Abstract
SIRT1 is a founding member of a sirtuin family of 7 proteins and histone deacetylases. It is involved in cellular resistance to stress, metabolism, differentiation, aging, and tumor suppression. SIRT1−/− mice demonstrate embryonic and postnatal development defects. We examined hematopoietic and endothelial cell differentiation of SIRT1−/− mouse embryonic stem cells (ESCs) in vitro, and hematopoietic progenitors in SIRT1+/++/−, and −/− mice. SIRT1−/− ESCs formed fewer mature blast cell colonies. Replated SIRT1−/− blast colony-forming cells demonstrated defective hematopoietic potential. Endothelial cell production was unaltered, but there were defects in formation of a primitive vascular network from SIRT1−/−-derived embryoid bodies. Development of primitive and definitive progenitors derived from SIRT1−/− ESCs were also delayed and/or defective. Differentiation delay/defects were associated with delayed capacity to switch off Oct4, Nanog and Fgf5 expression, decreased β-H1 globin, β-major globin, and Scl gene expression, and reduced activation of Erk1/2. Ectopic expression of SIRT1 rescued SIRT1−/− ESC differentiation deficiencies. SIRT1−/− yolk sacs manifested fewer primitive erythroid precursors. SIRT1−/− and SIRT1+/− adult marrow had decreased numbers and cycling of hematopoietic progenitors, effects more apparent at 5%, than at 20%, oxygen tension, and these progenitors survived less well in vitro under conditions of delayed growth factor addition. This suggests a role for SIRT1 in ESC differentiation and mouse hematopoiesis.
Introduction
Mouse embryonic stem cells (ESCs) are pluripotent with capacity for unlimited self-renewal or differentiation into endoderm, ectoderm, and mesoderm. Self-renewal behavior in vitro is sustained with leukemia inhibitory factor (LIF).1 With removal of LIF and in the absence of feeder layer cells, ESCs grow into spheres termed embryoid bodies (EBs), which generate hematopoietic and endothelial progeny recapitulating development of those populations in the yolk sac.2 Hemangioblasts generate blast colonies in vitro displaying hematopoietic and endothelial potential.3 The ESC/EB system provides a powerful in vitro model to explore cellular and molecular events that specify lineage choice and hematopoietic commitment.
Sirtuins, or Sir2 family proteins, are conserved from bacteria to humans.4 Sir2 modulates longevity and aging in yeast, Caenorhabditis elegans, and Drosophila.5 Mammalian homologs of Sir2 encompass a family of 7 proteins (SIRT1-SIRT7), among which SIRT1 is the closest human homolog of the yeast Sir2 protein.4 SIRT1 deacetylates proteins, including p53 and FOXO transcription factors, and plays many key functions including energy metabolism, differentiation, aging, and tumor suppression.6-10 SIRT1 is expressed at high levels in mouse embryos with the highest SIR2α mRNA expression is embryonic day (E) 4.5 embryos. Although expression is down-regulated during subsequent embryogenesis, high level expression remains detectable at E18.5.11
A number of SIRT1-deficient (−/−; knockout) mouse strains have been developed. Mice carrying a truncation mutation through targeted replacement of exons 5 and 6 with a hygromycin gene demonstrate 50% mortality at an early postnatal stage.12 Up to 90% of SIRT1 mutant mice carrying a deletion of exon 4 died perinatally, exhibiting developmental defects of retina and heart, the remaining 10% of these mutants still surviving at weaning.12-14 In another study,10 a majority of SIRT1−/− mice died at E9.5-E14.5; ∼ 1% of the mutants on the 129SvEv/FVB background and 9.3% on the 129SvEv/FVB/Black Swiss background survived to adulthood.10 These studies suggest that SIRT1 is important in embryonic development. Thus, functions of SIRT1 are worth investigating to better understand mammalian embryogenesis.
Endothelial and hematopoietic lineages develop in parallel and are most intertwined in yolk sac blood islands and the dorsal aorta in the embryo.15,16 In both the yolk sac and embryonic dorsal aorta, blood vessels give rise to the hematopoietic elements via specialized hemogenic endothelium.17 Although SIRT1 regulates vascular endothelial homeostasis controlling angiogenesis, vascular tone, and endothelial dysfunction,18,19 little information is available on SIRT1 activities in embryonic and adult stages of hematopoietic and endothelial development.
We demonstrated that SIRT1 regulates apoptosis and Nanog expression in mESCs in the presence of LIF and 2-mercaptoethanol-withdrawal–induced increase in reactive oxygen species by controlling p53 subcellular localization.20 Herein, we evaluated the effect of SIRT1−/− on differentiation of mESCs in the presence of 2-mercaptoethanol, but absence of LIF, and report a number of delays and/or defects, especially in hematopoietic development. Hematopoietic progenitor cell defects were also apparent in embryonic yolk sac and young (5 week) and adult (> 1 year) bone marrow from SIRT1−/− mice. Differences in growth of hematopoietic progenitor cells from EBs or adult marrow in SIRT1+/+ and SIRT1−/− cells were much greater and in some cases only apparent when cells were cultured under low (5% O2) oxygen tension compared with normoxia. This demonstrates a role for SIRT1 as a modulator for mouse ESC differentiation and early and later hematopoietic progenitor cell growth in mice.
Methods
ESC culture
Mouse ESC (mESC) line R1 and SIRT1−/− mESC derived from R1 parental cells (gift from Dr McBurney, Ottawa Health Research Institute, Ottawa, ON) were cultured21 on irradiated mouse embryonic fibroblasts in Dulbecco modified Eagle medium with 15% fetal bovine serum (FBS; Hyclone Laboratories), 1000 U/mL LIF (Chemicon International), 10 U/μL penicillin/streptomycin (Invitrogen), L-glutamine 200mM (100 ×; Gibco, Invitrogen), 0.1mM nonessential amino acids (Invitrogen), and 0.1mM β-mercaptoethanol (Invitrogen) at 37°C /5% CO2. Media was changed daily, and cultures passaged every 2 to 3 days.
Progenitor cell assays
Primitive erythroid and definitive progenitors generated from mESCs.
EBs were digested with 0.25% trypsin (Gibco, Invitrogen), passaged through a 20-gauge needle 2-3 ×, and plated at 25 000 cells/mL with 5 U/mL erythropoietin (EPO; R&D Systems), 100 ng/mL stem cell factor (SCF; R&D Systems), and 1 ng/mL interleukin-3 (IL-3; R&D Systems) for definitive erythroid progenitors, or with 5 U/mL EPO, 100 ng/mL SCF, 1 ng/mL IL-3, 10 ng/mL granulocyte-macrophage colony-stimulating factor (R&D Systems), and 5 ng/mL macrophage colony-stimulating factor (R&D Systems) for multipotential and granulocyte-macrophage progenitors. For primitive erythroid progenitors, cells were plated with 15% plasma-derived serum (Animal Technologies) and 5 U/mL EPO.22 Cells were cultured under normoxia (∼20% O2) and low (5%) O2.
Primitive erythroid yolk sac (E8-E8.25) progenitors.
Treatment with 0.25% collagenase (Sigma-Aldrich) plus 20% fetal calf serum for 10 mintues at 37°C with vigorous pipeting resulted in single cells that were plated with 15% plasma-derived serum (Animal Technologies) and 5 U/mL EPO. Colonies were counted at day 5.22 Embryos were generated as described.10
Adult marrow hematopoietic progenitors.
Bone marrow nucleated cells (5 × 104) were plated onto 35-mm Petri dishes in methylcellulose with 30% FBS (Hyclone), 1 U/mL human EPO, 5% pokeweed mitogen mouse spleen cell conditioned medium, and 50 ng/mL murine SCF.23 Colonies were scored after 7 days at ∼ 20% or 5% O2. Mating of mice were conducted as described.10
BL-CFC assays
EBs were collected at different days postdifferentiation in liquid culture, washed in phosphate-buffered saline, and treated with 0.25% trypsin for 3 minutes at 37°C. EBs were dissociated into single cells by passage 2-3 times through a 23-gauge needle and plated at 3 × 104 cells in 1 mL methycellulose medium with 10% FBS, 100 ng/mL SCF, 25% D4T endothelial cell-conditioned medium,24 5 ng/mL mouse vascular endothelial growth factor (VEGF; R&D Systems), 10 ng/mL human IL-6 (R&D Systems), and Iscove modified Dulbecco medium (IMDM). Cells were maintained at 37°C in a humidified 5% CO2 incubator under normoxia. After 3-4 days, developing Blast colony-forming cells (BL-CFCs) were counted.
Generation of hematopoietic and endothelial cells from BL-CFCs
Individual BL-CFCs were picked and transferred to Matrigel-coated (BD Biosciences) 96-well plates containing IMDM supplemented with 10% plasma-derived serum, 10% horse serum (Invitrogen), 150 μg/mL transferrin, 5 ng/mL VEGF, 10 ng/mL insulin-like growth factor 1 (Peprotech), 2 U/mL EPO, 10 ng/mL basic fibroblast growth factor (bFGF; Peprotech), IL-11 (50 ng/mL; R&D Systems), SCF (100 ng/mL), l-glutamine (2mM), and 4.5 × 10−4 monothioglycerol. After 4 days in culture, nonadherent cells of each well were harvested and cultured in 1 mL methycellulose containing the above mixture of cytokines used for growth of hematopoietic progenitors. Adherent cells were cultured an additional 1-2 weeks in IMDM with 10% fetal calf serum, 10% horse serum, 5 ng/mL VEGF, 10 ng/mL insulin-like growth factor 1, 10 ng/mL bFGF, 100 μg/mL endothelial cell growth supplement, 2mM l-glutamine, and 4.5 × 10−4 monothioglycerol.3
Immohistochemistry
Staining for endothelial cells derived from EBs was as described,25 except that we stained for 10 μg/mL Dil-Ac-LDL (BD Biosciences) and CD144 (BD Pharmingen) antibodies. Fluorescence was visualized using an Olympus FV1000 MPE fluorescence microscope (Olympus America). Images were taken with a 60× water (numeric aperature 1.2) objective lens and analyzed by Olympus Viewer 2.0C.
Vascular sprout formation assay
Flow cytometric analysis for surface markers
Staining was conducted as described.27 Analyses were performed with FACSCalibur (Becton Dickinson). Monoclonal antibodies used were ckit-APC (2B8; eBioscience) and Flk-1-PE (BD Bioscience).
Real-time PCR analysis
An RT2 Profile Custom PCR Array was used to simultaneously examine mRNA levels of 48 genes, including 3 housekeeping genes in 96-well plates according to the manufacturer (SuperArray Bioscience). Real-time polymerase chain reaction (PCR) was performed on an MX30000P Stratagene machine with SYBR Green PCR Master Mix (Superarray). PCR conditions consisted of a 10 minutes hot start at 95°C, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. The average threshold cycle for each gene was determined from triplicate reactions and 3 independent experiments. Values were exported to a template Excel file for analysis. Analyses of raw data were done through the Superarray Data Analysis Web Portal (http://www.sabiosciences.com/pcr/arrayanalysis.php).
Western blot analysis
Protein extracts from 2 × 106 mESCs were prepared, and immunoblots were performed according to standard techniques. To determine whether amounts of protein in each lane were comparable, the membrane was cut at approximately the expected molecular weight of target proteins and β-actin. The cut membranes were respectively probed with antibodies against target proteins or a rabbit polyclonal antibody against β-actin.
Statistical analysis
Significant differences were determined by Student t test comparisons for at least 3 experiments each, with triplicates performed for each experiment.
Results
Delayed hemangioblast development in absence of SIRT1
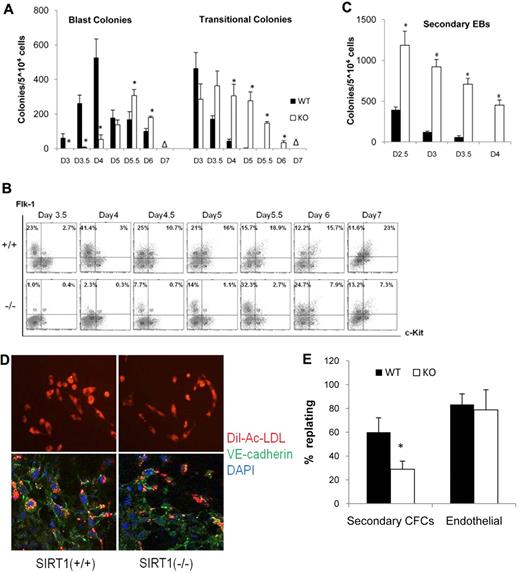
To assess whether SIRT1 plays a role in hemangioblast development, the ability of SIRT1 deficient ESCs to give rise to BL-CFCs was tested. BL-CFCs were analyzed on day 3, 3.5, 4, 5, 5.5, 6, and 7, respectively. As shown in Figure 1A, SIRT1+/+ EBs generated BL-CFCs from day 3 and peaked on day 4. SIRT1−/− EBs did not generate significant numbers of blast colonies typical of SIRT+/+ EBs until day 4; at day 4, SIRT1−/− BL-CFCs were still significantly decreased. SIRT1−/− BL-CFCs peaked on day 5.5. No colonies were generated in both cell lines on day 7. BL-CFC development in SIRT1−/− EBs is thus restricted to a narrower and later window of time, than found in SIRT1+/+ EBs. Meanwhile, transitional colonies, which have similar morphology as those developed in Scl−/− ESCs28 were most readily detected in the SIRT1−/− cultures (Figure 1A).
Blast cell colony development from WT (SIRT1+/+) and SIRT1-deficient (SIRT1−/−) ESCs. (A) Kinetics of blast and transitional colony development in SIRT1+/+ and SIRT1−/− EBs. Data are shown as mean ± SD. N = 3; *P < .01. Days (D) of differentiation are indicated. Δ = no colony grown. (B) fluorescence-activated cell sorting analysis of Flk1 and c-Kit expression on SIRT1+/+ and SIRT1−/− EB-derived cells. Days of differentiation are indicated. Numbers in each quadrant represent the percent of total population in each fraction. (C) Secondary EB colonies generated by SIRT1+/+ and SIRT1−/− EB cells. Days of differentiation are indicated. Data are shown as mean ± SD. N = 3; *P < .01. (D) Immunostaining and Dil-Ac-LDL uptake of adhesive cells generated from a single blast colony from SIRT1+/+ and SIRT1−/− EB-derived cells. VE-cadherin expression is indicated by green fluorescence and LDL uptake by red fluorescence. (E) Evaluation of the hematopoietic and endothelial potentials of BL-CFCs. The number of colonies that yielded secondary CFCs or adherent endothelial cells is divided by the total number of replated colonies. Bars represent SEM number from at least 3 experiments; *P < .01.
Blast cell colony development from WT (SIRT1+/+) and SIRT1-deficient (SIRT1−/−) ESCs. (A) Kinetics of blast and transitional colony development in SIRT1+/+ and SIRT1−/− EBs. Data are shown as mean ± SD. N = 3; *P < .01. Days (D) of differentiation are indicated. Δ = no colony grown. (B) fluorescence-activated cell sorting analysis of Flk1 and c-Kit expression on SIRT1+/+ and SIRT1−/− EB-derived cells. Days of differentiation are indicated. Numbers in each quadrant represent the percent of total population in each fraction. (C) Secondary EB colonies generated by SIRT1+/+ and SIRT1−/− EB cells. Days of differentiation are indicated. Data are shown as mean ± SD. N = 3; *P < .01. (D) Immunostaining and Dil-Ac-LDL uptake of adhesive cells generated from a single blast colony from SIRT1+/+ and SIRT1−/− EB-derived cells. VE-cadherin expression is indicated by green fluorescence and LDL uptake by red fluorescence. (E) Evaluation of the hematopoietic and endothelial potentials of BL-CFCs. The number of colonies that yielded secondary CFCs or adherent endothelial cells is divided by the total number of replated colonies. Bars represent SEM number from at least 3 experiments; *P < .01.
To further assess if SIRT1−/− affects in vitro differentiation potential of ESCs, we analyzed temporal expression patterns of cell surface Flk1 and c-Kit. Flk1 is the receptor 2 for VEGF (VEGF-R2). Others demonstrated that Flk-1 is expressed on BL-CFCs, and onset of Flk1 corresponds to BL-CFC development. c-Kit is expressed on ESCs and populations found at early stages of EB development;3,24 its expression is down-regulated after differentiation. Beyond the hemangioblast stage, c-Kit is coexpressed with Flk1 marking establishment of hematopoiesis.29 As shown in Figure 1B, a distinct Flk1+, c-Kit− cell population (Flk1+/c-Kit−) emerged in SIRT1+/+ EBs by day 3.5 of differentiation and increased during the next 24 hours. This window defines the hemangioblast stage of development, as demonstrated by presence of BL-CFCs at those times. In SIRT1−/− EBs, emergence of a distinct Flk1+/c-Kit− population was reproducibly detected at least 12 hours later than in SIRT1+/+ EBs, and the percent of SIRT1−/− Flk+/c-Kit− cells was significantly decreased on days 3.5 and 4. The average percentage and SEM in 3 independent experiments of Flk1+/c-kit− cells was: 27.7 ± 4.6 in SIRT1+/+ and 2.3 ± 1.3 in SIRT1−/− EBs at day 3.5 of differentiation; 45.7 ± 3.7 in SIRT1+/+ and 3.8 ± 0.8 in SIRT1−/− EBs at day 4; by day 5.5, there was a switch with 14.8 ± 1.8 in SIRT1+/+ and 31.7 ± 3.7 in SIRT1−/− EBs. Delayed emergence of Flk1+/c-Kit− cells in SIRT1−/− EBs was consistent with delayed BL-CFC development in SIRT1−/− EBs (Figure 1A). This delay appears to result from immature differentiation of the SIRT1−/− ESCs as kinetics of decline in transitional colonies and secondary EBs, is delayed compared with SIRT1+/+ ESCs (Figure 1A,C).
SIRT1-deficient BL-CFCs have reduced hematopoietic, but unaffected endothelial, replating potential
To assess developmental potential of blast colonies, single SIRT1+/+ and SIRT1−/− BL-CFCs were replated, transferred to microtiter wells, and cultured for 4 days to determine their potential to generate adherent (endothelial) and nonadherent (hematopoietic) cells. Considering the differing peak of blast colony formation in SIRT1+/+ and SIRT1−/− ESCs, day 4 SIRT1+/+ and day 5.5 SIRT1−/− blast colonies were used, respectively. After expansion, nonadherent cells of each well were replated in methycellulose to assay hematopoietic potential. Adherent cells were cultured for an additional week and then harvested and analyzed for expression of genes associated with the endothelial lineage. Endothelial cells express vascular endothelial cadherin (VE-cadherin, CD144) and take up acetylated low-density lipoprotein (Ac-LDL).25 Colonies from SIRT1+/+ and SIRT1−/− cells generated both types of cells. Adherent cells derived from SIRT1+/+ and SIRT1−/− BL-CFCs, both displayed Dil-Ac-LDL uptake (red) as well as CD144 expression (green; Figure 1D), indicating that adherent populations derived from SIRT1+/+ and SIRT1−/− individual blast colonies are of the endothelial lineage. Replating revealed that, whereas SIRT1+/+ and SIRT1−/− generated endothelial cells at a similar frequency, the nonadherent populations from SIRT1−/− blast colonies contained fewer secondary hematopoietic precursors with colony-forming potential (Figure 1E). Transitional colonies derived from SIRT1−/− ESCs were also replated and analyzed. They were able to give rise to adherent (endothelial) populations (data not shown). However, they did not generate round nonadherent (hematopoietic) cells after a 4-day culture, which suggests their inability to generate hematopoietic progeny.
Hematopoietic cell differentiation of SIRT1−/− cells is defective
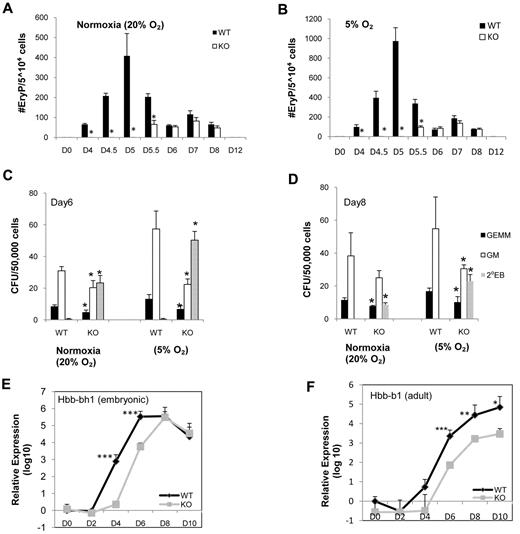
Defective hematopoietic development of blast colonies in SIRT1−/− EBs prompted us to investigate their primitive and definitive hematopoietic potential. ESCs were differentiated into EBs for 4-12 days, at which time cells were disrupted and plated for primitive erythroid (EryP) and definitive colonies. In addition to delay of hemangioblast development and limited hematopoietic potential from SIRT1−/− blast colonies, SIRT1−/− EBs displayed delayed and greatly decreased hematopoietic commitment as demonstrated by later generation of Flk1+/c-Kit+ cells (Figure 1B) and presence of primitive precursors by day 5.5 of differentiation compared with day 4 for SIRT1+/+ cells (Figure 2A). Low oxygen promotes erythropoiesis and other forms of hematopoiesis. Because low oxygen tension affects cellular redox state and SIRT1 is a redox-sensitive molecule,30 we assessed the ability of SIRT1+/+ and SIRT1−/− EBs to generate erythroid precursors under 5% O2. Lowered O2 allowed increased detection of EryP colonies from both SIRT1+/+ and SIRT1−/− cells. However, even under lowered O2, no SIRT1−/− cell-derived colonies were noticeable until day 5.5, even though the number of SIRT1+/+ colonies was greatly enhanced under the lowered O2. The defect in generation of SIRT1−/− EryP colonies was thus readily apparent at both oxygen tensions (Figure 2A-B; note different scales for colony numbers). We observed somewhat smaller, but still significant decreases in numbers of definitive granulocyte macrophage (CFU-GM) and multipotential (CFU-GEMM) progenitor cells obtained from SIRT1−/− compared with SIRT1+/+ cells at day 6 and 8 (Figure 1C-D). But the differences of CFU-GM (1.6620% O2 versus 2.565% O2 on day 6, 1.5320% O2 versus 1.795% O2 on day 8) between SIRT1+/+ and SIRT1−/− EBs are greater at low O2. Differences in fold changes of cells grown at normoxia and 5% O2 suggest that SIRT1−/− cells are not as responsive as SIRT1+/+ cells to the enhancing effect of lowered O2 tension. In addition, SIRT1−/− cells had higher numbers of secondary EBs (Figure 1C-D). We also evaluated mRNA levels of both embryonic and adult globin genes and observed reduction in expression of both genes in SIRT1−/− cells (Figure 2E-F). These results suggest a delay/defect in hematopoietic commitment upon SIRT1−/− ESC differentiation.
Kinetic analysis of primitive and definitive hematopoietic colonies generated from WT (SIRT1+/+) and knockout (KO; SIRT1−/−) EB cells under normoxia (20% O2) and 5%O2. Primitive erythroid colonies generated from SIRT1+/+ and SIRT1−/− EB-derived cells under (A) normoxia and (B) lower (5%) O2 tension. Definitive GEMM, multilineage progenitors of granulocytes, erythroid cells, macrophages, megakaryocytes; GM, multilineage progenitors of granulocytes and macrophages; and secondary EBs generated from SIRT1+/+ and SIRT1−/− EB-derived cells under normoxia condition and lower (5%) O2 tension at day 6 (C) and day 8 (D). Data are shown as mean ± SD. N = 3; *P < .05. Relative mRNA expression of embryonic globin (E) and adult globin (F) genes from SIRT1+/+ and SIRT1−/− day 0, 2, 4, 6, 8, and 10 EBs by real-time RT-PCR. Error bars indicate SDs from the average of 3 independent experiments, each performed in triplicate; ***P < .001; **P < .01; *P < .05.
Kinetic analysis of primitive and definitive hematopoietic colonies generated from WT (SIRT1+/+) and knockout (KO; SIRT1−/−) EB cells under normoxia (20% O2) and 5%O2. Primitive erythroid colonies generated from SIRT1+/+ and SIRT1−/− EB-derived cells under (A) normoxia and (B) lower (5%) O2 tension. Definitive GEMM, multilineage progenitors of granulocytes, erythroid cells, macrophages, megakaryocytes; GM, multilineage progenitors of granulocytes and macrophages; and secondary EBs generated from SIRT1+/+ and SIRT1−/− EB-derived cells under normoxia condition and lower (5%) O2 tension at day 6 (C) and day 8 (D). Data are shown as mean ± SD. N = 3; *P < .05. Relative mRNA expression of embryonic globin (E) and adult globin (F) genes from SIRT1+/+ and SIRT1−/− day 0, 2, 4, 6, 8, and 10 EBs by real-time RT-PCR. Error bars indicate SDs from the average of 3 independent experiments, each performed in triplicate; ***P < .001; **P < .01; *P < .05.
Deletion of SIRT1 results in defective formation of a vascular-like network in vitro
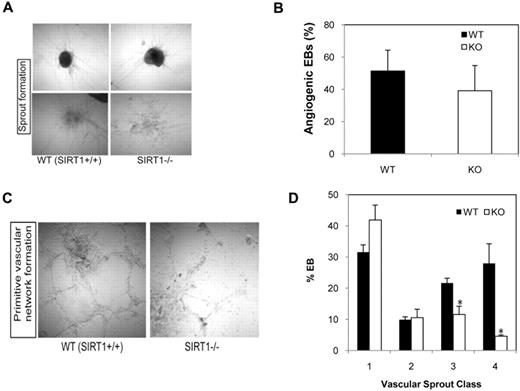
To assess a role for SIRT1 in angiogenesis, vascular sprout formation developed from day 6 EBs was assessed using a 3-dimensional spheroidal assay. SIRT1+/+ and SIRT1−/− EBs developed similar endothelial outgrowths when embedded into collagen gel (Figure 3A). Quantitative analysis on the percentage of angiogenic EBs revealed that loss of SIRT1 expression does not significantly affect endothelial sprout formation (Figure 3B). However, SIRT1−/− cells did not form a full vascular-like network in vitro (Figure 3C); they had significantly fewer class III (many sprouts but not network) and class IV (abundant networked tubules) vascular sprout formations than SIRT1+/+ EBs (Figure 3D).
Effect of SIRT1 deletion on in vitro vascular sprout formation in differentiating EBs. (A) Representative micrographs of 3-dimensional in vitro angiogenesis assays with collagen gel-embedded spheroids generated from WT (SIRT1+/+) or KO (SIRT1−/−) day 6 EBs. (B) Analysis of the percentage of angiogenic EBs was performed after 8 days of secondary culture in collagen gel. (C) Representative micrographs of in vitro matrigel assays with SIRT1+/+ or SIRT1−/− day 6 EBs. (D) Percentage of each class of vascular sprouting (see “Vascular sprout formation assay”). Data are shown as mean ± SEM. N = 3; *P = .01-.04. Image acquisition details: Nikon Diaphot microscope, 10×/0.25 numeric aperature objective lens, Nikon F3 camera, NIS-Elements D2.30 software.
Effect of SIRT1 deletion on in vitro vascular sprout formation in differentiating EBs. (A) Representative micrographs of 3-dimensional in vitro angiogenesis assays with collagen gel-embedded spheroids generated from WT (SIRT1+/+) or KO (SIRT1−/−) day 6 EBs. (B) Analysis of the percentage of angiogenic EBs was performed after 8 days of secondary culture in collagen gel. (C) Representative micrographs of in vitro matrigel assays with SIRT1+/+ or SIRT1−/− day 6 EBs. (D) Percentage of each class of vascular sprouting (see “Vascular sprout formation assay”). Data are shown as mean ± SEM. N = 3; *P = .01-.04. Image acquisition details: Nikon Diaphot microscope, 10×/0.25 numeric aperature objective lens, Nikon F3 camera, NIS-Elements D2.30 software.
SIRT1 deficiency affects expression of genes involved in mESC differentiation and hematopoietic commitment
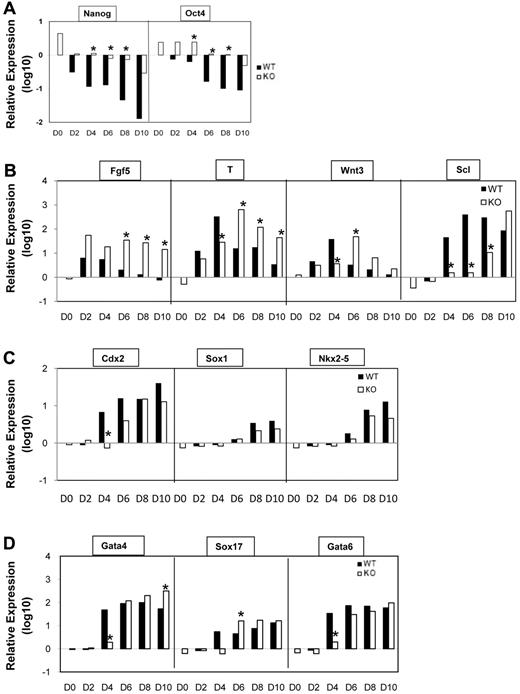
Given the inappropriate differentiation and reduced hematopoietic potential of SIRT1−/− ESCs, we collected RNA from EBs and quantified transcript levels of genes (by quantitative reverse transcription PCR [RT-PCR]) that might shed light on loss of ESC differentiation potential. This includes genes involved in development of epiblast-like cells, onset of mesoderm development, and markers for other germ layers in EBs at different stages. Expression of Oct4 (Pou5f1) and Nanog, well known pluripotency transcription factors, were rapidly shut down upon differentiation of SIRT1+/+ cells, consistent with rapid loss of potential to generate secondary EBs. However, down-regulation of Oct4 and Nanog was remarkably delayed in SIRT1−/− cells upon differentiation (Figure 4A). Notably Fgf5, a gene expressed in the epiblast of early embryos and down-regulated after prehemangioblast mesoderm stage27 was increased and maintained in SIRT1−/− EBs. Meanwhile, expression of T and Wnt3, indicative of mesoderm commitment and development, was delayed and sustained (Figure 4B). These gene expression pattern, combined with the results of blast and transitional colony formation in SIRT1−/− EBs, indicates the earliest stages of hemangioblast commitment are occurring, but not fully developed in SIRT1−/− ESCs at the appropriate time points. We further analyzed expression of Scl/Tal-1, a transcription factor essential for commitment of mesoderm to hematopoiesis.28 It was delayed at least 24 hours and greatly decreased on days 4, 6, and 8 in SIRT1−/− EBs (Figure 4B), which is consistent with the interpretation that progression of hematopoietic commitment was compromised in SIRT1−/− cultures. Trophectoderm marker Cdx2 (Figure 4B), and molecular markers for endoderm/mesoderm GATA-4, Sox17, and GATA-6 showed delayed expression in SIRT1−/− cells, but at later days, there was no difference between the expression of these genes in SIRT1+/+ and SIRT1−/− EBs (Figure 4C). Expression of a gene for cardiac lineage marker Nkx2.5 showed no difference. In addition, the hematopoietic-specific gene GATA-1 was reduced in day 5 SIRT1−/− EBs. Notably, GATA-1 at late stage and Runx-1 were nonsignificantly decreased (see supplemental Figure 2A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), suggesting that SIRT1 may largely affect early stages of ESC commitment to hematopoietic lineage. Together, these data suggest that SIRT1 is critically involved in gene expression for early commitment and differentiation.
Gene expression analysis of WT (SIRT1+/+) and KO (SIRT1−/−) EBs by real-time PCR analysis. (A) Quantitative RT-PCR (qRT-PCR) analysis of stem cell marker Oct4 and Nanog mRNA levels in day 0-10 SIRT1+/+ and SIRT1−/− EBs. (B) qRT-PCR analysis of mRNA levels of epiblast marker Fgf5, mesoderm markers T, Wnt3, and transcription factor Tal1/Scl in day 0-10 SIRT1+/+ and SIRT1−/− EBs. (C) qRT-PCR analysis of mRNA levels of trophectoderm marker Cdx2, neuroectoderm marker Sox1, and cardiac lineage marker Nkx2-5 in day 0-10 SIRT1+/+ and SIRT1−/− EBs. (D) qRT-PCR analysis of mRNA levels of endoderm/mesoderm markers GATA-4, GATA6, and Sox17 in day 0-10 SIRT1+/+ and SIRT1−/− EBs. Day (D) 0 represents undifferentiated ESCs. Graphs in panels A through D were plotted in logarithmic scale. Data are relative to SIRT1+/+ D0 control and mRNA levels of each gene were compared between SIRT1+/+ and SIRT1−/− EBs at each time point. Results were the average of 3 independent experiments, each performed in triplicate. *P < .05.
Gene expression analysis of WT (SIRT1+/+) and KO (SIRT1−/−) EBs by real-time PCR analysis. (A) Quantitative RT-PCR (qRT-PCR) analysis of stem cell marker Oct4 and Nanog mRNA levels in day 0-10 SIRT1+/+ and SIRT1−/− EBs. (B) qRT-PCR analysis of mRNA levels of epiblast marker Fgf5, mesoderm markers T, Wnt3, and transcription factor Tal1/Scl in day 0-10 SIRT1+/+ and SIRT1−/− EBs. (C) qRT-PCR analysis of mRNA levels of trophectoderm marker Cdx2, neuroectoderm marker Sox1, and cardiac lineage marker Nkx2-5 in day 0-10 SIRT1+/+ and SIRT1−/− EBs. (D) qRT-PCR analysis of mRNA levels of endoderm/mesoderm markers GATA-4, GATA6, and Sox17 in day 0-10 SIRT1+/+ and SIRT1−/− EBs. Day (D) 0 represents undifferentiated ESCs. Graphs in panels A through D were plotted in logarithmic scale. Data are relative to SIRT1+/+ D0 control and mRNA levels of each gene were compared between SIRT1+/+ and SIRT1−/− EBs at each time point. Results were the average of 3 independent experiments, each performed in triplicate. *P < .05.
Aberrant signaling pathways during differentiation of SIRT1−/− ESCs
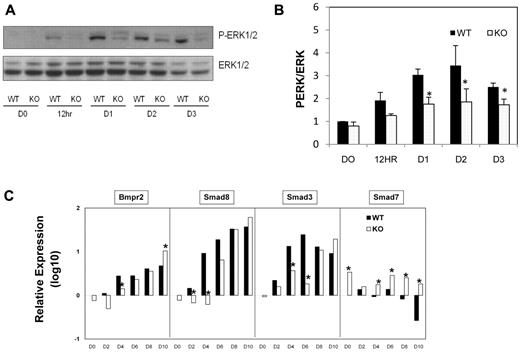
Activation of the Ras-Raf-Mek-Erk signaling cascade is important for morphology and gene expression changes indicative of early ESC differentiation by LIF withdrawal.31 When the Erk pathway is inhibited in LIF withdrawn cells, the pluripotent marker Oct4 shows delayed down-regulation.31 SIRT1 inhibition reduces Erk1/2 signaling in cultured neurons,32 so we assessed whether delay in loss of pluripotency markers in SIRT1−/− EBs is associated with effects on the Erk pathway during SIRT1−/− ESC commitment. Phosphorylated Erk1/2 was gradually increased during early SIRT1+/+ ESC differentiation, consistent with a previous report.31 However, Erk phosphorylation was greatly impaired in SIRT1−/− cells (Figure 5A-B). To evaluate signaling pathways for mesodermal differentiation within EBs, high-throughput mRNA expression profiling was used to examine gene expression involved in bone morphogenetic protein (BMP), FGF, and Wnt pathways. Expression of Bmpr, smad8, and smad3 was delayed in SIRT1−/− compared with SIRT1+/+ EBs. Smads are intracellular signal transducers of the BMP pathway. Among them, Smad7 is an inhibitory molecule for the BMP-SMAD pathway.33 Smad7 inhibits mesoderm formation and promotes neural cell fate in Xenopus embryos.34 Of note, Smad7 was persistently expressed at higher levels in SIRT1−/− than in SIRT1+/+ EBs (Figure 5C). This is interesting in light of Smad 7 down-regulation by SIRT1.35 Expression of several components of FGF and Wnt signal transduction pathways were altered in SIRT1−/− EBs (supplemental Figure 2A-B). FGF and Wnt pathways are implicated in hematopoietic development through protein network interactions,36,37 suggesting that SIRT1 deficiency may impair embryonic hematopoiesis indirectly through broad signal transduction networks. Our gene array expression analysis indicates a role for SIRT1 in intracellular signaling during hematopoietic cell development.
Western Blot analysis of Erk signaling pathways and qRT-PCR analysis of gene expression in day 0-10 SIRT1+/+ and SIRT1−/− EBs. (A) Representative blot and (B) quantification showing phosphorylation of Erk and Erk1/2 protein expression at different time points during EB development. Data are shown as mean ± SEM. N = 3; *P < .05. (C) qRT-PCR analysis of mRNA levels of genes (BMPr2, Smad8, Smad3, Smad7) involved in BMP pathways in day 0-10 SIRT1+/+ and SIRT1−/− EBs. D0 represents undifferentiated ESCs. Graphs in panel C were plotted in logarithmic scale. Data are relative to SIRT1+/+ D0 control and mRNA levels of each gene were compared between SIRT1+/+ and SIRT1−/− EBs at each time point. Results were of 3 independent experiments performed in triplicate. *P < .05.
Western Blot analysis of Erk signaling pathways and qRT-PCR analysis of gene expression in day 0-10 SIRT1+/+ and SIRT1−/− EBs. (A) Representative blot and (B) quantification showing phosphorylation of Erk and Erk1/2 protein expression at different time points during EB development. Data are shown as mean ± SEM. N = 3; *P < .05. (C) qRT-PCR analysis of mRNA levels of genes (BMPr2, Smad8, Smad3, Smad7) involved in BMP pathways in day 0-10 SIRT1+/+ and SIRT1−/− EBs. D0 represents undifferentiated ESCs. Graphs in panel C were plotted in logarithmic scale. Data are relative to SIRT1+/+ D0 control and mRNA levels of each gene were compared between SIRT1+/+ and SIRT1−/− EBs at each time point. Results were of 3 independent experiments performed in triplicate. *P < .05.
Reintroduction of SIRT1 gene into SIRT1−/− ESCs enhances rescue of normal hematopoietic phenotype of SIRT1−/− EBs
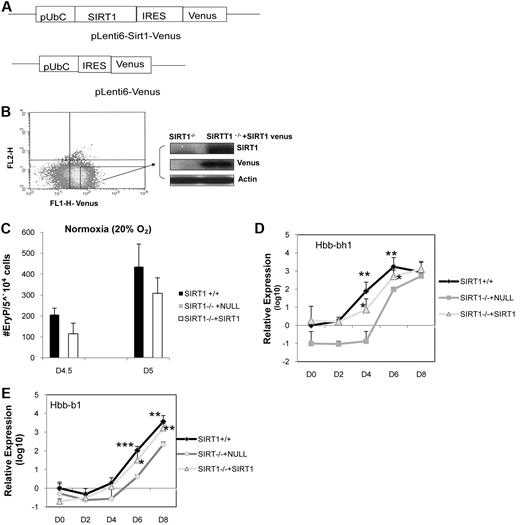
To examine whether the abnormal differentiation phenotype of SIRT1−/− cells can be corrected by adding back a wild-type SIRT1 gene, in vitro gene reconstitution studies were performed. Vectors were constructed containing Venus or the SIRT1 gene under control of a Ubc promoter (Figure 6A), giving levels of SIRT1 gene expression proportional to Venus fluorescence intensity (Figure 6B). Vector containing Venus gene alone was used as a control. ESCs were efficiently infected, as shown by flow cytometry 48 hours after infection (Figure 6B). ESCs with high level expression of Venus were sorted and analyzed for SIRT1 expression. Western blot analysis demonstrated that SIRT1 was expressed in SIRT1−/− cells transfected with the SIRT1 vector (Figure 6B). SIRT1−/− cells generated few EryP on day 4.5, but numbers of primitive progenitors in SIRT1−/− cells were significantly increased with reconstitution of SIRT1 to a level comparable with SIRT1+/+ cells (Figure 6C). In addition, expression of hemoglobin genes, Hbb-bh1 (Figure 6D) and Hbb-b1 (Figure 6E) was recovered in SIRT1−/− cells with the SIRT1 gene insert vector, compared with SIRT1+/+ cells. This demonstrates that the altered hematopoietic differentiation potential observed for SIRT1−/− cells was specific to deletion of SIRT1.
Ectopic expression of exogenous SIRT1 in SIRT1−/− ESCs. (A) Diagram of pLenti6-SIRT1-Venus lentiviral vector. (B) Transfected SIRT1−/− ESCs were sorted, and expression of SIRT1 protein was determined by Western blotting. (C) Number of primitive erythroid colonies generated by day 4.5 and day 5 SIRT1+/+, SIRT1−/− ESCs. (D-E) qRT-PCR analysis of mRNA levels of embryonic globin (Hbb-bh1) and adult globin (Hbb-b1) in day (D) 0-8 SIRT1+/+, SIRT1−/− plus null and SIRT1−/− +SIRT1 venus EBs. Data represent fold changes in EBs, relative to SIRT1+/+ D0 control and comparison between SIRT1+/+, SIRT1−/− plus null, and SIRT1−/− plus SIRT1 EBs at each time point. Results are average of 3 independent experiments. Each data point denotes 6 biological replicates; *P < .05; **P < .01; ***P < .001.
Ectopic expression of exogenous SIRT1 in SIRT1−/− ESCs. (A) Diagram of pLenti6-SIRT1-Venus lentiviral vector. (B) Transfected SIRT1−/− ESCs were sorted, and expression of SIRT1 protein was determined by Western blotting. (C) Number of primitive erythroid colonies generated by day 4.5 and day 5 SIRT1+/+, SIRT1−/− ESCs. (D-E) qRT-PCR analysis of mRNA levels of embryonic globin (Hbb-bh1) and adult globin (Hbb-b1) in day (D) 0-8 SIRT1+/+, SIRT1−/− plus null and SIRT1−/− +SIRT1 venus EBs. Data represent fold changes in EBs, relative to SIRT1+/+ D0 control and comparison between SIRT1+/+, SIRT1−/− plus null, and SIRT1−/− plus SIRT1 EBs at each time point. Results are average of 3 independent experiments. Each data point denotes 6 biological replicates; *P < .05; **P < .01; ***P < .001.
Defective primitive hematopoiesis during early development and decreased adult hematopoiesis in SIRT1−/− mice
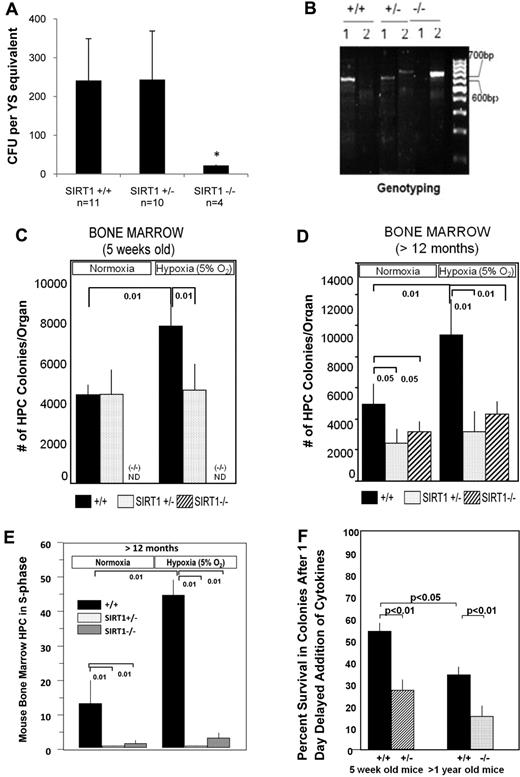
Based on above in vitro studies, we evaluated embryo and adult bone marrow hematopoiesis in SIRT1+/+, SIRT1+/−, and SIRT1−/− mice.10 We performed in vitro colony-forming assays on yolk sac cells from E8 to E8.25 embryos. We observed significant decreases in absolute numbers of primitive erythroid progenitors from SIRT1−/−, but not SIRT1+/−, E8 to E8.25 yolk sacs under normoxic conditions compared with SIRT1+/+ yolk sacs (Figure 7A). Genotypes of the yolk sacs were confirmed (Figure 7B). Less than 10% of SIRT1−/− mice survive to birth,10 but SIRT1+/− mice are born with normal Mendelian genetics. We assessed absolute numbers of hematopoietic progenitor cells (CFU-GM, BFU-E, and CFU-GEMM) per femur of 5-week-old SIRT1+/− versus SIRT1+/+ mice detected via culturing bone marrow cells in vitro at both ∼ 20% and 5% O2 tension. In that way, we could determine whether there were differences in progenitor cell numbers in the SIRT1+/− and SIRT1+/+ cells, and whether or not the growth of these cells could be enhanced by growth in lowered O2. While we detected no significant differences in colony numbers of SIRT1+/+ and SIRT1+/− cells grown in vitro under normoxic conditions from bone marrow of 5-week-old mice, we found that SIRT1+/+, but not SIRT1+/− cells were enhanced in numbers at lowered O2 (Figure 7C). Thus, SIRT1+/− cells do not sense enhanced growth conditions of lowered O2. We were also able to evaluate SIRT1+/+, SIRT1+/−, and SIRT1−/− mice that were more than 1 year old. As shown in Figure 7D, absolute numbers of SIRT1+/− and SIRT1−/− progenitors were significantly decreased by almost half when cells were cultured under normoxic conditions, suggesting an age-related decrease in SIRT+/− numbers. Consistent with the SIRT1+/− data from 5-week-old mice (Figure 7C), neither SIRT1+/− nor SIRT1−/− progenitors from the more aged mice responded to the enhancing effects of lowered O2 that the SIRT1+/+ cells responded to, confirming that SIRT1+/−, and also SIRT1−/− progenitors from adult bone marrow fail to respond to lowered O2. At lowered O2, the differences detected in numbers of progenitor cells in S-phase from SIRT1+/+ mice to that of either SIRT1+/− or SIRT1−/−, was substantially greater than that seen at normoxia (Figure 7E). Nucleated bone marrow cellularities/femur did not show significant differences between SIRT1+/+, SIRT1+/−, or SIRT1−/− mice (supplemental Table 1). We also assessed the in vitro survival of hematopoetic progenitor cells (CFU-GM) from 5-week- and more than 1-year-old mice growing at 5% O2 in response to growth factor deprivation and delayed addition of EPO, PWMSCM, and TPO at day 1 after culture in the presence of FBS. Seven days after addition of cytokines there was a significantly greater decrease in colony formation for 5-week-old bone marrow of SIRT1+/− mice and SIRT1−/− marrow of > 1-year-old mice compared with their comparable aged matched SIRT1+/+ mice (Figure 7F). This demonstrates that SIRT1+/− and SIRT1−/− progenitors survive less well than the SIRT1+/+ progenitors, under these conditions of growth factor deprivation. In addition, there was a significantly greater decrease in progenitor cells from older compared with younger SIRT1+/+ mice.
Hematopoietic progenitor cell colony forming assay from yolk sac and bone marrow of SIRT1+/+, SIRT1+/−, and SIRT1−/− mice. (A) Primitive erythroid colony formation from yolk sac (YS) cells generated from E8 to E8.25 SIRT1+/+, SIRT+/−, and SIRT1−/− embryos (SIRT1+/+, n = 11; heterozygous, n = 10; SIRT1−/−, n = 4) were grown in methylcellulose-based medium. Data are shown as mean ± SD; *P < .01. (B) PCR shows the genotyping of SIRT1 mutant embryos. The primers are: forward-5′-TCCTTGCCACAGTCACTCAC-3′, reverse for wild-type 5′-CATCTAAACTTTGTTGGCTGC-3′, reverse for deletion 5′-ACAGTCCCATTCCCATACC-3′. (C) Hematopoietic progenitor cell colony formation from bone marrow of 5-week-old SIRT1 heterozygote (SIRT1+/−) or wild-type controls (SIRT1+/+) under normoxia (20% O2) and 5% O2 conditions. Data are shown as mean ± SD; *P < .01; ND = SIRT1−/− cells were not available for use and were not done. (D) Hematopoietic progenitor cell colony formation from bone marrow of 12-month-old SIRT1 heterozygote (SIRT1+/−) or homozygote (SIRT1−/−) null mice or from wild-type controls (SIRT1+/+) under normoxia (20% O2) or 5% O2 conditions. (E) Cycling status of mouse bone marrow progenitor cells generated from 12-month-old SIRT1+/+, SIRT1+/−, and SIRT1−/− mice under normoxia (20% O2) and 5% O2 conditions. This shows percent progenitors in DNA synthesis (S-phase) as determined by high specific activity tritiated thymidine kill technique.23 P values were calculated with Student t test. ND = not done. Data are the average of 3-5 mice/group. (F) Survival of hematopoietic progenitor cells in vitro after 1 day delayed addition of cytokines as denoted by decreased colony formation. Results are expressed as mean ± ISEM for 4-5 mice per group.
Hematopoietic progenitor cell colony forming assay from yolk sac and bone marrow of SIRT1+/+, SIRT1+/−, and SIRT1−/− mice. (A) Primitive erythroid colony formation from yolk sac (YS) cells generated from E8 to E8.25 SIRT1+/+, SIRT+/−, and SIRT1−/− embryos (SIRT1+/+, n = 11; heterozygous, n = 10; SIRT1−/−, n = 4) were grown in methylcellulose-based medium. Data are shown as mean ± SD; *P < .01. (B) PCR shows the genotyping of SIRT1 mutant embryos. The primers are: forward-5′-TCCTTGCCACAGTCACTCAC-3′, reverse for wild-type 5′-CATCTAAACTTTGTTGGCTGC-3′, reverse for deletion 5′-ACAGTCCCATTCCCATACC-3′. (C) Hematopoietic progenitor cell colony formation from bone marrow of 5-week-old SIRT1 heterozygote (SIRT1+/−) or wild-type controls (SIRT1+/+) under normoxia (20% O2) and 5% O2 conditions. Data are shown as mean ± SD; *P < .01; ND = SIRT1−/− cells were not available for use and were not done. (D) Hematopoietic progenitor cell colony formation from bone marrow of 12-month-old SIRT1 heterozygote (SIRT1+/−) or homozygote (SIRT1−/−) null mice or from wild-type controls (SIRT1+/+) under normoxia (20% O2) or 5% O2 conditions. (E) Cycling status of mouse bone marrow progenitor cells generated from 12-month-old SIRT1+/+, SIRT1+/−, and SIRT1−/− mice under normoxia (20% O2) and 5% O2 conditions. This shows percent progenitors in DNA synthesis (S-phase) as determined by high specific activity tritiated thymidine kill technique.23 P values were calculated with Student t test. ND = not done. Data are the average of 3-5 mice/group. (F) Survival of hematopoietic progenitor cells in vitro after 1 day delayed addition of cytokines as denoted by decreased colony formation. Results are expressed as mean ± ISEM for 4-5 mice per group.
Discussion
Although functional requirements for SIRT1 during embryogenesis and gametogenesis have been demonstrated in mammals,12 roles for SIRT1 during ESC differentiation commitment are poorly understood. In this study, using an ESC/EB in vitro differentiation system, we demonstrate that loss of SIRT1 affects the potential and timing of ESCs to exit the pluripotent stem cell program upon LIF withdrawal. The most apparent defect identified is likely delayed shut off of Oct4 and Nanog and persistent Fgf5 expression, which could account for diminished mesoderm commitment. Inactivation of Oct4 occurs concomitantly with deacetylation of its promoter region shortly after differentiation begins38 and the level of Nanog decreases rapidly after LIF withdrawal.39 Upon removal of LIF or induction of differentiation by addition of external signals, SIRT1+/+ ESCs are able to rapidly down-regulate these genes in response to external signals. However, as demonstrated herein, the ability to switch off Oct4 and Nanog expression is delayed by loss of SIRT1. Early differentiation of ESCs into primitive ectoderm, key to formation of the 3 germ layers, was unaffected, as suggested by the presence of Oct4 and Fgf5 expression in day 2 EBs. However, SIRT1−/− EBs persistently express high levels of Fgf5 from day 6 to 10. Further analysis of EB transcriptional profiles indicates delayed and sustained expression of specific mesoderm markers (T and Wnt3) in SIRT1−/− EBs. The delayed/sustained Fgf5/T/Wnt3 gene expression supports functional data regarding delays/defects of development of BL-CFCs and generation of Flk-1+ c-Kit− cells in SIRT1−/− EBs. SIRT1−/− ESCs did not show marked differences in morphology and expression of mES-specific markers (supplemental Figure 3), indicating that in unperturbed conditions, SIRT1 is not required to maintain the undifferentiated phenotype state. This concurs with previous findings12 and studies suggesting that elimination of SIRT1 in mouse TC1 ESCs has little impact on expression of pluripotency factors.40 However, consistent with the latter work40 indicating SIRT1 regulation of developmental genes during differentiation of mouse and human ESCs, SIRT1 depletion most dramatically affected target gene expression profiles during early stage ESC commitment to differentiation.
Our results also suggest that SIRT1 deficiency impairs proper differentiation of blast cell colonies, the hematopoietic developmental potential of blast cells in vitro and, to a lesser extent, of endothelial cells where primitive vascular network formation is impaired. Hematopoietic progenitor differentiation of SIRT1−/− cells is defective. Low-level mRNA expression of genes associated with hematopoietic development such as Scl, β-H1 globin, and β-major globin was detected at days 4, 6, 8 in SIRT1−/− EBs, consistent with the early defect in primitive and definitive hematopoiesis. Basic helix-loop-helix transcription factor Scl is necessary for early stages of in vitro differentiation of mesoderm to hematopoietic lineages and acts during a limited time window.41 Scl−/− ESCs were not able to form blast colonies, but did form transitional colonies.28 Transitional colonies derived from Scl−/− ESCs are unable to generate hematopoietic precursors but do form cells with endothelial characteristics.28 Induction of Scl at later than day 4 could not restore competence to differentiation to hematopoietic progenitor cell fate.41 Mesodermal cells that failed to express Scl at the right stage appear to be irreversibly excluded from a hematopoietic progenitor fate. SIRT1−/− ESCs generated a greater number of transitional colonies instead of blast colonies. SIRT1−/− ESCs were able to form endothelial lineages, but were defective in formation of a full vascular-like network. The progression of hematopoiesis of blast cells was defective in SIRT1−/− EBs. Decreased expression of Scl, combined with sustained expression of Fgf5/T, at the mesoderm stage in SIRT1−/− EBs may explain its inability to adequately generate mature blast colonies and progress into primitive hematopoietic commitment.
ESC pluripotency and cell fate determination are profoundly affected by MAPK, BMP, FGF, and Wnt signaling pathways.42 The Erk pathway is involved in ESC differentiation. As noted, phosphorylation of Erk is less activated in SIRT1−/− cells. An inhibitory Smad for BMP signaling, Smad7, is persistently expressed in SIRT1−/− EBs. Smad7 is involved in early patterning events. Low levels of Smad7 block activation of dorsal mesoderm genes, and high levels block all mesoderm gene expression in Xenopus embryos.34 In mesangial cells, SIRT1 interacts with Smad7. Smad7 expression level was increased by SIRT1 knockdown.35 Persistent expression of Smad7 in SIRT1−/− EBs may account for defective differentiation in SIRT1−/− EBs.
Evidence from mouse models indicates that SIRT1 is a central regulator of embryonic and somatic stem cell function.20,43 We found that lack of SIRT1 expression led to decreases in primitive erythroid progenitor cells in E8 to E8.25 yolk sacs. Because the majority of SIRT1 null embryos die between E9.5 and E14.5, we did not have access to SIRT1−/− embryos at later time points. We cannot exclude the possibility that decreased Ery-P is because there is a developmental delay in SIRT1−/− embryos. Consistent with studies of Wang et al,10 SIRT1−/− embryos were abnormally small. In addition, these embryos show nuclear fragmentation and cell death. This could be another reason for differences in numbers of Ery-P in SIRT1−/− embryos. Analysis of bone marrow of SIRT1−/− adult mice demonstrated decreased hematopoietic progenitor cell numbers and cell cycle status. The decrease was more apparent when cells were cultured under lowered O2 tension. This is of interest because during hypoxia, activated SIRT1 augments hypoxia-inducible factor-2α signaling and consequently participates in regulation of the hypoxia-inducible factor-2α target gene, Epo.44 Congenital SIRT1 deficiency affects fetal and adult Epo gene expression in mice.44 Epo, generally considered an erythropoietic growth factor, is also a potent prosurvival factor that protects developing stem and progenitor cells in a variety of organs.45 Renal Epo gene expression differs between SIRT1+/− and SIRT1+/+ mice exposed to 6% O2, while both groups of mice have similar amounts of renal Epo mRNA under ambient O2.44 Similarly, our data showed that hematopoietic progenitor colony formation from adult mice significantly differed between SIRT1+/+ and SIRT1+/− mice under 5% O2, while both groups of mice had similar numbers under normoxia. We also found that hematopoietic progenitor cells from SIRT1+/+ and SIRT1−/− mice survive less well in vitro at lowered O2 tension after the stress of delayed addition of growth factors. Decreased survival is directly associated with enhanced apoptosis.46
Sirtuins are implicated in aging.47 SIRT1+/− mice are born with apparently normal mendelian genetics, while less than 10% of SIRT1−/− mice live to birth. It is not yet clear why some SIRT1−/− mice live to birth while others do not. We detected no significant differences in primitive erythroid progenitors generated between SIRT1+/− and SIRT1−/− embryos, similar to hematopoietic progenitor cell numbers from 5-week-old SIRT1+/− and SIRT1+/+ mice when cells were cultured under normoxia. That SIRT1+/− cells from adult mice (> 12 months) demonstrate decreased hematopoietic progenitor cell numbers at normoxia, similar to that of SIRT1−/− cells and that SIRT1+/− and SIRT1−/− cells in the aged mice show even greater differences compared with SIRT1+/+ mice when cells are cultured at lowered O2 allows us to evaluate a role for SIRT1 in the hematopoietic aging process, as there will be more SIRT1+/− than SIRT1−/− mice surviving to older ages.
Analyses of SIRT1−/− mice revealed that this protein serves essential functions during embryonic and postnatal development, as well as for several homeostatic programs during adulthood.12-14,48 Our results demonstrate that SIRT1 is an important regulator of mESC differentiation and hematopoiesis, and suggest its role in the earliest stages of hematopoietic and endothelial development. Because hematopoietic and endothelial development is not extinguished by knockout of SIRT1 but rather is delayed, and definitive hematopoiesis is less affected compared with primitive hematopoiesis, there might be distinct pathways of differentiation for primitive and definitive hematopoiesis, or compensation by other sirtuin, or nonsirtuin family members.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Dr Michael W. McBurney (Ottawa Health Research Institute) for providing SIRT1−/− mESCs. We thank Drs Anna Roman and Louis Pelus for advice and support. We thank Myung-Kwan Han, Ying Liu, Tim Campbell, and Yan Fan for technical suggestions.
These studies were supported by US Public Health Service grant nos. RO1 HL56416, RO1 HL67384, and a project in PO1 HL53586 to H.E.B., and R01 AI080759 to M.C.Y. from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: X.O. contributed toward conception and design, the setup and scoring of experiments, collection and assembly of data, data analysis, interpretation, and manuscript writing; H.-D.C. contributed to setup and scoring of experiments; H.-D.C. and R.-H.W. contributed toward data collection, analysis, and interpretation; W.C.S. contributed to setup of experiments and critical suggestions for experiments; S.C. contributed to setup of experiments; T.T., Y.-J.K., C.-X.D., and M.C.Y. contributed toward critical suggestions for experiments and manuscript editing; and H.E.B. contributed toward conception, scoring of experiments and design, financial support, data analysis and interpretation, and manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hal E. Broxmeyer, Department of Microbiology and Immunology, Indiana University School of Medicine, 950 W Walnut St R2-302, Indianapolis, IN 46202; e-mail: hbroxmey@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal