1-2 indicated that treatment of mice with IL-7 under conditions of high viral load exerted minimal effects on virus-specific CD8 T-cell responses to LCMV-Clone 13. After day 15 PI, circulating levels of LCMV-Clone 13 showed a gradual decline (Figure 2A). Therefore, we examined the effect of IL-7 therapy between days 15 and 25 PI on T-cell responses to LCMV-Clone 13. Mice were infected with LCMV-Clone 13, and then IL-7 or PBS was administered between days 16 and 25 PI (Figure 2A). At different days PI, we quantified CD8 and CD4 T-cell responses to LCMV. IL-7 treatment induced a significant enhancement in the total number of activated CD8 and CD4 T cells in the spleen at day 26 PI (Figure 2B). The elevated numbers of activated CD8 T cells in IL-7–treated mice were maintained for at least 20 days after the termination of IL-7 treatment (day 45 PI) compared with those in PBS-treated mice. IL-7 treatment appeared to have little effect on the number of CD8 T cells specific to the immunodominant epitopes NP396, GP33, and GP276 (Figure 2C). Conversely, in the IL-7–treated mice, the numbers of CD8 T cells specific to the subdominant epitopes (L2062, GP118, and NP205) were significantly higher at day 26 PI, and the elevated number of these cells persisted for at least until another 20 days before declining to levels seen in PBS-treated mice at day 85 PI (Figure 2C). Likewise, IL-7 treatment led to a substantial increase in the number of cytokine-producing, LCMV-specific CD4 T cells at days 26 and 45 PI (Figure 2C). In summary, IL-7 treatment between days 16 and 25 PI delayed the contraction of CD4 T cells and CD8 T cells specific to the subdominant epitopes. Surprisingly, despite enhanced virus-specific T-cell responses, IL-7 therapy did not have a significant effect on viral titers in the serum (data not shown).
IL-7 therapy during the late contraction phase augments LCMV-specific T-cell responses. LCMV-Clone 13–infected mice were treated daily with either IL-7 or PBS between days 15 and 25 PI, as illustrated by the shaded area (A). At days 8, 26 (1 day after cessation of therapy), 45 (20 days after completion of IL-7 therapy), and 85 (60 days after IL-7 therapy) PI, CD8 and CD4 T-cell responses in the spleens of IL-7 (●)– or PBS (▴)–treated mice were quantified by flow cytometry. (B) The numbers of naive and activated CD8 or CD4 T cells. (C) LCMV epitope-specific CD8/CD4 T cells were quantified by intracellular staining for IFNγ. Data for each time point was obtained from 4-5 mice per group. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
IL-7 therapy during the late contraction phase augments LCMV-specific T-cell responses. LCMV-Clone 13–infected mice were treated daily with either IL-7 or PBS between days 15 and 25 PI, as illustrated by the shaded area (A). At days 8, 26 (1 day after cessation of therapy), 45 (20 days after completion of IL-7 therapy), and 85 (60 days after IL-7 therapy) PI, CD8 and CD4 T-cell responses in the spleens of IL-7 (●)– or PBS (▴)–treated mice were quantified by flow cytometry. (B) The numbers of naive and activated CD8 or CD4 T cells. (C) LCMV epitope-specific CD8/CD4 T cells were quantified by intracellular staining for IFNγ. Data for each time point was obtained from 4-5 mice per group. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
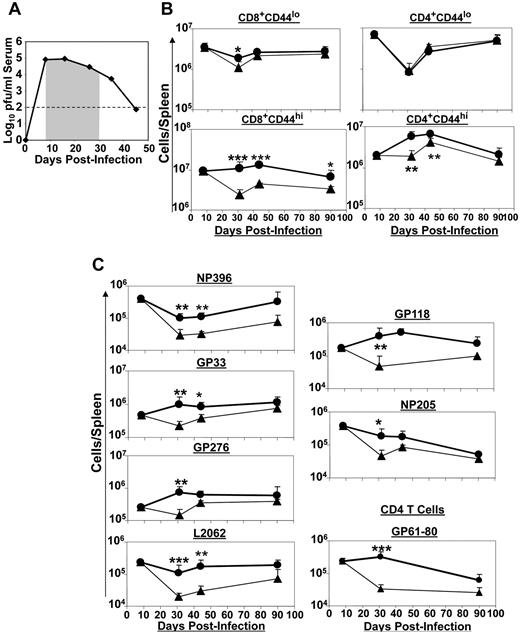
Extended duration of IL-7 treatment is required to enhance LCMV-specific CD8 and CD4 T cells during chronic LCMV infection
The data presented in Figures 1 and 2 showed that short-term IL-7 therapy might not enhance or sustain CD8 T-cell responses to effect improved viral control, especially when viral titers are high. Therefore, we investigated whether a longer duration of IL-7 treatment during the phase of clonal contraction might be necessary to effectively augment CD8 T-cell responses and improve viral control during a chronic LCMV infection. LCMV-Clone 13–infected mice were treated with either PBS or IL-7 daily between days 8 and 30 PI (Figure 3A), and T-cell responses were quantified at different days after IL-7 treatment. As shown in Figure 3B, the number of activated CD8 T cells declined after day 8 PI in PBS-treated mice, which is reminiscent of programmed contraction.32 However, the number of activated CD8 T cells did not noticeably decline between days 8 and 44 PI in IL-7–treated mice. Moreover, IL-7 administration led to a transient increase in the number of activated CD4 T cells compared with PBS-treated mice (Figure 3B). The kinetics of CD8 T-cell responses to MHC I–restricted epitopes in IL-7– and PBS–treated mice is shown in Figure 3C. In PBS-treated mice, the number of CD8 T cells specific to the majority of epitopes exhibited various levels of contraction between days 8 and 30 PI. In comparison, depending on the epitope specificity, CD8 T cells showed substantially reduced contraction (NP396 and NP205 specific) or no apparent contraction (for other CTL epitopes) in IL-7–treated mice. Consequently, there was a significant (P < .05) increase in the number of LCMV-specific CD8 T cells in the spleens of IL-7–treated mice at days 31 and 44 PI. By day 90 PI, differences in the number of CD8 T cells between IL-7– and PBS–treated mice were less remarkable. Similarly, IL-7 treatment delayed the contraction in the number of LCMV-specific CD4 T cells (Figure 3C). The data shown in Figure 3 suggested that supraphysiologic levels of IL-7 sustained virus-specific CD8 and CD4 T-cell responses, possibly by antagonism of cellular mechanisms that drive programmed contraction of CD8 T cells during a chronic LCMV infection.
Extended duration of IL-7 therapy during clonal contraction enhances the LCMV-specific T-cell response. Cohorts of LCMV-Clone 13–infected mice were treated daily with either IL-7 or PBS between days 8 and 30 PI, as illustrated by the shaded area (A). At days 8, 31, 44, and 90 PI, LCMV-specific CD8 and CD4 T-cell responses were quantified by flow cytometry. (B) The kinetics of naive and activated CD8 or CD4 T cells in IL-7– and PBS–treated mice. (C) LCMV epitope-specific CD8 and CD4 T cells were quantified by staining for intracellular IFNγ. Data for each time point was obtained from an analysis of 4-5 IL-7 (●)– or PBS (▴)–treated mice and are representative of 2 experiments. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
Extended duration of IL-7 therapy during clonal contraction enhances the LCMV-specific T-cell response. Cohorts of LCMV-Clone 13–infected mice were treated daily with either IL-7 or PBS between days 8 and 30 PI, as illustrated by the shaded area (A). At days 8, 31, 44, and 90 PI, LCMV-specific CD8 and CD4 T-cell responses were quantified by flow cytometry. (B) The kinetics of naive and activated CD8 or CD4 T cells in IL-7– and PBS–treated mice. (C) LCMV epitope-specific CD8 and CD4 T cells were quantified by staining for intracellular IFNγ. Data for each time point was obtained from an analysis of 4-5 IL-7 (●)– or PBS (▴)–treated mice and are representative of 2 experiments. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
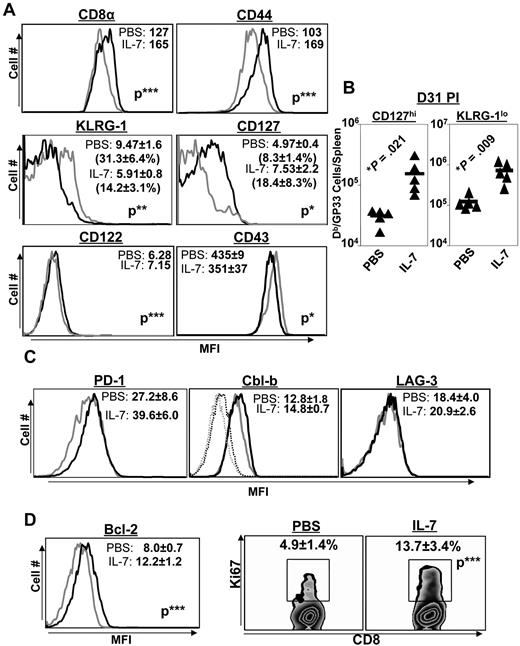
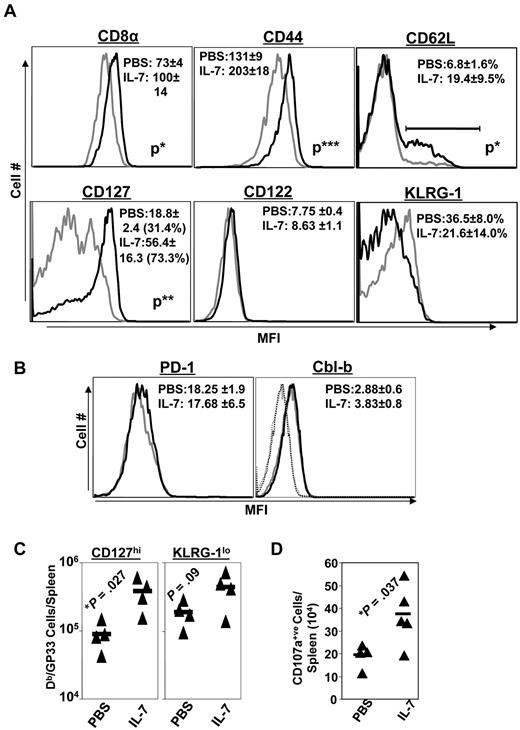
IL-7 treatment alters the differentiation of memory phenotype CD8 T cells
Functionally exhausted effector CD8 T cells in LCMV-infected mice exhibit phenotypic characteristics, including the high-level expression of the senescence marker KLRG-1, in association with diminished levels of cell-surface CD44 and the IL-7R CD127.34 To assess the effect of IL-7 on the phenotype of CD8 T cells, LCMV-Clone 13–infected mice were treated with either PBS or IL-7 daily between days 8 and 30 PI. In addition to the increase in the number of LCMV-specific CD8 T cells (Figure 3), IL-7 treatment induced marked alterations in the expression of key cell-surface molecules at day 31 PI. LCMV-specific CD8 T cells in IL-7–treated mice expressed higher levels of cell-surface CD8 and CD44 molecules than CD8 T cells from PBS-treated mice (Figure 4A). The data in Figure 4A also show that a substantially larger fraction of virus-specific CD8 T cells in IL-7–treated mice exhibited the “nonexhausted” phenotype (ie, expressed lower levels of KLRG-1 and elevated levels of CD127) compared with those in PBS-treated mice at day 31 PI. The absolute numbers of CD127hi or KLRG-1lo LCMV-specific CD8 T cells in the spleens of IL-7–treated mice were ∼ 5-fold (P < .05) greater than in the spleens of PBS-treated mice (Figure 4B). The data shown in Figure 4 suggest that IL-7 treatment promoted the development of nonsenescent, nonexhausted memory phenotype CD8 T cells during a chronic LCMV infection.
Extended duration of IL-7 treatment during the clonal contraction phase alters phenotypic attributes of LCMV-specific CD8 T cells. Mice were infected with LCMV-Clone 13 and treated with either IL-7 or PBS between days 8 and 30 PI, as described in Figure 3. At day 31 PI, splenocytes were stained with anti-CD8, anti-CD44, and Db/GP33 tetramers in combination with antibodies against KLRG-1, CD127, CD122, CD43, PD-1, and LAG-3 (A-C). Splenocytes were stained with anti-CD8, Db/GP33 tetramers, and antibodies against intracellular Bcl-2, Cbl-b, and Ki-67 molecules (D). Data were analyzed by flow cytometry, and the FACS plots are gated on tetramer-binding CD8 T cells. The numbers in panels A, C, and D are the mean fluorescence intensity (MFI) and/or percentages among tetramer-binding CD8 T cells. Data are from analysis of 4-5 IL-7–treated (black line) or PBS-treated (gray line) mice and are representative of 2 experiments. Stainings with anti–Cbl-b antibodies after incubation with the specific immunogenic Cbl-b peptide are shown as dotted lines. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
Extended duration of IL-7 treatment during the clonal contraction phase alters phenotypic attributes of LCMV-specific CD8 T cells. Mice were infected with LCMV-Clone 13 and treated with either IL-7 or PBS between days 8 and 30 PI, as described in Figure 3. At day 31 PI, splenocytes were stained with anti-CD8, anti-CD44, and Db/GP33 tetramers in combination with antibodies against KLRG-1, CD127, CD122, CD43, PD-1, and LAG-3 (A-C). Splenocytes were stained with anti-CD8, Db/GP33 tetramers, and antibodies against intracellular Bcl-2, Cbl-b, and Ki-67 molecules (D). Data were analyzed by flow cytometry, and the FACS plots are gated on tetramer-binding CD8 T cells. The numbers in panels A, C, and D are the mean fluorescence intensity (MFI) and/or percentages among tetramer-binding CD8 T cells. Data are from analysis of 4-5 IL-7–treated (black line) or PBS-treated (gray line) mice and are representative of 2 experiments. Stainings with anti–Cbl-b antibodies after incubation with the specific immunogenic Cbl-b peptide are shown as dotted lines. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
PD-1, LAG-3, and Cbl-b are known to promote functional exhaustion of CD8 T cells during a chronic LCMV infection.2,35,36 Because IL-7 has been reported to down-regulate the expression of PD-1 and Cbl-b,22 we assessed whether IL-7 treatment altered the expression of PD-1, LAG-3, and Cbl-b in LCMV-specific CD8 T cells. Figure 4C shows that the expression of PD-1, LAG-3, and Cbl-b in LCMV-specific CD8 T cells from IL-7–treated mice was comparable to that in PBS-treated mice. Therefore, IL-7 did not alter the expression of PD-1, LAG-3, or Cbl-b in LCMV-specific CD8 T cells during a chronic LCMV infection.
The sustenance of CD8− and CD4− T-cell responses in IL-7–treated mice could have resulted from increased proliferation and/or reduced apoptosis.37 To address these possibilities, we examined whether IL-7 treatment altered the proliferation or expression of the prosurvival molecule Bcl-2 at day 31 PI in LCMV-specific CD8 T cells. Not only did LCMV-specific CD8 T cells from IL-7–treated mice expressed elevated levels of intracellular Bcl-2, a larger proportion of these cells was Ki67-positive compared with those in PBS-treated mice (Figure 4D). These data suggested that IL-7 might have increased the number of LCMV-specific CD8 T cells by enhancing proliferation and/or by reducing cellular apoptosis.
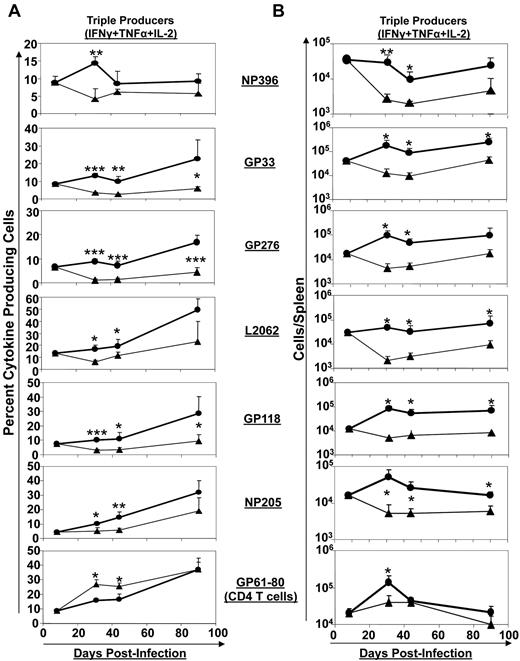
Extended duration of IL-7 treatment augments polycytokine production and promotes the development of memory phenotype CD8 T cells
Depending on the magnitude of the viral load, the availability of CD4 T-cell help, and the induction or lack of cytokines such as TGF-β, IL-10, and IL-21, CD8 T cells in LCMV-Clone 13–infected mice fail to elaborate their full complement of effector functions and exhibit graded levels of functional exhaustion, which distinguishes these cells from the polyfunctional effector CD8 T cells that are induced in acute viral infections.3,4,38-40 Typically, infection of immunocompetent mice with LCMV-Clone 13 results in “partial exhaustion,” in which most virus-specific CD8 T cells produce IFNγ, albeit at reduced levels; however, depending on the viral load, these cells might also lose their capacity to produce TNFα and IL-2 in response to antigenic stimulation.41 Because IL-7 treatment has been reported to inhibit several negative regulators of T-cell function,22 we investigated whether IL-7 administration (days 8-30 PI) affected the cytokine-producing ability of CD8 T cells during a chronic LCMV infection. The percentages of epitope-specific CD8 T cells that produced IFNγ, TNFα, and IL-2, that is, triple cytokine producers, at different days after LCMV infection are shown in Figure 5A. A very small percentage (< 10%) of LCMV-specific CD8 T cells produced all 3 cytokines at day 8 PI, but the fraction of triple cytokine–producing CD8 T cells (specific to certain epitopes) gradually increased with time (Figure 5A). In PBS-treated mice, the triple cytokine–producing cells constituted an average of only 4.4% of cells among epitope-specific CD8 T cells (regardless of epitope specificity) at days 31 and 44 PI. The percentages of triple cytokine–producing CD8 T cells in the spleens of IL-7–treated mice were significantly (∼ 3-fold; P < .05) higher than in PBS-treated mice at days 31 and 44 PI (Figure 5A). Figure 5B illustrates the kinetics of triple cytokine–producing CD8 T cells in PBS– and IL-7–treated mice. The data shown in Figure 5B show a precipitous decrease in the numbers of triple cytokine–producing CD8 T cells in PBS-treated mice after day 8 PI. Strikingly, in IL-7–treated mice, depending on the epitope specificity, the numbers of triple cytokine–producing CD8 T cells were either sustained or significantly (P < .005) elevated after day 8 PI. These data suggested that IL-7 supplementation promoted the expansion and/or maintenance of polycytokine-producing CD8 T cells during a chronic LCMV infection.
Extended duration of IL-7 treatment during the clonal contraction phase results in durable enhancement of the quality of LCMV-specific CD8 T cells. Mice were infected with LCMV-Clone 13 and treated with either IL-7 (●) or PBS (▴) between days 8 and 30 PI, as described in Figure 3. At days 8, 31, 44, and 90 PI, LCMV-specific CD8− and CD4− T-cell responses in the spleen were assessed by flow cytometry. LCMV epitope–specific, triple cytokine (IFNγ, TNFα, and IL-2)–producing cells were enumerated by intracellular staining (A-B). (A) The percentages of triple cytokine–producing cells of epitope-specific CD8 or CD4 T cells at different days PI. (B) The total numbers of triple cytokine–producing epitope-specific CD8 or CD4 cells. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
Extended duration of IL-7 treatment during the clonal contraction phase results in durable enhancement of the quality of LCMV-specific CD8 T cells. Mice were infected with LCMV-Clone 13 and treated with either IL-7 (●) or PBS (▴) between days 8 and 30 PI, as described in Figure 3. At days 8, 31, 44, and 90 PI, LCMV-specific CD8− and CD4− T-cell responses in the spleen were assessed by flow cytometry. LCMV epitope–specific, triple cytokine (IFNγ, TNFα, and IL-2)–producing cells were enumerated by intracellular staining (A-B). (A) The percentages of triple cytokine–producing cells of epitope-specific CD8 or CD4 T cells at different days PI. (B) The total numbers of triple cytokine–producing epitope-specific CD8 or CD4 cells. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
The beneficial effects of IL-7 administration on the cell-surface characteristics of LCMV-specific CD8 T cells observed at day 31 PI (Figure 4A) were largely preserved until at least 90 days PI, 60 days after the cessation of IL-7 treatment (Figure 6A). Interestingly, LCMV-specific CD8 T cells in IL-7–treated mice continued to express elevated levels of cell-surface CD8 and CD44 molecules compared with those in PBS-treated mice (Figure 6A). In addition, a substantially larger fraction of virus-specific CD8 T cells in IL-7–treated mice expressed high levels of CD127 and low levels of KLRG-1 than those in PBS-treated mice. Remarkably, IL-7 treatment led to a significant increase in the total numbers of CD127hi and KLRG-1lo LCMV-specific CD8 T cells during a chronic LCMV infection (Figure 6C). Loss of polyfunctionality coupled with low-level expression of CD127 is considered a signature feature of functionally exhausted CD8 T cells.20 In addition, the spleens of IL-7–treated mice contained substantially greater numbers of virus-specific CD8 T cells capable of degranulation and cytotoxicity (Figure 6D). The data shown in Figures 5B and 6A through D suggested that IL-7 treatment during a chronic LCMV infection promoted the development of functionally competent, nonexhausted CD8 T cells. Moreover, the percentages of LCMV-specific CD8 T cells expressing higher levels of CD62L (central memory phenotype) in IL-7–treated mice were substantially higher than in PBS-treated mice (Figure 6A). In summary, the increased numbers of CD62hi/KLGR-1lo/CD127hi LCMV-specific CD8 T cells in IL-7–treated mice indicated that IL-7 supplementation might affect the differentiation of effector CD8 T cells and augment the number of polyfunctional CD8 T cells during a chronic LCMV infection. However, the enhancement in the number of LCMV-specific CD8 and CD4 T cells by IL-7 was not associated with alterations in the expression of PD-1 or Cbl-b (Figure 6B).
Extended duration of IL-7 treatment during the clonal contraction phase alters the phenotypic attributes of LCMV-specific CD8 T cells. Mice were infected with LCMV-Clone 13 and treated with either IL-7 or PBS between days 8 and 30 PI, as described in Figure 3. At day 90 PI, splenocytes were stained with anti-CD8, anti-CD44, anti-CD122, anti-CD127, anti-CD62L, anti-KLRG-1, anti–Cbl-b, anti-PD-1, and Db/GP33 tetramers (A-C). (D) At day 44 PI (14 days after cessation of IL-7 therapy), splenocytes were incubated with anti-CD107a (anti–LAMP-1) and DbGP33 tetramer and stimulated with LCMV-specific cognate peptide for 1 hour at 37°C. After incubation, cells were washed and stained with anti-CD8 antibody. Data were analyzed by flow cytometry, and FACS plots were gated on tetramer-binding CD8 T cells. The numbers in panels A and B are the mean fluorescence intensity (MFI) and/or percentages of tetramer-binding CD8 T cells. Data are from an analysis of 4-5 IL-7–treated (black line) or PBS-treated (gray line) mice. Stainings with anti–Cbl-b antibodies preincubated with the specific immunogenic Cbl-b peptide are shown as dotted lines. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
Extended duration of IL-7 treatment during the clonal contraction phase alters the phenotypic attributes of LCMV-specific CD8 T cells. Mice were infected with LCMV-Clone 13 and treated with either IL-7 or PBS between days 8 and 30 PI, as described in Figure 3. At day 90 PI, splenocytes were stained with anti-CD8, anti-CD44, anti-CD122, anti-CD127, anti-CD62L, anti-KLRG-1, anti–Cbl-b, anti-PD-1, and Db/GP33 tetramers (A-C). (D) At day 44 PI (14 days after cessation of IL-7 therapy), splenocytes were incubated with anti-CD107a (anti–LAMP-1) and DbGP33 tetramer and stimulated with LCMV-specific cognate peptide for 1 hour at 37°C. After incubation, cells were washed and stained with anti-CD8 antibody. Data were analyzed by flow cytometry, and FACS plots were gated on tetramer-binding CD8 T cells. The numbers in panels A and B are the mean fluorescence intensity (MFI) and/or percentages of tetramer-binding CD8 T cells. Data are from an analysis of 4-5 IL-7–treated (black line) or PBS-treated (gray line) mice. Stainings with anti–Cbl-b antibodies preincubated with the specific immunogenic Cbl-b peptide are shown as dotted lines. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
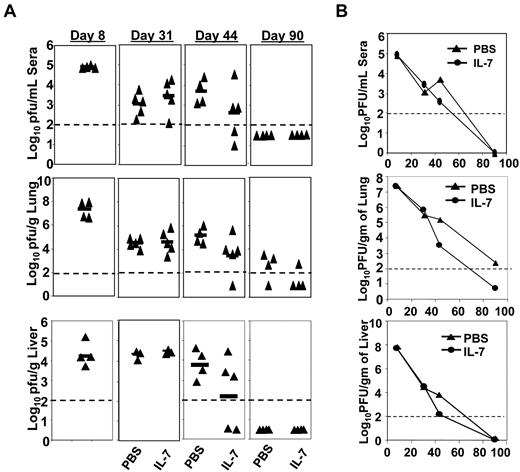
Extended IL-7 therapy accelerates viral clearance
The data shown in Figures 4–5 show that IL-7 therapy enhanced the quantity and quality of virus-specific CD8 T cells during a chronic LCMV infection. Therefore, we examined the therapeutic effects of IL-7 on viral control. Figure 7 shows the kinetics of viral clearance in PBS– and IL-7–treated mice in the serum, lungs, and liver. Viral titers in serum, lungs, and liver at days 8 and 31 PI were comparable between PBS– and IL-7–treated mice. Fifteen days after the cessation of IL-7 therapy (day 44), the serum, lungs, and liver of all PBS–treated mice contained high levels of infectious LCMV. Conversely, in 4 of 5 mice treated with IL-7, the viral titers in the serum and liver were ∼10-fold lower than in PBS-treated mice or were below the level of detection (Figure 7A). At day 90 PI, whereas 75% of PBS-treated mice harbored LCMV in the lungs, viral titers in the lungs of 75% of IL-7–treated mice were below the level of detection (Figure 7A). Although the differences in viral titer between PBS– and IL-7–treated groups at days 44 and 90 PI were not statistically significant, these data suggest that IL-7 therapy might accelerate viral clearance during a chronic LCMV infection.
Extended duration of IL-7 treatment after the clonal expansion phase promotes accelerated viral clearance. Mice were infected with LCMV-Clone 13 and treated with either IL-7 or PBS as described in Figure 3. At days 8, 31, 44, and 90 PI, the virus titers in tissues of IL-7–treated or PBS-treated mice were quantified by plaque assay. Panel A shows virus titers in serum, lungs, and liver at different days PI in PBS- or IL-7–treated mice, and panel B illustrates the overall kinetics of viral clearance.
Extended duration of IL-7 treatment after the clonal expansion phase promotes accelerated viral clearance. Mice were infected with LCMV-Clone 13 and treated with either IL-7 or PBS as described in Figure 3. At days 8, 31, 44, and 90 PI, the virus titers in tissues of IL-7–treated or PBS-treated mice were quantified by plaque assay. Panel A shows virus titers in serum, lungs, and liver at different days PI in PBS- or IL-7–treated mice, and panel B illustrates the overall kinetics of viral clearance.
Discussion
Viruses use several immune evasion mechanisms to establish chronic infections in mice and humans,1,42 including deletion and/or functional exhaustion of virus-specific CD8 T cells.41 Therefore, there is considerable interest in developing effective immune therapies to enhance T-cell responses in patients with chronic viral infections. Suppression of CD8 T-cell responses during chronic viral infections is multifactorial in causation and involves diverse cellular mechanisms, including induction of immunosuppressive cytokines (TGF-β and IL-10), expression of inhibitory receptors (PD-1, LAG-3, and TIM-3) on T cells, and deficiency of cytokines such as IL-21.3,4,26,38,40 IL-7 is a unique cytokine that has the potential to down-regulate several of the cellular pathways known to suppress CD8 T-cell responses during chronic viral infections.22 In the present study, we documented that IL-7 treatment effectively enhanced the number of polyfunctional virus-specific T cells and expedited viral control in mice undergoing a chronic LCMV infection. We also found that viral load and the duration and timing of IL-7 administration were critical factors that influenced the therapeutic efficacy of IL-7 therapy. These findings have significant implications in the clinical use of IL-7 to treat patients with chronic viral infections.
We also showed that IL-7 treatment during the first 15 days PI increased the numbers of LCMV-specific CD8 T cells, albeit in the short term, with no effect on LCMV control. However, there was a noticeable improvement in the immune-enhancing effects of IL-7 when administered between days 15 and 25 PI. LCMV-Clone 13–infected animals harbored a very high viral load in the first 15 days PI, and viral titers gradually receded thereafter (Figure 1A). The modest effects of IL-7 on T-cell responses during the first 15 days PI could have been linked to the high viral load and antigen-triggered down-regulation of IL-7R expression. With reducing viral load and reexpression of the IL-7R, at least a subset of T cells might have become sensitive to the effects of IL-7. Indeed, IL-7 treatment for at least 3 weeks under conditions of decreasing viral load potently augmented the proportions of IL-7R (CD127hi)–expressing, LCMV-specific CD8 T cells. Therefore, we propose that down-regulation of the IL-7R induced by high viral load might pose a significant constraint on the effects of IL-7 on T-cell responses. One plausible strategy to improve the immunotherapeutic benefits of IL-7 under conditions of high viral load is to combine IL-7 administration with an antiviral drug such as ribavirin.43 It has been reported that LCMV-Clone 13 might dampen antiviral T-cell responses by inducing immunosuppressive cytokines such as IL-10 and TGF-β, especially early in the infection.3,4 Therefore, it is also possible that IL-7 therapy might not be able to overcome the immunosuppressive effects of IL-10 and/or TGF-β, at least early in the infection. Anther possible scenario is that under conditions of high viral load and intense antigenic stimulation, augmentation of TCR signaling by concomitant signaling via the IL-7R might enhance proliferation, but may also lead to activation-induced T cell death, culminating in no net increase in the number of LCMV-specific CD8 T cells.
Treatment of mice with IL-7 between days 15 and 25 PI had a more pronounced effect on CD8 T cells specific to the LCMV-subdominant epitopes (L2062, GP118, and NP205) and less impressive effects on the number of CD8 T cells reacting with immunodominant epitopes (Figure 2). The mechanism(s) underlying the potent effect of IL-7 on subdominant epitopes is unclear. It has been reported that IL-7 enhanced CD8 T-cell responses to subdominant epitopes in a murine tumor model.44 We theorize that, despite declining viral load, high numbers of immunodominant epitope–specific CD8 T cells outcompete the less frequent subdominant epitope–specific CD8 T cells for interactions with peptide/MHC complexes on APCs, thus continuing to receive strong antigenic stimulation and expressing lower levels of IL-7R. Conversely, subdominant epitope–specific CD8 T cells might receive lower levels of antigenic stimulation and thus maintain sufficient levels of IL-7R expression and remain receptive to IL-7–mediated effects.
Our studies show that daily administration of IL-7 for 3 weeks exerted a multitude of effects on virus-specific CD8 and CD4 T cells. First, IL-7 therapy prevented the decline in the number of LCMV-specific CD8 T cells after day 8 PI. Consequently, a larger number of LCMV-specific CD8 T cells were maintained during a chronic LCMV infection. The number of CD8 or CD4 T cells at a given time point during a chronic LCMV infection is dictated by the rate of cellular proliferation and apoptosis. High doses of IL-7 have been shown to drive T-cell proliferation by down-regulating the cell-cycle inhibitor p27Kip1.45 As evident by higher Ki67 expression, a substantially larger fraction of LCMV-specific CD8 T cells were in the active cycle in IL-7–treated mice than in PBS-treated mice. Therefore, increased cellular proliferation induced by IL-7 might be responsible, at least in part, for the increase in the number of LCMV-specific CD8 T cells. However, we cannot exclude the possibility that reduced cellular apoptosis also contributed to the increase in the number of LCMV-specific CD8 and CD4 T cells in IL-7–treated mice.22 IL-7 might enhance T-cell proliferation by several mechanisms. Signaling by the IL-7R is known to trigger the PI3-K pathway, which leads to phosphorylation of AKT and its downstream substrates GSK3β, FOXO1, and FOX03; phosphorylation of FOXO1 and FOXO3 in turn leads to down-regulation of p27Kip1 expression and cell-cycle entry.45 In addition, IL-7 might have enhanced the proliferation of LCMV-specific CD8 T cells by rendering these cells refractory to the inhibitory effects of cytokines such as TGF-β,22 which is known to suppress CD8 T-cell responses to LCMV-Clone 13.3
Functional exhaustion of CD8 T cells during a chronic viral infection is associated with low-level expression of cell-surface CD127 and CD44, and reduced or complete loss of the ability to produce cytokines such as IFNγ, TNFα, and IL-2.2,31 Our studies show that in LCMV-infected mice treated with IL-7, a substantially larger fraction of virus-specific CD8 T cells expressed high levels of CD8, CD127, and CD44, coupled with a marked increase in the number of CD8 T cells that produced IFNγ, TNFα, and IL-2 at day 90 PI. The cell-surface expression of KLRG-1 in CD8 T cells has been associated with cellular senescence or terminal differentiation.46 Interestingly, the percentages of KLRG-1hi cells among LCMV-specific CD8 T cells were considerably reduced in IL-7–treated mice. These data suggest that IL-7 treatment increased the number of functionally competent, nonsenescent, virus-specific CD8 T cells in LCMV-infected mice. Functional exhaustion of CD8 T cells has been linked to the expression of a panel of inhibitory molecules, including PD-1 and LAG-3, during a chronic viral infection.2,35 Moreover, the E3 ubiquitin ligase Cbl-b has been implicated in the causation of CD8 T-cell functional exhaustion during LCMV infection.36 Although IL-7 treatment has been reported to reduce PD-1 and Cbl-b expression in CD8 T cells in tumor-bearing mice,22 our studies show that IL-7 administration did not alter PD-1 or Cbl-b levels in virus-specific CD8 T cells in LCMV-Clone 13–infected mice. More detailed studies are warranted to determine whether IL-7 enhances the number of functionally competent, nonexhausted CD8 T cells by affecting PD-1 and/or Cbl-b. It is possible that IL-7 enhances the number of polyfunctional CD8 T cells by modulating mechanisms that are independent of PD-1 or Cbl-b. In that case, IL-7 treatment and PD-1 blockade would be expected to have additive effects in enhancing T-cell responses to LCMV-Clone 13.
The precise mechanism(s) underlying the accelerated viral control in IL-7–treated mice is unclear. Both CD8 and CD4 T cells are essential for effective control of LCMV-Clone 13, and the presence of greater number of polyfunctional CD8 and CD4 T cells in IL-7–treated mice were strongly correlated with accelerated viral control. We propose that the increased number of functional LCMV-specific CD8 T cells mediated enhanced viral control by cell-mediated cytotoxicity and/or by production of effector cytokines such as IFN-γ and TNF. The increased number of LCMV-specific CD4 T cells may have contributed to improved viral control by fostering the maintenance of functional CD8 T cells and/or by direct antiviral effects.
In summary, the present study has identified IL-7 as an effective therapeutic agent to augment the quantity and quality of T-cell responses to a chronic viral infection. Specifically, we showed that IL-7 therapy administered for at least 3 weeks led to a durable increase in the number of polyfunctional CD8 T cells with a “nonexhausted” phenotype and promoted viral clearance. Moreover, our studies emphasize the critical importance of the timing and duration of IL-7 therapy to achieve the desired immunotherapeutic effects during a chronic viral infection. These findings pave the way for the clinical use of IL-7 to treat humans with chronic viral infections or cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Erin Plisch for excellent technical assistance and Jane Walent for making the MHC I tetramers.
This work was supported by Public Health Service grants AI48785, AI59804, and AI68841 to M.S.
National Institutes of Health
Authorship
Contribution: S.G.N conducted and analyzed the experiments; E.H.K. conducted experiments; and S.G.N. and M.S. designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr M. Suresh, Department of Pathobiological Sciences, University of Wisconsin-Madison, 2015 Linden Dr, Madison, WI 53706; e-mail: sureshm@svm.vetmed.wisc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal